Abstract
Background
From 2008–2013, the European indication for panitumumab required that patients’ tumor KRAS exon 2 mutation status was known prior to starting treatment. To evaluate physician awareness of panitumumab prescribing information and how physicians prescribe panitumumab in patients with metastatic colorectal cancer (mCRC), two European multi-country, cross-sectional, observational studies were initiated in 2012: a physician survey and a medical records review. The first two out of three planned rounds for each study are reported.
Methods
The primary objective in the physician survey was to estimate the prevalence of KRAS testing, and in the medical records review, it was to evaluate the effect of test results on patterns of panitumumab use. The medical records review study also included a pathologists’ survey.
Results
In the physician survey, nearly all oncologists (299/301) were aware of the correct panitumumab indication and the need to test patients’ tumor KRAS status before treatment with panitumumab. Nearly all oncologists (283/301) had in the past 6 months of clinical practice administered panitumumab correctly to mCRC patients with wild-type KRAS status. In the medical records review, 97.5% of participating oncologists (77/79) conducted a KRAS test for all of their patients prior to prescribing panitumumab. Four patients (1.3%) did not have tumor KRAS mutation status tested prior to starting panitumumab treatment. Approximately one-quarter of patients (85/306) were treated with panitumumab and concurrent oxaliplatin-containing chemotherapy; of these, 83/85 had confirmed wild-type KRAS status prior to starting panitumumab treatment. All 56 referred laboratories that participated used a Conformité Européenne-marked or otherwise validated KRAS detection method, and nearly all (55/56) participated in a quality assurance scheme.
Conclusions
There was a high level of knowledge amongst oncologists around panitumumab prescribing information and the need to test and confirm patients’ tumors as being wild-type KRAS prior to treatment with panitumumab, with or without concurrent oxaliplatin-containing therapy.
Introduction
Anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (mAbs), such as panitumumab (Vectibix®, a recombinant, fully human IgG2 mAb) and cetuximab (Erbitux®, a recombinant, chimeric mouse/human IgG1 mAb), bind with high affinity and specificity to the EGFR, and have been shown to be effective across all lines of treatment in metastatic colorectal cancer (mCRC) [1–7]. Reports of improved efficacy with panitumumab and cetuximab in patients with wild-type versus mutant or unknown KRAS exon 2 status [8–15] led to the requirement for physicians to determine a patient’s tumor KRAS mutation status prior to starting treatment with EGFR inhibitors. Physicians can now determine the most appropriate treatment option for individual patients with mCRC, based upon the molecular profile of their tumor.
Panitumumab was first approved in Europe in December 2007 as monotherapy to treat patients with wild-type KRAS exon 2 mCRC who had failed on prior fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens, based upon phase III clinical data [6,8]. Educational materials for panitumumab have been distributed to physicians since 2009, making them aware of the appropriate prescribing information and the need for KRAS mutation status to be determined by an experienced laboratory prior to prescribing panitumumab. In November 2011, data from two additional phase III studies led to the European panitumumab license being expanded to include use in patients with wild-type KRAS exon 2 mCRC in the first-line setting combined with FOLFOX [16], and the second-line setting combined with FOLFIRI [17]. Within the panitumumab licensed indication, concurrent treatment with oxaliplatin-containing chemotherapy in patients with mutant or unknown KRAS mCRC status was contraindicated, due to detrimental effects on progression free survival and overall survival.
Previous physician surveys have found that in 2010, when guidelines first recommended testing for KRAS status [18,19], the adoption of KRAS testing prior to treating patients with EGFR inhibitors varied widely [20–22]. In 2010, 73% (326/448) of participating physicians in Europe reported undertaking appropriate KRAS testing when mCRC was diagnosed, compared with 63% (160/256) in Latin America and 20% (28/139) in Asia [20]. However, there was a rapid and widespread adoption of KRAS testing prior to treating patients with EGFR inhibitors (3% in 2008; 47% in 2009; 69% in 2010), with results available quickly (within 15 days) for more than 80% of patients [20]. In the US, a survey carried out in 2010 reported that of 1,242 physicians responding, only one-fifth of those who had treated mCRC had ordered or recommended KRAS testing [21]. In contrast in another study, a more targeted identification of oncologists treating mCRC found that in 2010 all oncologists (34/34) tested tumor KRAS status [22].
Medical records review studies carried out in 2010 reported that over 94% of patients were being tested for tumor KRAS status prior to being treated with EGFR-targeted therapies [23,24]. In 93 sites from France, 94% of 1,044 patients being treated with cetuximab for CRC had been tested for tumor KRAS status, with wild-type KRAS confirmed in 95% of tested patients [23]. In the US, there was rapid uptake of KRAS tumor testing by 2010 in one study, with 97% of 1,188 patients with mCRC being tested in 2010 compared with 7% of patients being tested in 2006 [24].
In order to evaluate physician awareness of the prescribing information for panitumumab and how physicians prescribe panitumumab in Europe, two studies were initiated in 2012: a physician survey evaluating oncologists’ knowledge of the licensed indication for panitumumab, and a medical records review of patients with mCRC treated with panitumumab to evaluate how oncologists prescribe panitumumab. The first two rounds focused upon KRAS exon 2 status and are reported here. In July 2013, based upon improved outcomes in patients with wild-type versus mutant or unknown tumor RAS status [25], the European license for panitumumab was refined to target panitumumab treatment to patients with wild-type RAS mCRC. The ongoing third and final rounds of each study are focusing on tumor RAS (KRAS exons 2/3/4 and NRAS exons 2/3/4) testing, and will be reported separately in a future publication. Both studies will help to understand the level of acceptance of tumor KRAS/RAS testing by clinicians during their decision processes around treating with panitumumab.
Materials and Methods
Study participants
A physician survey and a medical records review were designed in Europe: both were multi-country, cross-sectional, observational studies with practicing oncologists who had prescribed panitumumab to mCRC patients in the previous 6 months. Rounds 1 and 2 (evaluating KRAS testing) were conducted between September 2012 and December 2013. Round 3 (evaluating RAS testing) is currently being conducted for both studies in 2014 and 2015.
In Round 1, physicians in five countries (France, Germany, Italy, Spain, and the Czech Republic) were invited to participate. Four additional countries (Belgium, Denmark, The Netherlands, and Sweden) were included in Round 2 to increase representation of countries in Europe. In both rounds of each study, physicians were screened by telephone using standardized questionnaires to determine eligibility.
The sampling list used in Round 1 was based on a healthcare industry database provider (Cegedim) medical marketing database and included physician contact details that were not filtered by specialty. Physicians were randomly selected for inclusion in the study from this list. The inclusion of physicians who were not oncologists in the study population partly explains the low response rate observed in Round 1 of the study. In Round 2 of the medical chart review the sampling list used included the medical marketing database filtered by specialty in addition to lists of colorectal cancer physicians. This approach allowed for a more targeted sampling of oncologists and led to a higher physician response rate in Round 2 of the chart review than observed in Round 1.
In order to obtain data on pathology testing, participating oncologists in the medical records review study were asked permission for the investigator to approach the pathology laboratories which carried out KRAS mutation tests, in order to send them a pathology survey to complete.
Study protocols and informed consent forms (ICF) were reviewed and approved by the local Institutional Review Board or Ethical Review Board in each country before the studies began, as required. As agreed with the ethical committees, in France and The Netherlands signed ICFs were not required, only verbal ICF (recorded in the patient’s file). ICFs were required in Germany (reviewed and approved by the Ethik-Kommission der Bayerischen Landesärztekammer), Italy (reviewed and approved by the EC Azienda AOU S. Maria della Misericordia di Udine), Spain (reviewed and approved by the Comite Etico de Investigacion Clinica H.G.U. Gregorio Maranon), Czech Republic (reviewed and approved by the Eticka Komise Masarykova Onkologickeho Ustavu, Brno), Belgium (reviewed and approved by the Medical Ethics Committee des Cliniques Saint-Joseph, Liege) and Sweden (reviewed and approved by the Regional Ethic Committee Board in Sweden—Karolina Institutet/ Solna). Ethics Committee approval was not required in Denmark, but a signed ICF was required in all Danish sites.
Inclusion criteria for participating physicians
Physician survey
Practicing oncology specialists were eligible if they had treated at least five (Round 1) or three (Round 2) new or continuing patients with mCRC in the last 3 months and prescribed panitumumab in the previous 6 months.
Medical records review
Practicing oncologists who had treated at least five (Round 1) or three (Round 2) new or continuing patients with mCRC in the last 3 months, and had prescribed panitumumab to treat new or continuing patients with mCRC in the past 6 months were eligible. Only one oncologist per medical center could participate for each round of the study.
Exclusion criteria for participating physicians
In Round 2 of each study, physicians were excluded if they had already taken part in Round 1. Physicians were excluded from the physician survey if they had taken part in the medical record review (Round 1 or 2). Physicians were excluded from the medical record review if they had taken part in the physicians survey (Round 1 only).
Note that in Round 2 of the medical records review, a more targeted sampling of oncologists was introduced with physicians from specialties that do not treat mCRC being excluded. In addition, in both studies, the number of new or continuing patients with mCRC requiring treatment in the last 3 months was reduced from at least five in Round 1, to at least three in Round 2. These changes were introduced in order to improve physician response rates.
Inclusion criteria for patients
In the medical records review, eligible patients must have received panitumumab (outside of a clinical trial setting) for the treatment of mCRC during the 6-month period prior to the time when medical records were obtained, and have provided written consent to allow access to their medical records (if local laws required it). Informed consent was obtained from a legally acceptable representative of deceased patients, where necessary, to allow access to their medical records for the purpose of this study.
Study objectives
Physician survey
The primary objective is to assess oncologists’ knowledge of the appropriate licensed indication for panitumumab in mCRC patients regarding tumor KRAS status in selected European countries. Specific primary objective measures are to evaluate oncologists’ knowledge: of the licensed indication for panitumumab, which is for the treatment of patients with wild-type KRAS mCRC in first line combined with FOLFOX, in second line combined with FOLFIRI in patients who have received first–line fluoropyrimidine-based chemotherapy (excluding irinotecan), and as monotherapy after failure of fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens; that testing tumor KRAS mutation status should be performed prior to starting treatment with panitumumab; that panitumumab is not indicated for mCRC patients with mutant or unknown KRAS tumor status; that panitumumab should not be administered in combination with oxaliplatin-containing chemotherapy in mCRC patients with mutant or unknown KRAS tumor status. An additional objective measure is to characterize the results of the survey in each round to evaluate whether there are any differences between rounds.
Medical records review
The primary objective is to estimate the prevalence of KRAS testing and the impact of KRAS test results on patterns of panitumumab use in patients with mCRC treated with panitumumab in selected European countries, following changes in the licensed indication regarding the risk of panitumumab use in mCRC patients with mutant KRAS tumors. The primary objective measures include: the estimated proportion of patients with mCRC tested for KRAS status prior to treatment with panitumumab; the proportion of tested patients treated with panitumumab who had mutant, wild-type, or unknown tumor KRAS status; the estimated proportion of patients with mCRC tested for KRAS status prior to treatment with panitumumab who were treated concurrently with oxaliplatin-containing chemotherapy; the proportion of tested patients treated with panitumumab concurrently with oxaliplatin-containing chemotherapy who had mutant, wild-type, or unknown tumor KRAS status. Secondary objective measures include the estimated proportion of: oncologists who agree to participate in the study; oncologists who conduct a KRAS test prior to prescribing panitumumab (in those patients with mCRC who had been treated with panitumumab); laboratories that tested mCRC KRAS status for those patients who were treated with panitumumab who i) participate in the European Society of Pathology (ESP) Quality Assurance (QA) Scheme, or are certified by an ESP-approved accreditation body, and ii) use a Conformité Européenne (CE)-marked or otherwise validated KRAS detection method. An additional objective measure is to characterize the results of each round of the medical records review, and to evaluate whether there are any differences between rounds.
Data collection
Physician survey
In each round of survey, telephone interviews with eligible participating oncologists were conducted by trained study staff using a standardized questionnaire and following consistent procedures and data collection. The questionnaire was carefully translated to ensure that the content and the way of asking questions was consistent across countries. Two pilot interviews were conducted in each country in Round 1 to ensure that all questions were well targeted and correctly understood; questions were reviewed and modified as needed, depending on the outcome of the pilot interviews.
Medical records review
Medical information relating to panitumumab (including but not limited to 3 months before and after the first dose of panitumumab) and tumor KRAS testing was abstracted and anonymized at the site from medical records for transfer into standardized electronic case report forms. Abstracted and anonymized data were checked by trained data extractors prior to being included in the data analysis. Two pilot abstractions were conducted in each country prior to Round 1, to ensure all questions were well targeted and correctly understood, with modifications made as required.
Statistical analysis
In both studies, no formal hypothesis testing was conducted and the data analysis was descriptive in nature. For the categorical study endpoints described above, the count and proportion (%) in each category, based on the appropriate denominator, were calculated. The 95% confidence intervals for the proportions are based on a normal approximation to the binomial distribution.
Results
Physician response rate and baseline characteristics
Physician survey
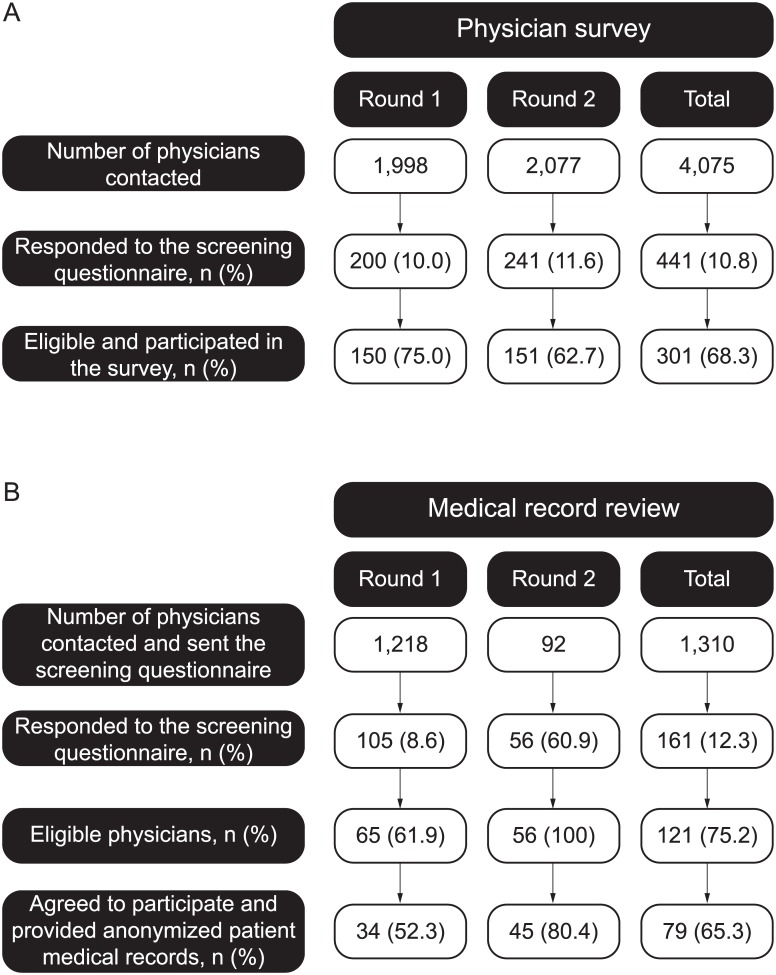
There were low responses in the first two rounds of the physician survey, with 10.8% (441/4,075) of physicians responding to the screening questionnaire (Fig 1A).
Fig 1. Physician disposition for the A) physician survey and B) medical records review studies.
In Rounds 1 and 2 combined, 301 eligible physicians who responded to the screening questionnaire agreed to participate (Fig 1A), and their characteristics were generally similar between rounds (Table 1). Participating oncologists were mainly from university/training hospitals (41.5%; n = 125) and general/regional hospitals (30.2%; n = 91), with the median (Q1–Q3) number of in-patient beds being 400 (100–773) (Table 1). The median (Q1–Q3) number of years of experience as a practicing oncologist specialist was 11 (7–16), with oncologists treating 40 (25–70) mCRC patients in the last 3 months (Table 1). Over three quarters (78.1%; 235/301) of participating oncologists recalled having received educational material regarding KRAS testing.
Table 1. Oncologist demographics in the physician survey and medical records review studies.
| Physician survey | Medical records review | |||||
|---|---|---|---|---|---|---|
| Round 1 | Round 2 | Total | Round 1 | Round 2 | Total | |
| N = 150 | N = 151 | N = 301 | N = 34 | N = 45 | N = 79 | |
| Country, n (%) | ||||||
| France | 50 (33.3) | 39 (25.8) | 89 (29.6) | 14 (41.2) | 9 (20.0) | 23 (29.1) |
| Germany | 35 (23.3 | 44 (29.1) | 79 (26.2) | 15 (44.1) | 11 (24.4) | 26 (32.9) |
| Italy | 28 (18.7) | 18 (11.9) | 46 (15.3) | 1 (2.9) | 5 (11.1) | 6 (7.6) |
| Spain | 25 (16.7) | 19 (12.6) | 44 (14.6) | 3 (8.8) | 5 (11.1) | 8 (10.1) |
| Czech Republic | 12 (8.0) | 6 (4.0) | 18 (6.0) | 1 (2.9) | 4 (8.9) | 5 (6.3) |
| Belgium | 6 (4.0) | 6 (2.0) | 3 (6.7) | 3 (3.8) | ||
| Denmark | 5 (3.3) | 5 (1.7) | 3 (6.7) | 3 (3.8) | ||
| Netherlands | 9 (6.0) | 9 (3.0) | 2 (4.4) | 2 (2.5) | ||
| Sweden | 5 (3.3) | 5 (1.7) | 3 (6.7) | 3 (3.8) | ||
| Type of institution, n (%) | ||||||
| General or regional | ||||||
| hospital | 52 (34.7) | 39 (25.8) | 91 (30.2) | 8 (23.5) | 12 (26.7) | 20 (25.3) |
| Oncology clinic/institute | 16 (10.7) | 14 (9.3) | 30 (10.0) | 0 | 7 (15.6) | 7 (8.9) |
| Private clinic/hospital | 27 (18.0) | 23 (15.2) | 50 (16.6) | 20 (58.8) | 13 (28.9) | 33 (41.8) |
| University/training | ||||||
| hospital | 51 (34.0) | 74 (49.0) | 125 (41.5) | 4 (11.8) | 9 (20.0) | 13 (16.5) |
| Other | 4 (2.7) | 1 (0.7) | 5 (1.7) | 2 (5.9) | 4 (8.9) | 6 (7.6) |
| Size of intitution (number of inpatient beds) | ||||||
| n | 141 | 151 | 292 | 28 | 42 | 70 |
| Mean (SD) | 383.4 (377.1) | 596.5 (515.5) | 493.6 (465.6) | 319.3 (496.1) | 315.3 (549.9) | 316.9 (525.4) |
| Median (Q1–Q3) | 300.0 (50.0–600.0) | 550.0 (200.0–800.0) | 400.0 (100.0–772.5) | 69.5 (7.0–350.0) | 50.0 (17.0–400.0) | 50.0 (17.0–400.0) |
| Number of years' experience as a practicing oncologist | ||||||
| n | 149 | 151 | 300 | 34 | 45 | 79 |
| Mean (SD) | 12.8 (7.7) | 12.2 (6.5) | 12.5 (7.1) | 12.3 (6.1) | 18.2 (9.1) | 15.7 (8.5) |
| Median (Q1–Q3) | 12.0 (7.0–16.0) | 11.0 (7.0–15.0) | 11.0 (7.0–16.0) | 12.5 (6.0–16.0) | 19.0 (13.0–24.0) | 15.0 (10.0–20.0) |
| Number of mCRC patients treated by the oncologist in the previous 3 months | ||||||
| n | 150 | 151 | 301 | 34 | 45 | 79 |
| Mean (SD) | 58.7 (58.3) | 59.4 (57.3) | 59.0 (57.7) | 75.1 (96.3) | 45.1 (33.1) | 58.0 (69.0) |
| Median (Q1–Q3) | 45.0 (20.0–60.0) | 40.0 (25.0–70.0) | 40.0 (25.0–70.0) | 47.5 (20.0–90.0) | 30.0 (20.0–60.0) | 36.0 (20.0–70.0) |
| Number of mCRC patients treated with panitumumab in the last 6 months | ||||||
| n | NsR | NR | NR | 34 | 45 | 79 |
| Mean (SD) | 9.4 (6.0) | 9.0 (10.9) | 9.2 (9.1) | |||
| Median (Q1–Q3) | 8.0 (6.0–10.0) | 6.0 (5.0–8.0) | 6.0 (5.0–10.0) | |||
mCRC, metastatic colorectal cancer; NR, not recorded; SD, standard deviation.
Medical records review
In Round 1, low responses to the screening questionnaire (8.6%; 105/1,218) were observed (Fig 1B). However, in Round 2 response rates increased (60.9%; 56/92) as a result of changes in methodology and inclusion criteria outlined in the Methods section, with all 56 oncologists responding to the screening questionnaire being eligible (40 were not eligible in Round 1).
Seventy-nine eligible physicians who responded to the screening questionnaire agreed to participate (Fig 1B). The characteristics of those who participated were generally similar in Rounds 1 and 2, though the proportion based in private practice halved from 58.8% (20/34) in Round 1 to 28.9% (13/45) in Round 2 (Table 1). The median (Q1–Q3) number of years of experience as a practicing oncologist specialist was 13 (6–16) and 19 (13–24) years in Rounds 1 and 2, respectively.
Three hundred and six patient records from the 79 participating oncologists in both rounds of the medical records review were evaluated. Two-thirds of patients were male (66.7%; 204/306), and approximately one-quarter (23.9%; 73/306) were 75 years of age or older (Table 2). Just over one-quarter were receiving concurrent panitumumab and oxaliplatin-containing therapy (27.8%; 85/306) (Table 2).
Table 2. Patient demographics in the medical records review study.
| All patients | Round 1 | Round 2 | Total |
| N = 155 | N = 151 | N = 306 | |
| Sex—male, n (%) | 96 (61.9) | 108 (71.5) | 204 (66.7) |
| Age (years)—mean (SD) | 66.1 (11.0) | 66.8 (10.9) | 66.4 (10.9) |
| Age ≥65 years, n (%) | 93 (60.0) | 96 (63.6) | 189 (61.8) |
| Age ≥75 years, n (%) | 39 (25.2) | 34 (22.5) | 73 (23.9) |
| Patients receiving concurrent oxaliplatin a | Round 1 | Round 2 | Total |
| N = 53 | N = 53 | N = 85 | |
| Sex—male, n (%) | 39 (73.6) | 26 (81.3) | 65 (76.5) |
| Age (years)—mean (SD) | 63.5 (9.5) | 64.4 (13.7) | 63.8 (11.2) |
| Age ≥65 years, n (%) | 29 (54.7) | 19 (59.4) | 48 (56.5) |
| Age ≥75 years, n (%) | 7 (13.2) | 8 (25.0) | 15 (17.6) |
SD, standard deviation.
aReceived oxaliplatin-containing chemotherapy during the interval from 7 days prior to the date of first dose of panitumumab, until 7 days after the last dose of panitumumab.
Physician’s understanding of who, and how, to treat with panitumumab
Physician survey results: Awareness of the licensed indication and testing for KRAS status before starting treatment with panitumumab
Nearly all oncologists were aware of the need to test patients with mCRC for KRAS status before treatment with panitumumab (99.3%; 299/301) (Table 3). Of the two remaining oncologists, one did not consider KRAS testing to be appropriate, and the other provided no response. Nearly all oncologists (99.0%; 298/301) were aware of the correct indication for panitumumab for treatment of mCRC patients with wild-type KRAS tumors; two oncologists responded for the treatment of patients with mutant mCRC KRAS tumors, and one gave an invalid multiple response. Almost all (97.7%; 294/301) oncologists were aware of all their patients’ tumor KRAS status prior to treatment with panitumumab (Table 3). Six oncologists were unaware of their patients’ tumor KRAS mutation status prior to treatment with panitumumab, and the other gave a missing response.
Table 3. Outcomes of testing for KRAS exon 2 in the physician survey study.
| n (%) | |||
|---|---|---|---|
| (95% CI: %) | |||
| Round 1 | Round 2 | Total | |
| All oncologists | N = 150 | N = 151 | N = 301 |
| Aware KRAS testing should be performed prior to | 149 (99.3) | 150 (99.3) | 299 (99.3) |
| initiation of panitumumab a | (98.0–100.0) | (98.0–100.0) | (98.4–100.0) |
| Aware of the correct indication for panitumumab | 148 (98.7) | 150 (99.3) | 298 (99.0) |
| for treatment of mCRC patients with wild-type | (96.8–100.0) | (98.0–100.0) | (97.9–100.0) |
| KRAS tumors b | |||
| Aware of patients tumor KRAS status prior to | 144 (96.0) | 150 (99.3) | 294 (97.7) |
| initiation of panitumumab treatment in the past | (92.9–99.1) | (98.0–100.0) | (96.0–99.4) |
| 6 months of routine clinical practice c | |||
| Administered panitumumab to only mCRC | 143 (95.3) | 140 (92.7) | 283 (94.0) |
| patients with wild-type KRAS in the past 6 months | (92.0–98.7) | (88.6–96.9) | (91.3–96.7) |
| of routine clinical practice d | |||
| Subset of oncologists who administered | |||
| panitumumab concurently with oxaliplatin- | Round 1 | Round 2 | Total |
| containing chemotherapy | N = 74 | N = 90 | N = 164 |
| Administered panitumumab with concurrent | 70 (94.6) | 83 (92.2) | 153 (93.3) |
| oxaliplatin-containing chemotherapy to only | (89.4–99.7) | (86.7–97.8) | (89.5–97.1) |
| mCRC patients with wild-type KRAS in the past | |||
| 6 months of routine clinical practice e | |||
CI, confidence interval; mCRC, metastatic colorectal cancer.
aOne oncologist responded that KRAS testing was not appropriate (Round 1), and one oncologist did not respond (Round 2).
bTwo oncologists responded for treatment of patients with mutant mCRC KRAS tumors (one in each round), and one gave an invalid multiple response (Round 1).
cSix oncologists were unaware of patients’ tumor KRAS status prior to initiation of panitumumab treatment (Round 1), and one oncologist gave a missing response (Round 2).
dFifteen oncologists had administered panitumumab to mCRC patients with mutant KRAS tumors or with tumor KRAS status unknown (seven in Round 1 and eight in Round 2), two oncologists gave a not sure response (Round 2), and one oncologist did not give a response (Round 2).
eTen oncologists had administered panitumumab with concurrent oxaliplatin-containing chemotherapy to mCRC patients with mutant KRAS tumors or with tumor KRAS status unknown (three in Round 1 and seven in Round 2), and one oncologist gave a not sure response (Round 1).
Correct administration of panitumumab: Nearly all oncologists (94.0%; 283/301) had administered panitumumab correctly to all their mCRC patients in the past 6 months of clinical practice (those with known wild-type KRAS status prior to first dose of panitumumab) (Table 3). Of the responses from the remaining 18 oncologists, 15 (5.0%) had administered panitumumab to at least one patient with mutant or unknown KRAS status in the past 6 months of clinical practice, two (0.7%) were unsure, and one (0.3%) gave a missing response. The most frequent influencing factors cited for treating patients with mutant KRAS status were the patient’s medical status (n = 8) and other (n = 6: not concerned with KRAS mutation type [n = 3]; administered when KRAS status was unknown [n = 1]; special [13] mutation [n = 1]; good results with other patients [n = 1]). The most frequent influencing factors cited for treating patients with KRAS status unknown were time to obtain KRAS results (n = 6), tissue not available / insufficient tissue available (n = 4) and patient’s status or medical condition (n = 4). It should be noted that multiple reasons could be given.
Of the 301 participating oncologists, 164 (54.5%) had administered panitumumab concurrently with oxaliplatin-containing therapy to at least one mCRC patient in the past 6 months of routine clinical practice (Table 3). Of these, 10 (6.1%) oncologists had administered panitumumab simultaneously with oxaliplatin-containing chemotherapy to mCRC patients with KRAS status mutant or unknown, and one oncologist was unsure (Table 3). There was no indication from the free-text responses that these oncologists were unaware of the contraindication of administering panitumumab simultaneously with oxaliplatin-containing chemotherapy to mCRC patients with tumor KRAS status unknown.
Differences in survey responses between rounds: There were no noticeable differences in the results obtained for the physician survey between Rounds 1 and 2.
How oncologists treat patients with panitumumab based upon KRAS testing
Medical records review results: Proportion of oncologists testing patients’ tumors for KRAS status prior to treatment with panitumumab
Nearly all oncologists (97.5%; 77/79) conducted a KRAS mutation test for all their mCRC patients prior to prescribing panitumumab.
Almost all patients (98.7%; 302/306) had been tested for KRAS mutation status prior to their first panitumumab dose, with wild-type KRAS mutation status confirmed in 97.7% (299/306) of patients (Table 4). Four patients (1.3%; all from two oncologists) had not been tested for KRAS mutation status prior to their first dose of panitumumab. One patient had a KRAS test result (wild-type) dated after the first panitumumab dose (this patient was also receiving concurrent oxaliplatin-containing chemotherapy). Three patients had no KRAS test result available (tissue was not available/insufficient tissue available [1 patient], unaware of the need for KRAS test [1 patient]; patient’s situation [1 patient]).
Table 4. Outcomes of testing for KRAS exon 2 in the medical records review study.
| n (%) | |||
|---|---|---|---|
| (95% CI: %) | |||
| Round 1 | Round 2 | Total | |
| All patients | N = 155 | N = 151 | N = 306 |
| Tested for KRAS status prior to first dose of | 153 (98.7) | 149 (98.7) | 302 (98.7) |
| panitumumab a | (96.9–100.0) | (96.9–100.0) | (97.4–100.0) |
| Wild-type KRAS test result confirmed prior to | 152 (98.1) | 147 (97.4) | 299 (97.7) |
| first dose of panitumumab b | (95.9–100.0) | (94.8–99.9) | (96.0–99.4) |
| Subset of patients treated with concurrent | Round 1 | Round 2 | Total |
| oxaliplatin-containing chemotherapy c | N = 53 | N = 32 | N = 85 |
| Tested for KRAS status prior to first dose of | 51 (96.2) | 32 (100) | 83 (97.6) |
| panitumumab d | (91.1–100.0) | 100.0–100.0) | (94.4–100.0) |
| Wild-type KRAS test result confirmed prior to | 51 (96.2) | 32 (100) | 83 (97.6) |
| first dose of panitumumab | (91.1–100.0) | (100.0–100.0) | (94.4–100.0) |
CI, confidence interval; mCRC, metastatic colorectal cancer.
aThree patients did not have a KRAS test performed (one in Round 1 and two in Round 2), and one patient had a KRAS test performed after the first dose of panitumumab (Round 1).
bTwo patients had a mutant KRAS test result confirmed prior to first dose of panitumumab (one in Round 1 and one in Round 2) and one patient had an unknown KRAS test result (Round 2).
cReceived oxaliplatin-containing chemotherapy at any time during the interval from 7 days prior to the date of first dose of panitumumab, until 7 days after the last dose of panitumumab.
dOne patient did not have a KRAS test performed (Round 1), and one patient had a KRAS test performed after the first dose of panitumumab (Round 1).
Three (1.0%) of the 302 patients tested for KRAS tumor status prior to their first dose of panitumumab had mutant (n = 2) or unknown (n = 1) KRAS status (none were receiving concurrent oxaliplatin-containing therapy (Table 4).
Testing patients’ tumors for KRAS status prior to treatment with panitumumab and concurrent oxaliplatin-containing chemotherapy: Approximately one quarter of patients (27.8%; 85/306) were treated with panitumumab and concurrent oxaliplatin-containing chemotherapy, with nearly all of these patients (97.6%; 83/85) having a confirmed wild-type KRAS result prior to starting treatment (Table 4).
Laboratory use of validated KRAS detection methods: Participating oncologists were asked to name the corresponding laboratories that analyzed the tumor samples. Of the 95 named laboratories, 56 (58.9%) responded to the survey. All 56 laboratories (100%) used a CE-marked or otherwise validated KRAS detection method, with 34 (60.7%) laboratories using non-commercial tests that were not CE-marked, but were otherwise validated. Nearly all (98.2%; 55/56) participated in at least one QA scheme (e.g. ESP QA scheme [10 (17.9%)], Directory of Molecular Genetic External QA schemes in Europe [8 (14.3%)], United Kingdom National External QA Service [4 (7.1%)], local or regional QA schemes such as the French Gen&Tiss scheme [25 (44.6%)], or other QA schemes [21 (37.5%)]).
Differences in medical record review responses between rounds: There were no noticeable differences in the results obtained for the medical records review study between Rounds 1 and 2.
Discussion
In the physician survey, amongst participating oncologists, nearly all those surveyed in 2012 and 2013 (99.3%; 299/301) were aware of the need to test tumor KRAS status before administration of panitumumab to patients with mCRC. Nearly all oncologists (94.0%; 283/301) had administered panitumumab correctly to all of their mCRC patients with wild-type KRAS status, according to the label, in the past 6 months of clinical practice. In the medical records review, nearly all oncologists (97.5%) conducted a KRAS test for all of their patients prior to prescribing panitumumab. Importantly, tumor KRAS status was known in nearly all (98.7%) patients prior to prescribing panitumumab (with or without oxaliplatin-containing therapy), with tumor wild-type KRAS confirmed in 97.7% of patients. All corresponding laboratories that responded to the pathologist survey used validated KRAS testing methods, and regularly checked their testing methods versus QA schemes in place.
Fifteen of the 301 oncologists in the physician survey said that in the last 6 months of clinical practice they had administered at least once panitumumab to mCRC patients when the KRAS result was mutant or unknown. This proportion (5%) is higher in the survey than in the chart review study in which 1% of the patients had mutant or unknown KRAS results at the initiation of panitumumab treatment. The physician survey records the number of physicians who had administered panitumumab outside the label indication once or more in the last 6 months and therefore, did not capture the number patients treated according to the label as the chart review did. Oncologists quoted a number of reasons for prescribing panitumumab (with or without concurrent oxaliplatin-containing therapy) to patients with mutant or unknown KRAS status, with the most frequent influencing factors being the patients’ medical condition, that they were unconcerned by KRAS mutation status, the availability of tumor tissue, and the time taken for KRAS test results to be received. It appears that on rare occasions physicians are using their clinical judgment and they do not adhere with the guidelines despite that they seem to have a good knowledge of the label indication. Characteristics of patients such as co-morbidity is a factor that has been shown in the past to influence physicians implementation of clinical guidelines [25].
Low response rates are not uncommon for physician telephone surveys. A physician survey carried out in 12 countries assessing their knowledge and application of COPD showed that contacting physicians by telephone had low response rate (USA 10%, Italy 21%) [26]. A telephone survey amongst neurologists in 12 European countries to assess awareness of clinical guidelines had a response rate of 27.2% [27]. Low response rates also occur in chart review studies, with 10% of invited physicians participating in a US-based community chart review study [28], and 34% of invited urologists participating in a retrospective on-line chart review [29].
The sampling list used for the physicians’ survey and Round 1 of chart review was based on a medical marketing database and included physician contact details that were not filtered by specialty. The inclusion of physicians who were not oncologists in the initial study population may give an underestimate of the true response rate observed in the study. For Round 2 of the chart review study a more targeted method of sampling was used where the medical marketing database was filtered by specialty. With the different sampling methods used and the similarities in baseline characteristics of physicians between rounds, the results are considered to be representative of oncologist prescribing practice in Europe and any perceived selection bias resulting from the low response rate is unlikely to be impacting the study results.
Responding and non-responding physicians have been found to have similar demographic characteristics, perhaps due to the high level of homogeneity in the physician population (comparable education, socio-economic status, etc.) [30]. In the physician survey, more participating oncologists had ≥10 years’ experience (62.1% [187/301] vs 42.7% [44/103], respectively) and had treated ≥40 mCRC patients in the previous quarter (58.1% [175/301] vs 12.6% [13/103], respectively) than screened oncologists who were not eligible for the study. Approximately three quarters of physicians could recall receiving educational materials on KRAS testing and this result was encouraging. Overall, we consider that the participating oncologists were likely to be representative of those treating mCRC patients in the EU.
Within the EU, individual countries are likely to have their own strategies for improving the care of patients with mCRC. In general there should be equal access to treatments and a need to develop platforms that enable patients to receive the most appropriate treatment (e.g. molecular genetics and testing). Within individual countries, the proportion of patients treated in each type of facility (institution / hospital / private practice) will differ, and the use of KRAS testing may also vary between facility types. In the physician survey, we have shown that by 2012, the percentage of participating physicians adopting tumor KRAS testing was nearly 100% in Europe. Previous physician surveys in 2010 following published guidance recommending the adoption of KRAS testing prior to EGFR-targeted treatment [18,19] found that the range of adoption varied widely (20–100%) across countries [20–22]. In Europe in 2010, physicians used clinical judgment when deciding whether or not to test mCRC KRAS status prior to treatment with EGFR-targeted therapies, with 73% of participating physicians testing mCRC KRAS status prior to prescribing EGFR-targeted therapies [20]. Of those specialists who did not test KRAS status, reasons cited were that the tests were not considered relevant for the patients (47%) or the specialist was unfamiliar with the test (40%) [21]. In Round 3 of the physician survey, more information regarding influencing factors will be collected to gain as much insight as possible into why, on rare occasions, physicians might prescribe panitumumab to patients with mutant or unknown RAS tumor status.
The data we report in our medical records review are supported by previous studies, which showed that, in 2010, physicians were beginning to adopt KRAS testing prior to EGFR-targeted treatment following published guidance [23,24]. Of those specialists in France who did not test KRAS status, reasons for not testing were that the patient had received prior anti-EGFR-treatment (63% of patients) or there was an absence of tumor/technical issues (27% of patients) [23]. Interestingly, in the US in 2010, of those patients with mutant KRAS tumor status, only 86% were not treated with EGFR-targeted therapies [24], suggesting that 14% received anti-EGFR-treatment. In comparison, by 2012 in our medical records review, 1% (3/302) of patients tested for KRAS tumor status prior to their first dose of panitumumab had mutant or unknown KRAS status (none were receiving concurrent oxaliplatin-containing therapy).
We have shown that by 2012 in Europe, there was a high level of knowledge amongst oncologists around the need to test and confirm patients’ mCRC tumors as being wild-type for KRAS prior to treatment with panitumumab, with or without concurrent oxaliplatin-containing therapy. Nearly all oncologists surveyed followed the licensed indication for panitumumab, but a minority still used their clinical judgment when deciding whether to treat patients with mutant or unknown KRAS mCRC status. While their decisions conflict with current prescribing information, discussions between individual physicians and their patients around the risks and benefits (e.g. additional biopsy, denial of treatment) may contribute to these decisions. Both studies report very good compliance with the licensed indication for panitumumab in terms of KRAS testing at the time of Rounds 1 and 2 of the studies. There was also a very good level of knowledge amongst oncologists around the need to test and confirm wild-type KRAS tumor status prior to treatment with panitumumab, with or without concurrent oxaliplatin-containing therapy. Nearly all patients treated with panitumumab (with or without concurrent oxaliplatin-containing therapy) had wild-type KRAS tumor status confirmed prior to first dose. It is important that tests for predictive molecular markers such as KRAS are carried out using validated tests in accredited laboratories that have quality controls in place. All participating laboratories used a CE-marked or otherwise validated KRAS detection method, and nearly all participated in a QA scheme.
In Round 3 of the physician survey and medical records review, which is evaluating knowledge of the need to test for an expanded number of RAS mutations prior to treating with panitumumab, the physician questionnaires will capture additional free-text details to describe the oncologists’ rationale that led to patients with mutant or unknown tumor RAS status receiving panitumumab. Distribution of the educational materials continues. Data from Round 3 for both studies evaluating RAS testing are awaited with interest.
Acknowledgments
The authors would like to thank and acknowledge all of the physicians who participated in the physician survey and in the medical records review study. In addition, we would like to thank the following for their contributions to the two studies and this article: James Bennett MSc (Amgen Ltd) for statistical support; Rachel Bowman BSc and Paul Knowles BSc (Amgen Ltd) for SAS programming support; Paula Haynes BSc RGN and Pari Parker BSc (Amgen Ltd) for study management support; Adelphi Research for conduct and data management of the Physician Survey study; Quintiles for data management of the Medical Records Review study; Carolyn Bowler PhD (Bioscript Medical Ltd) for medical writing assistance (funded by Amgen Ltd).
Data Availability
All relevant data are within the paper.
Funding Statement
These studies were funded by Amgen. Amgen was also involved in the study design, data collection and analysis, decision to publish, and preparation of the manuscript. They also provided funding for medical writing assistance and for the journal publication fee.
References
- 1. Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, et al. (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27: 663–671. 10.1200/JCO.2008.20.8397 [DOI] [PubMed] [Google Scholar]
- 2. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, et al. (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351: 337–345. [DOI] [PubMed] [Google Scholar]
- 3. Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, et al. (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357: 2040–2048. [DOI] [PubMed] [Google Scholar]
- 4. Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, et al. (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26: 2311–2319. 10.1200/JCO.2007.13.1193 [DOI] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, et al. (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360: 1408–1417. 10.1056/NEJMoa0805019 [DOI] [PubMed] [Google Scholar]
- 6. Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, et al. (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25: 1658–1664. [DOI] [PubMed] [Google Scholar]
- 7. Wilke H, Glynne-Jones R, Thaler J, Adenis A, Preusser P, et al. (2008) Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: MABEL Study. J Clin Oncol 26: 5335–5343. 10.1200/JCO.2008.16.3758 [DOI] [PubMed] [Google Scholar]
- 8. Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, et al. (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26: 1626–1634. 10.1200/JCO.2007.14.7116 [DOI] [PubMed] [Google Scholar]
- 9. Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, et al. (2007) Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67: 2643–2648. [DOI] [PubMed] [Google Scholar]
- 10. De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, et al. (2008) KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 19: 508–515. [DOI] [PubMed] [Google Scholar]
- 11. Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, et al. (2007) Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer 96: 1166–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freeman DJ, Juan T, Reiner M, Hecht JR, Meropol NJ, et al. (2008) Association of K-ras mutational status and clinical outcomes in patients with metastatic colorectal cancer receiving panitumumab alone. Clin Colorectal Cancer 7: 184–190. 10.3816/CCC.2008.n.024 [DOI] [PubMed] [Google Scholar]
- 13. Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG (2009) KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol 27: 1130–1136. 10.1200/JCO.2008.19.8168 [DOI] [PubMed] [Google Scholar]
- 14. Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, et al. (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359: 1757–1765. 10.1056/NEJMoa0804385 [DOI] [PubMed] [Google Scholar]
- 15. Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, et al. (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26: 374–379. 10.1200/JCO.2007.12.5906 [DOI] [PubMed] [Google Scholar]
- 16. Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, et al. (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28: 4697–4705. 10.1200/JCO.2009.27.4860 [DOI] [PubMed] [Google Scholar]
- 17. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, et al. (2010) Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28: 4706–4713. 10.1200/JCO.2009.27.6055 [DOI] [PubMed] [Google Scholar]
- 18. Van Cutsem E, Nordlinger B, Cervantes A (2010) Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol 21 Suppl 5: v93–v97. 10.1093/annonc/mdq222 [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network (2009) Clinical practice guidelines in oncology: colon cancer. http://wwwnccnorg/professionals/physician_gls/PDF/colonpdf. [DOI] [PubMed]
- 20. Ciardiello F, Tejpar S, Normanno N, Mercadante D, Teague T, et al. (2011) Uptake of KRAS mutation testing in patients with metastatic colorectal cancer in Europe, Latin America and Asia. Target Oncol 6: 133–145. 10.1007/s11523-011-0181-x [DOI] [PubMed] [Google Scholar]
- 21. Cox SL, Zlot AI, Silvey K, Elliott D, Horn T, et al. (2012) Patterns of cancer genetic testing: a randomized survey of Oregon clinicians. J Cancer Epidemiol 2012: 294730 10.1155/2012/294730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris JN, Liljestrand P, Alexander GL, Goddard KA, Kauffman T, et al. (2013) Oncologists' attitudes toward KRAS testing: a multisite study. Cancer Med 2: 881–888. 10.1002/cam4.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith D, Rouyer M, Francois E, Mitry E, Monnereau A, et al. (2011) Use of cetuximab in a real-life setting in France with respect to KRAS status: Preliminary results of EREBUS cohort study. J Clin Oncol 29: Abstract e14094. [Google Scholar]
- 24. Webster J, Kauffman TL, Feigelson HS, Pawloski PA, Onitilo AA, et al. (2013) KRAS testing and epidermal growth factor receptor inhibitor treatment for colorectal cancer in community settings. Cancer Epidemiol Biomarkers Prev 22: 91–101. 10.1158/1055-9965.EPI-12-0545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Francke AL, Smit MC, de Veer AJ, Mistiaen P (2008) Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak 8: 38 10.1186/1472-6947-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis KJ, Landis SH, Oh YM, Mannino DM, Han MK, et al. (2015) Continuing to Confront COPD International Physician Survey: physician knowledge and application of COPD management guidelines in 12 countries. Int J Chron Obstruct Pulmon Dis 10: 39–55. 10.2147/COPD.S70162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lledo A, Dellva MA, Strombom IM, Wilkie JL, Jungemann ME, et al. (2007) Awareness of potential valvulopathy risk with pergolide and changes in clinical practice after label change: a survey among European neurologists. Eur J Neurol 14: 644–649. [DOI] [PubMed] [Google Scholar]
- 28. Goldberg SL, Chen L, Guerin A, Macalalad AR, Liu N, et al. (2013) Association between molecular monitoring and long-term outcomes in chronic myelogenous leukemia patients treated with first line imatinib. Curr Med Res Opin 29: 1075–1082. 10.1185/03007995.2013.812034 [DOI] [PubMed] [Google Scholar]
- 29. Witjes JA, Palou J, Soloway M, Lamm D, Kamat AM, et al. (2013) Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): results of an international individual patient data survey (IPDS). BJU Int 112: 742–750. 10.1111/bju.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kellerman SE, Herold J (2001) Physician response to surveys. A review of the literature. Am J Prev Med 20: 61–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.