Theobroma cacao is a tropical understory tree that is one of the most important perennial crops in agriculture. Treasured by ancient civilizations in Mesoamerica for over 3,000 years, the cocoa bean now supports a multibillion-dollar industry that is involved in the production and commercialization of chocolate, a treat appreciated worldwide. The cacao tree is originally from the Amazon rainforest and is currently grown in more than 50 countries throughout the humid tropics, serving as a major source of income for over 40 million people. Each year, more than 3 million tons of cocoa beans are produced, mostly by smallholder farmers in areas of high biodiversity. Notably, the cacao tree does not require direct sunlight and naturally grows under the canopy of other, taller trees. This characteristic often encourages farmers to preserve existing forests and to plant additional trees to shelter their cacao plants [1], thereby reducing the environmental impacts of cacao cultivation. Despite its great importance, the cacao tree is affected by a number of untreatable diseases that reduce fruit production and threaten our global supply of cacao. Among them, witches’ broom disease (WBD) stands out as one of the most severe problems that affect this crop, accounting for production losses of up to 90%.
WBD Is a Devastating Tropical Disease
WBD was first described in 1785 by the naturalist Alexandre Rodrigues Ferreira during his epic expedition across the Amazon basin. This disease is currently present in nearly all cacao-producing countries in the Americas, and it considerably reduces fruit production and bean quality, making it a major limiting factor for cacao cultivation. A striking example of the devastating impact of WBD occurred in Brazil [2], which was one of the largest cocoa exporters by the end of the 1980s. The introduction of the disease in its main producing area (the state of Bahia) in 1989 decreased the production by 70%, turning the country into a net importer of cocoa beans. Consequently, many farms were abandoned and workers were forced to move to cities that were not prepared to receive such a migration, resulting in a scenario of intense poverty and misery that persists today. Moreover, much of the Atlantic rainforest that protected cacao farms was replaced by pasture. Almost three decades after the emergence of WBD in Bahia, the Brazilian cocoa production has not yet recovered from the significant negative impact of the disease, reaching in 2013 only 65% of what was produced in 1989. Fortunately, WBD is still absent from West Africa, which currently accounts for nearly 70% of all cacao produced in the world. However, the introduction of this disease in African countries poses a real threat that would decimate the worldwide chocolate industry and cause severe socioeconomic damage. This is especially relevant because most of the cacao cultivated in Africa belongs to the highly susceptible “Amelonado” genetic group.
WBD Is Caused by a Peculiar Fungal Pathogen
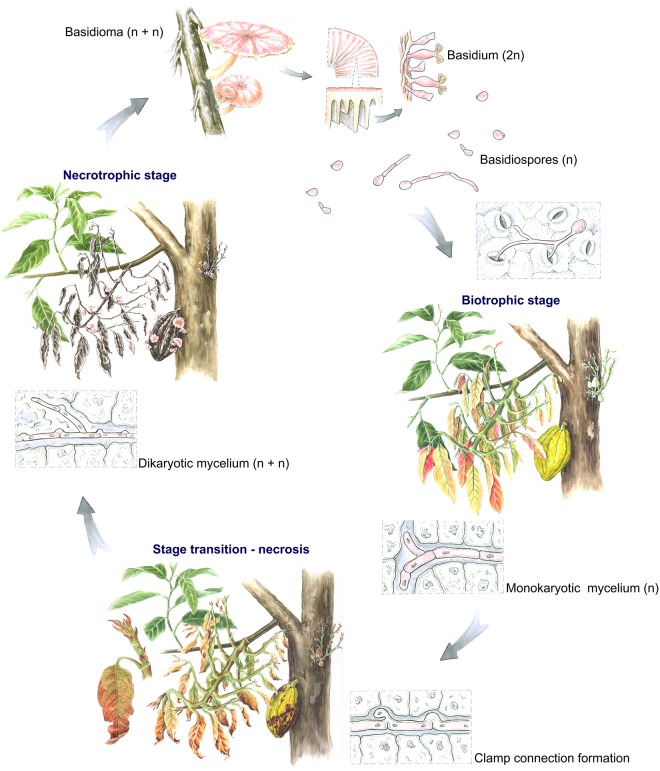
Witches’ broom disease is caused by the basidiomycete Moniliophthora perniciosa. This pathogen displays a hemibiotrophic lifestyle, meaning that it initially grows on the living cacao tissues (biotrophic stage) before killing and feeding off of the dead tissue (necrotrophic stage) [3]. In comparison to other hemibiotrophic interactions, the M. perniciosa life cycle is regarded as atypical. Whereas most hemibiotrophs display a short and asymptomatic biotrophic stage (lasting for only a few days), M. perniciosa establishes a long-term biotrophic interaction with cacao that lasts for one to three months and is responsible for the main symptoms of the disease. These symptoms include hyperplasia and hypertrophy of infected tissues, loss of apical dominance, and proliferation of axillary shoots, resulting in the formation of abnormal stems called green brooms. Eventually, the green broom becomes necrotic and dies, and after alternate rainy and dry periods, dry brooms produce basidiomata, completing the fungal life cycle (Fig 1). Notably, infection of the shoots’ apical and axillary meristems results in the most distinctive symptoms of WBD. However, M. perniciosa can also infect other parts of the plant, including developing flowers and fruits.
Fig 1. The Moniliophthora perniciosa life cycle in Theobroma cacao.
Infection begins when fungal basidiospores penetrate the plant through stomata or wounds. In the first stage of the disease, M. perniciosa develops as a swollen monokaryotic mycelium that grows exclusively in the extracellular space of the living plant tissue. Infection of shoots induces drastic morphological alterations resulting in the characteristic “green broom” structure, though infection can also occur in other tissues (fruits and flowers). After one to three months of biotrophic infection, necrosis of the plant tissue occurs, giving rise to the “dry broom” structure. Necrotic tissue is colonized intracellularly by thin dikaryotic mycelium, which is characterized by the presence of clamp connections—a cross structure formed by hyphal cells that ensures the presence of two nuclei in each fungal cell. After alternating rainy and dry periods, basidiomata are formed from necrotrophic hyphae, completing the pathogen life cycle. Illustrations by Diana Carneiro.
To invade the host plant, M. perniciosa does not form specialized infection structures (e.g., appressorium), but it penetrates cacao tissues through wounds and stomata. Germinated basidiospores form swollen monokaryotic hyphae that grow intercellularly and feed on nutrients derived from the plant apoplast. In contrast with many (hemi)biotrophs, M. perniciosa does not employ nutrient-absorbing structures (e.g., haustorium and invasive hyphae), and it manipulates host metabolism to increase nutrient availability in the site of infection. In the late stages of WBD, M. perniciosa grows intracellularly as a necrotrophic mycelium, which is dikaryotic and exhibits clamp connections for nuclear transfer (Fig 1) [4].
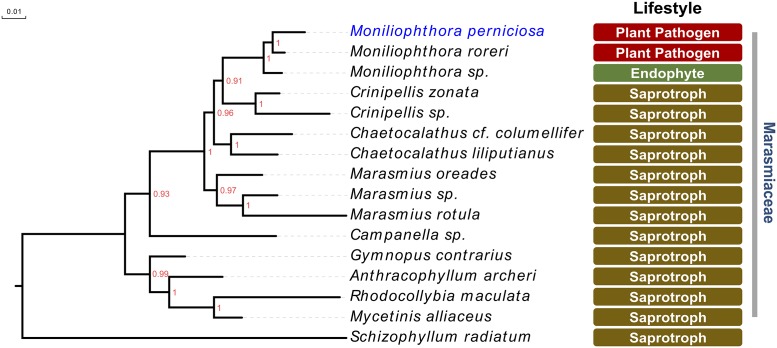
In addition to its intriguing life cycle, there are some other remarkable features of M. perniciosa biology and pathogenicity. Although displaying a pathogenic lifestyle, M. perniciosa belongs to the family Marasmiaceae, which is known for its predominantly saprotrophic species (Fig 2). Interestingly, the closest species to M. perniciosa evolutionarily, M. roreri, is also a cacao pathogen that exclusively infects the host fruits. The genus Moniliophthora also includes a grass endophyte that was isolated in New Mexico, suggesting that the pathogenic lifestyle in this group may have evolved from a biotrophic ancestor [5]. More recently, a saprotrophic Moniliophthora species, named M. canescens, was isolated in Asia [6]. Additional Moniliophthora species need to be identified and characterized in order to fully support hypotheses regarding the evolution of pathogenicity in this genus. Even so, M. perniciosa and its related Marasmiaceae species constitute a very interesting model by which to understand the evolution of pathogenicity in fungi.
Fig 2. The pathogenic lifestyle of M. perniciosa is an exception within the Marasmiaceae family of basidiomycetes, which is mostly composed of saprotrophic litter and wood-decomposing fungi.
The genus Moniliophthora includes the hemibiotrophic sister species M. perniciosa and M. roreri, the two major pathogens of Theobroma cacao. Notably, it also encompasses a still poorly characterized grass endophyte, suggesting that the pathogenic lifestyle of M. perniciosa may have evolved from an endophyte ancestral. The tree was constructed based on Bayesian inference using regions of the genes 25S, 18S, ITS/5.8S and Rbp1 (large fragment of the RNA polymerase II). Sequences were retrieved from Aime & Phillips-Mora (2005) [5] and Matheny et al. (2006) [34]. Numbers next to the branches represent the posterior probabilities. The species Schizophyllum radiatum was used as outgroup.
Also intriguing is the fact that, in addition to cacao, M. perniciosa is found in association with other plant species in the genus Theobroma and in plants from unrelated families, such as Solanaceae. Based on that, M. perniciosa is classified in three biotypes according to host specificity: the C-biotype infects plants of the family Malvaceae (e.g., cacao), the S-biotype infects members of the family Solanaceae (e.g., tomato), and the L-biotype is found in association with members of the family Bignoniaceae (e.g., lianas) [7–9]. Remarkably, there is no evidence that these biotypes constitute separate species. In particular, biotypes C and S seem to be very close genetically, and they even cause similar symptoms in the host plant, indicating that they might employ conserved pathogenicity strategies. Despite the evident scientific relevance, the mechanisms associated with host adaptation are still poorly understood and thus present an exciting topic for future research.
Important Advances in WBD Research
WBD has been the focus of attention from the scientific community for several decades. Early studies performed mostly in the 1980s and 1990s were of great importance to establishing basic knowledge about the biology of M. perniciosa, including the characterization of its hemibiotrophic lifestyle and the infection process in cacao [3,4,10–12]. Molecular biology research on this pathogen started mainly with the WBD Genome Project, an initiative launched by Brazilian laboratories in 2000 that unveiled the first DNA sequences of M. perniciosa [13]. This initiative revealed a number of fungal genes with potential roles in WBD, which have been the focus of several specific studies [14–18]. Likewise, the cacao genome sequence was released in 2010 and has been a valuable resource for WBD research [19,20].
Until the mid-2000s, our knowledge regarding the interaction between T. cacao and M. perniciosa was quite incipient. In 2005, a comprehensive biochemical characterization of WBD progression provided a first big picture of this complex pathosystem [21]. Subsequent studies also increased our knowledge about various aspects of the disease, including the importance of plant sugars as signaling molecules in WBD, mechanisms regulating the phase transition process in M. perniciosa, and the role of some fungal proteins in M. perniciosa virulence [16,22,23]. In particular, NEPs (Necrosis and Ethylene-inducing Proteins) constitute a very intriguing class of proteins, since they induce necrosis in plant tissues and seem to have a major role in the death of cacao tissues during the development of WBD [18,24]. Remarkably, Tiburcio et al. (2010) verified that NEP genes in M. perniciosa (and in its sister species M. roreri) were acquired by horizontal transfer from oomycetes of the genus Phytophthora [25]. Indeed, several Phytophthora species infect cacao, supporting the idea that these oomycetes coexisted with an ancestral Moniliophthora species. It seems likely that acquisition of NEP genes was an important event in the development/improvement of a pathogenic hemibiotrophic lifestyle in the genus Moniliophthora.
Another fungal gene characterized during the WBD research program was the MpAOX gene, which encodes a mitochondrial alternative oxidase (AOX) [16]. AOX constitutes an alternative respiratory route that is resistant to many inhibitors of the main respiratory chain, such as the plant defense molecule nitric oxide and strobilurin fungicides. Remarkably, the biotrophic and necrotrophic stages of M. perniciosa seem to employ different respiratory pathways: whereas the monokaryotic (biotrophic) hyphae use the alternative route, the dikaryotic (necrotrophic) hyphae rely mostly on the main respiratory chain. Notably, pharmacological inhibition of AOX completely blocks the development of the monokaryotic mycelium, whereas the dikaryotic stage is still able to grow in the presence of AOX inhibitors. Moreover, inhibition of the main respiratory pathway in monokaryotic hyphae delays the transition to the dikaryotic stage, indicating that the phase transition in M. perniciosa is linked to the cell energetic status. Importantly, the dual inhibition of the main and alternative respiratory chains blocks fungal development and represents a promising alternative to control WBD [16].
Recently, a dual RNA-seq analysis of the biotrophic stage of WBD allowed an unprecedented characterization of the transcriptomes of both the cacao plant and M. perniciosa during their interaction [26]. Significant transcriptional alterations that correlate with symptom development were identified in infected plants, as well as a set of putative fungal pathogenicity factors. The transcriptional reprogramming associated with hormonal metabolism was remarkable and is compatible with auxin, gibberellin, cytokinin, and ethylene unbalance. Interestingly, auxin-responsive genes were strongly up-regulated in green brooms, but plant genes required for the biosynthesis of this hormone were not differentially expressed. These data indicate that M. perniciosa may interfere directly with the cacao hormonal metabolism, which is in agreement with the finding that this fungus is able to produce the plant hormone auxin [27]. In addition, a clear carbon deprivation signature in the transcriptomes of infected plants was verified, including the up-regulation of the glyoxylate cycle, lipid degradation, asparagine biosynthesis, and reduction of photosynthetic rates. This nutrient-starving condition appears to trigger a premature senescence process in infected plant tissues, which is responsible for the first signs of necrosis observed during WBD. Based on this transcriptomic analysis, we now have a model of the molecular events underlying the biotrophic stage of WBD as well as new insights on the plant metabolic processes associated with the transition to the dry broom/necrotrophic stage of the disease.
This detailed transcriptional analysis of the biotrophic stage of WBD is part of the WBD Transcriptome Atlas initiative (http://www.lge.ibi.unicamp.br/wbdatlas). This database comprises a continuously growing set of RNA-seq libraries covering the transcriptomes of both M. perniciosa and T. cacao across multiple biological conditions (e.g., pathogen life cycle and stages of WBD). With this initiative, we aim to provide the WBD research community with access to a valuable resource that might be used as an additional line of evidence in the study of gene function.
Searching for WBD-Resistant Varieties
The development of resistant cacao varieties can be a durable and sustainable approach to meet the increasing demand for cocoa beans. The genetic improvement of disease resistance in cacao started in the 1930s, when Frederick J. Pound undertook expeditions to the Upper Amazon region to search for wild cacao trees with an absence of WBD symptoms. Since then, many of these varieties have been used in breeding programs for WBD resistance. Notably, Scavina 6 (SCA6) has been one of the most widely used sources of resistance to WBD identified so far. However, because of its poor agronomic characteristics, it is not directly used as a clone, but is usually crossed with other high-quality varieties in order to obtain hybrids with disease resistance as well as high yield and large bean weight.
Although SCA6 has been a recognized source of WBD resistance, it is susceptible to M. perniciosa in some regions of Ecuador, Peru, and Brazil. This geographical variation in disease susceptibility is assumed to be due to regional variability in the pathogen populations. In this regard, the exploration of novel cacao varieties is essential to expand the genetic resources of WBD resistance. Indeed, high resistance to WBD has been identified in other germplasm groups. A study by Sereno et al. (2006) identified resistant accessions in wild germplasm originally collected from distinct river basins in the Brazilian Amazon [28]. These resistant accessions are CAB0208 and CAB0214 and constitute promising parental lines to be used in breeding programs. Another outstanding source of resistance to WBD is the CCN51 variety, which is the product of an independent breeding program developed in the 1960s by Homero Castro in Ecuador. Despite its poor organoleptic quality, CCN51 is valued for its high productivity and disease resistance, which make it another promising parental line to be used in the cacao-breeding programs worldwide. So far, the molecular basis of WBD resistance in all these varieties remains completely unknown, and a classic gene-for-gene relationship between plant resistance and fungal avirulence genes remains to be identified and characterized.
Bottlenecks in WBD Research and Exciting New Directions
WBD results from the interaction between two non-model organisms. Therefore, a number of methodological tools that are commonly used for model organisms are still missing in this system. In particular, the routine application of genetic manipulation techniques constitutes the main bottleneck in WBD research. In 2009, RNAi-based gene silencing was reported for the necrotrophic mycelium of M. perniciosa [29]. However, stable gene silencing could not be obtained in the infective stages of the pathogen. More recently, Barau et al. transformed M. perniciosa protoplasts with a cassette containing the hygromycin resistance gene and a Green Fluorescent Protein—tagged version of the ATG8 gene [23]. Even so, genetic manipulation is not a standard practice in M. perniciosa, and a number of basic methods still need to be established and validated. Particularly, gene-specific mutagenesis has never been reported, which limits the study of gene function in this pathogen. The development of the CRISPR/Cas9 editing tool provides new perspectives and stands as a very promising approach for efficient genetic manipulation of M. perniciosa.
Transformation protocols have already been developed for cacao somatic embryos [30]. Nevertheless, as a perennial plant, the cacao tree has a quite long life cycle (3–4 years from seed to seed), which considerably hinders the use of classical genetics approaches in the study of this plant. In this context, the infection of tomatoes by isolates belonging to the S-biotype of M. perniciosa presents a more amenable and promising model system for WBD [31,32]. In this regard, it is of primary importance to understand how the disease development compares in tomatoes and cacao. Comparative genomics and transcriptomics of M. perniciosa isolates belonging to different biotypes offers an exciting direction in which to expand our knowledge of the mechanisms involved in host adaptation in this pathogen.
Finally, results from the WBD research program may eventually translate into strategies to control the disease more efficiently. Several strategies have been developed and tested with limited success over the past years. Currently, two promising approaches include the following: (1) the use of biofungicides based on the mycoparasite Trichoderma stromaticum, which can antagonize M. perniciosa [33], and (2) the use of drugs to simultaneously block the main and the alternative respiratory chains of the pathogen, which showed high effectiveness in in vitro assays [16]. These strategies must be carefully evaluated under field conditions and, along with the disciplined management of the farms, they may constitute important approaches to fight off WBD in the near future. Moreover, the characterization of resistance genes (e.g., Nucleotide-Binding Leucine-Rich Repeats [NLRs]) in resistant cacao cultivars (or even in its related wild plant species) may, through biotechnology, provide additional effective ways to tackle WBD in the long term. With the advent of the CRISPR/Cas9 technology, the development of disease-resistant cacao plants is certainly a very promising and sustainable strategy to control WBD. However, the success of this strategy still depends on the regulation of this new technology and on the public’s approval.
Acknowledgments
The authors would like to thank Alex Schultink for critical reading of the manuscript and Diana Carneiro for preparing the illustrations in Fig 1.
Funding Statement
Research on witches’ broom disease at Pereira’s lab is supported by the São Paulo Research Foundation (FAPESP, grant 09/50119-9) and the National Council for Scientific and Technological Development (CNPq, grant 475535/2013-8). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rice RA, Greenberg R (2000) Cacao cultivation and the conservation of biological diversity. AMBIO: A Journal of the Human Environment 29: 167–173. [Google Scholar]
- 2. Pereira JL, de Almeida LCC, Santos SM (1996) Witches' broom disease of cocoa in Bahia: attempts at eradication and containment. Crop Protection 15: 743–752. [Google Scholar]
- 3. Evans HC (1980) Pleomorphism in Crinipellis perniciosa, causal agent of witches' broom disease of cocoa. Transactions of the British Mycological Society 74: 515–523. [Google Scholar]
- 4. Calle H, Cook A, Fernando S (1982) Histology of witches'-broom caused in cacao by Crinipellis perniciosa . Phytopathology 72: 1479–1481. [Google Scholar]
- 5. Aime MC, Phillips-Mora W (2005) The causal agents of witches' broom and frosty pod rot of cacao (chocolate, Theobroma cacao) form a new lineage of Marasmiaceae. Mycologia 97: 1012–1022. [DOI] [PubMed] [Google Scholar]
- 6. Kerekes J, Desjardin D (2009) A monograph of the genera Crinipellis and Moniliophthora from Southeast Asia including a molecular phylogeny of the nrITS region. Fungal Diversity 37: 101. [Google Scholar]
- 7. Bastos CN, Evans HC (1985) A new pathotype of Crinipellis perniciosa (witches' broom disease) on solanaceous hosts. Plant Pathology 34: 306–312. [Google Scholar]
- 8. Griffith GW, Hedger JN (1994) Spatial distribution of mycelia of the liana (L-) biotype of the agaric Crinipellis perniciosa (Stahel) Singer in tropical forest. New Phytologist 127: 243–259. [DOI] [PubMed] [Google Scholar]
- 9. Hedger JN, Pickering V, Aragundi J (1987) Variability of populations of the witches' broom disease of cocoa (Crinipellis perniciosa). Transactions of the British Mycological Society 88: 533–546. [Google Scholar]
- 10. Griffith GW, Hedger JN (1994) The breeding biology of biotypes of the witches' broom pathogen of cocoa, Crinipellis perniciosa . Heredity 72: 278–289. [Google Scholar]
- 11. Griffith GW, Hedger JN (1994) Dual culture of Crinipellis perniciosa and potato callus. European Journal of Plant Pathology 100: 371–379. [Google Scholar]
- 12. Frias G, Purdy L, Schmidt R (1991) Infection biology of Crinipellis perniciosa on vegetative flushes of cacao. Plant Disease 75: 552–556. [Google Scholar]
- 13. Mondego JM, Carazzolle MF, Costa GG, Formighieri EF, Parizzi LP, et al. (2008) A genome survey of Moniliophthora perniciosa gives new insights into Witches' Broom Disease of cacao. BMC Genomics 9: 548 10.1186/1471-2164-9-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de O Barsottini MR, de Oliveira JF, Adamoski D, Teixeira PJ, do Prado PF, et al. (2013) Functional diversification of cerato-platanins in Moniliophthora perniciosa as seen by differential expression and protein function specialization. Mol Plant Microbe Interact 26: 1281–1293. 10.1094/MPMI-05-13-0148-R [DOI] [PubMed] [Google Scholar]
- 15. Teixeira PJ, Thomazella DP, Vidal RO, do Prado PF, Reis O, et al. (2012) The fungal pathogen Moniliophthora perniciosa has genes similar to plant PR-1 that are highly expressed during its interaction with cacao. PLoS One 7: e45929 10.1371/journal.pone.0045929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomazella DP, Teixeira PJ, Oliveira HC, Saviani EE, Rincones J, et al. (2012) The hemibiotrophic cacao pathogen Moniliophthora perniciosa depends on a mitochondrial alternative oxidase for biotrophic development. New Phytol 194: 1025–1034. 10.1111/j.1469-8137.2012.04119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Oliveira BV, Teixeira GS, Reis O, Barau JG, Teixeira PJ, et al. (2012) A potential role for an extracellular methanol oxidase secreted by Moniliophthora perniciosa in Witches' broom disease in cacao. Fungal Genet Biol 49: 922–932. 10.1016/j.fgb.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 18. Garcia O, Macedo JA, Tiburcio R, Zaparoli G, Rincones J, et al. (2007) Characterization of necrosis and ethylene-inducing proteins (NEP) in the basidiomycete Moniliophthora perniciosa, the causal agent of witches' broom in Theobroma cacao . Mycol Res 111: 443–455. [DOI] [PubMed] [Google Scholar]
- 19. Argout X, Salse J, Aury JM, Guiltinan MJ, Droc G, et al. (2011) The genome of Theobroma cacao . Nat Genet 43: 101–108. 10.1038/ng.736 [DOI] [PubMed] [Google Scholar]
- 20. Motamayor JC, Mockaitis K, Schmutz J, Haiminen N, Livingstone D 3rd, et al. (2013) The genome sequence of the most widely cultivated cacao type and its use to identify candidate genes regulating pod color. Genome Biol 14: r53 10.1186/gb-2013-14-6-r53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scarpari LM, Meinhardt LW, Mazzafera P, Pomella AW, Schiavinato MA, et al. (2005) Biochemical changes during the development of witches' broom: the most important disease of cocoa in Brazil caused by Crinipellis perniciosa . J Exp Bot 56: 865–877. [DOI] [PubMed] [Google Scholar]
- 22. de Oliveira Ceita G, Macêdo JNA, Santos TB, Alemanno L, da Silva Gesteira A, et al. (2007) Involvement of calcium oxalate degradation during programmed cell death in Theobroma cacao tissues triggered by the hemibiotrophic fungus Moniliophthora perniciosa . Plant Science 173: 106–117. [Google Scholar]
- 23. Barau J, Grandis A, Carvalho VM, Teixeira GS, Zaparoli GH, et al. (2015) Apoplastic and intracellular plant sugars regulate developmental transitions in witches' broom disease of cacao. J Exp Bot 66: 1325–1337. 10.1093/jxb/eru485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zaparoli G, Barsottini MR, de Oliveira JF, Dyszy F, Teixeira PJ, et al. (2011) The crystal structure of necrosis- and ethylene-inducing protein 2 from the causal agent of cacao's Witches' Broom disease reveals key elements for its activity. Biochemistry 50: 9901–9910. 10.1021/bi201253b [DOI] [PubMed] [Google Scholar]
- 25. Tiburcio RA, Costa GG, Carazzolle MF, Mondego JM, Schuster SC, et al. (2010) Genes acquired by horizontal transfer are potentially involved in the evolution of phytopathogenicity in Moniliophthora perniciosa and Moniliophthora roreri, two of the major pathogens of cacao. J Mol Evol 70: 85–97. 10.1007/s00239-009-9311-9 [DOI] [PubMed] [Google Scholar]
- 26. Teixeira PJ, Thomazella DP, Reis O, do Prado PF, do Rio MC, et al. (2014) High-resolution transcript profiling of the atypical biotrophic interaction between Theobroma cacao and the fungal pathogen Moniliophthora perniciosa . Plant Cell 26: 4245–4269. 10.1105/tpc.114.130807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kilaru A, Bailey Ba, Hasenstein KH (2007) Moniliophthora perniciosa produces hormones and alters endogenous auxin and salicylic acid in infected cocoa leaves. FEMS microbiology letters 274: 238–244. [DOI] [PubMed] [Google Scholar]
- 28. Sereno ML, Albuquerque PS, Vencovsky R, Figueira A (2006) Genetic diversity and natural population structure of cacao (Theobroma cacao L.) from the Brazilian Amazon evaluated by microsatellite markers. Conservation Genetics 7: 13–24. [Google Scholar]
- 29. Caribe dos Santos AC, Sena JA, Santos SC, Dias CV, Pirovani CP, et al. (2009) dsRNA-induced gene silencing in Moniliophthora perniciosa, the causal agent of witches' broom disease of cacao. Fungal Genet Biol 46: 825–836. 10.1016/j.fgb.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 30. Maximova S, Miller C, Antunez de Mayolo G, Pishak S, Young A, et al. (2003) Stable transformation of Theobroma cacao L. and influence of matrix attachment regions on GFP expression. Plant Cell Rep 21: 872–883. [DOI] [PubMed] [Google Scholar]
- 31. Marelli J-P, Maximova SN, Gramacho KP, Kang S, Guiltinan MJ (2009) Infection Biology of Moniliophthora perniciosa on Theobroma cacao and Alternate Solanaceous Hosts. Tropical Plant Biology 2: 149–160. [Google Scholar]
- 32. Deganello J, Leal GA, Rossi ML, Peres LEP, Figueira A (2014) Interaction of Moniliophthora perniciosa biotypes with Micro-Tom tomato: a model system to investigate the witches' broom disease of Theobroma cacao . Plant Pathology 63: 1251–1263. [Google Scholar]
- 33. Samuels GJ, Pardo-Schultheiss R, Hebbar KP, Lumsden RD, Bastos CN, et al. (2000) Trichoderma stromaticum sp. nov., a parasite of the cacao witches broom pathogen. Mycological Research 104: 760–764. [Google Scholar]
- 34. Matheny PB, Curtis JM, Hofstetter V, Aime MC, Moncalvo J-M, et al. (2006) Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98: 982–995. [DOI] [PubMed] [Google Scholar]