Abstract
Background
Akkermansia muciniphila and Desulfovibrio spp. are commensal microbes colonising the mucus gel layer of the colon. Both species have the capacity to utilise colonic mucin as a substrate. A. muciniphila degrades colonic mucin, while Desulfovibrio spp. metabolise the sulfate moiety of sulfated mucins. Altered abundances of these microorganisms have been reported in ulcerative colitis (UC). However their capacity to bind to human colonic mucin, and whether this binding capacity is affected by changes in mucin associated with UC, remain to be defined.
Methods
Mucin was isolated from resected colon from control patients undergoing resection for colonic cancer (n = 7) and patients undergoing resection for UC (n = 5). Isolated mucin was purified and printed onto mucin microarrays. Binding of reference strains and three clinical isolates of A. muciniphila and Desulfovibrio spp. to purified mucin was investigated.
Results
Both A. muciniphila and Desulfovibro spp. bound to mucin. The reference strain and all clinical isolates of A. muciniphila showed increased binding capacity for UC mucin (p < .005). The Desulfovibrio reference strain showed increased affinity for UC mucin. The mucin binding profiles of clinical isolates of Desulfovibrio spp. were specific to each isolate. Two isolates showed no difference in binding. One UC isolate bound with increased affinity to UC mucin (p < .005).
Conclusion
These preliminary data suggest that differences exist in the mucin binding capacity of isolates of A. muciniphila and Desulfovibrio spp. This study highlights the mucin microarray platform as a means of studying the ability of bacteria to interact with colonic mucin in health and disease.
Introduction
The mucus gel layer (MGL) forms a protective barrier between colonic epithelium and colonic contents, preventing entry of bacteria while allowing diffusion of essential nutrients [1]. Mucins constitute the functional units of the MGL, with MUC2 representing the principal component of colonic mucus [2, 3]. Due to a high degree of glycosylation, mucins provide an energy source and a growth medium for mucus-associated microbiota [2], that in health exist in a symbiotic relationship with the host [4]. Due to their close proximity to the epithelium, microbes present within the MGL of the colon exert a greater effect on the host than luminal microbes [5]. The intestinal microbiota modulates a variety of host responses, including those related to metabolism which the host has not developed for itself [4, 6, 7]. Through foraging of carbohydrates from both dietary sources and colonic mucins, the microbiota provides an energy source through the production of short chain fatty acids (SCFAs) [7]. Interaction between the microbiota and colonic mucins warrants investigation, as this represents the true host microbial interface in the colon.
In ulcerative colitis (UC), changes occur in the MGL that may alter its protective capacity [8–10]. These include physical changes in the mucus barrier [11], altered mucin gene expression [12] and biochemical changes affecting the mucins [13]. Increased microbial colonisation of the MGL has been reported in UC [14]. Changes in colonic mucus and mucin may influence bacterial colonisation in the inflamed colon, due to altered availability of mucin-derived substrate, leading to an altered microenvironment. In addition there is substantial evidence of a dysbiosis in UC [15–17]. As a result, changes in relative abundances of certain bacterial taxa in the UC setting may affect mucin production and secretion [18].
This study focuses on two colonic commensals A. muciniphila and Desulfovibrio spp., both of which have the potential to metabolise colonic mucin. A. muciniphila is known to have mucolytic potential in-vitro [19] and may have a role in stimulation of the immune system and maintenance of tolerance to commensal microbes [20, 21]. A. muciniphila may also be involved in a “positive feedback loop” whereby mucolytic properties may stimulate mucus renewal [22]. Furthermore, this microbe binds to colonic cell lines and may contribute to maintenance of the integrity of the colonic epithelial cell layer [23]. In UC, the abundance of A. muciniphila is reduced [24, 25]. However, it remains to be determined whether this change is related to altered binding to colonic mucin.
Desulfovibrio spp. may contribute to mucosal inflammation in UC through production of potentially toxic hydrogen sulfide, released as a by-product of metabolism of sulfated mucin [26–31]. It is not known whether Desulfovibrio spp. is capable of directly binding mucin or whether it metabolises sulfate from mucin that has been cleaved by other bacteria.
The present study utilises a mucin microarray platform as a means of testing the hypothesis that mucolytic microbes bind to human colonic mucin and investigates the affinity for mucin in health and UC.
Materials and Methods
Ethical approval, patient recruitment and sample collection
Ethical approval was obtained from St. Vincent’s University Hospital Ethics and Medical Research Committee. All individuals gave informed, written consent prior to the procedure.
For the collection of mucin specimens, seven control patients undergoing colonic resection for cancer and five patients with UC undergoing colectomy were recruited. For the control mucin, paired biopsies of approximately 2 cm2 of mucosal tissue were resected from the excised colon at least 5 cm from the tumour. Similar paired biopsies of mucosa from patients with UC were obtained from the caecum, transverse, left colon and rectum of fresh surgical resection specimens. Patients had not received bowel preparation prior to undergoing surgery. In each case, one of the paired samples for mucin isolation was freshly frozen and one for histological analysis was stored in formalin.
Mucus was harvested and mucin purified as previously described [32, 33]. In brief, mucus was suspended in guanidine hydrochloride (final concentration 4M) to form a solution. Samples were reduced with dithiothreitol (DTT) (Sigma Aldrich) at a final concentration of 0.01M at 37°C for 5 hours and were alkylated with iodoacetamide (0.025M) (Sigma Aldrich). Mucin was purified by CsCl density gradient separation and size exclusion chromatography.
For bacterial isolation, mucosal biopsies were obtained from one control patient, and three patients with active UC. The healthy volunteer was asymptomatic and undergoing a screening colonoscopy for family history of colorectal carcinoma. This patient had no mucosal evidence of pathology. Bowel preparation was sodium picosulfate based. Exclusion criteria included: antibiotic usage or hospital admission in the six weeks prior to colonoscopy, a history of bleeding per rectum, personal history of irritable bowel syndrome or colorectal carcinoma. The biopsy was obtained using a RadialJaw 3 biopsy forceps (Boston Scientific, Natick, MA, USA) and was retrieved with a sterile needle to prevent external contamination. Biopsies from patients with UC were obtained from rectal mucosa at the time of surgical resection for disease refractory to medical management. Approximately 1 cm2 of mucosa was resected using sterile instruments.
Bacterial strains, bacterial isolations and culture
The A. muciniphila reference strain ATCCBAA-835 (American Type Culture Collection, Manassas, VA) and Desulfovibrio desulfuricans reference strain ATCC 27774 (American Type Culture Collection) were cultured according to the suppliers guidelines using BHI (Sigma Aldrich, Dublin, Ireland) and a modified Postgate’s medium respectively. Modified Postgate’s medium was prepared as follows: K2PO4 0.5g/L, NH4CL 0.5 g/L, CaSO4 1 g/L, MgSO4.7H2O 2 g/L, sodium lactate 3.5 g/L, yeast extract 1 g/L, 30 g/L fastidious anaerobic broth (Lab M Ltd., Bury, Manchester, UK), and ascorbic acid 0.1 g/L and autoclaving at 121°C for 15 min. Filter sterilised FeSO4 was added to the modified Postgate’s medium at a concentration of 0.5 g/L immediately prior to use. All reagents were sourced from Sigma Aldrich unless otherwise stated. Cultures were placed in a shaking incubator at 200 rpm at 37°C for 16 hours under anaerobic conditions achieved by the use of AnaeroGen anaerobic gas packs (Oxoid, Basingstoke, UK).
Fresh colonic mucosal samples were placed directly into 5 ml of sterile phosphate buffered saline (PBS) immediately after resection and stored at 4°C until culturing. Immediately prior to culturing, samples were vortexed for 30 seconds and 1 ml of the PBS solution was inoculated into 50 ml of Brain Heart Infusion(BHI) broth and 50 ml of modified Postgate’s medium broth for isolation of A. muciniphila and Desulfovibrio spp. respectively. Cultures of A. muciniphila were incubated for 16 hours and Desulfovibrio spp. for up to 72 hours. Following growth, as evidenced by a cloudy appearance of BHI broth and a black precipitate accompanied by the odour of hydrogen sulfide in the case of modified Postgate’s medium, the cultures were sub-cultured onto BHI and a modified Postgate’s medium agar. The modified Postgate’s medium agar was prepared as described above plus the addition of 15 g/L of bacteriologic agar. Cultures were incubated at 37°C overnight under anaerobic conditions achieved by the use of AnaeroGen anaerobic gas packs (Oxoid). Sub-culturing was repeated until a pure growth of each isolate was obtained.
Growth of A. muciniphila was characterised by white colonies measuring approximately 0.7 mm in diameter as previously described [19]. Gram staining was performed to confirm the presence of gram negative oval-shaped cells characteristic of A. muciniphila. Colonies of Desulfovibrio spp. were identified as described above. Isolates of A. muciniphila and Desulfovibrio spp. were stored at -80°C on cryopreservative beads (MicroBank, ProLab Diagnostics, ON, Canada) until further analysis.
To confirm the identity of clinical isolates, PCR using an assay specific for each bacterial target was performed. DNA extraction was performed on a single colony from each culture by re-suspending in PBS followed by four heat/freeze cycles at 100°C and -80°C. Conventional PCR targeting the 16S rRNA gene of each target was performed using oligonucleotide primers targeting A. muciniphila (forward primer 5’- CAGCACGTGAAGGTGGGGAC– 3’ reverse primer 5’- CCTTGCGGTTGGCTTCAGAT-3’) [24] and Desulfovibrio spp. (forward primer 5’- CCGTAGATATCTGGAGGAACATCAG -3’, reverse primer 5’-ACARCTAGCATCCATCGTTTACAGC-3’) [34] respectively. All PCR reactions contained 1X My Taq Red Mix (Bioline, London, UK), forward primer and reverse primer at a final concentration of 200 nM. For Desulfovibrio spp., each reaction contained 5 μl of DNA template and for A. muciniphila reactions contained 10 μl of DNA template. Each assay run incorporated a negative control and a reference sample of cloned 16S rRNA gene from Desulfovibrio spp. or A. muciniphila as a positive control. All reactions were carried out on a Multigene thermocycler (Labnet International Inc., Woolbridge, NJ, U.S.A.) under the following cycling conditions: A. muciniphila 95°C for 10 seconds initially, followed by 30 cycles of 95°C for one minute, 50°C for one minute, 68°C for 10 seconds. PCR conditions for Desulfovibrio spp. were: 95°C for 5 minutes initially, followed by 35 cycles of 95°C for 1 minute, 62°C for 1 minute, 72°C for 45 seconds and a final extension step at 72°C for 5 minutes. PCR products were analysed by electrophoresis in a 2% agarose gel stained with 0.5 μg/ml ethidium bromide (Sigma) and visualised under ultra violet light immediately after electrophoresis. Products of A. muciniphila and Desulfovibrio spp. were visualised at 327 and 135 base pairs, respectively.
Histological analysis of specimens from which mucin was isolated
Formalin fixed, paraffin embedded mucosal biopsy specimens for each mucin sample were stained using Haematoxylin and eosin stain (H&E) and High Iron Diamine-Alcian Blue (HID-AB) staining to quantify degree of inflammation and percentage sulfation as previously described [35]. For each specimen, the quantity of sulfated mucin was determined and results expressed as the percentage relative to the total mucin content for a given specimen. For histological analysis, UC specimens were scored as mild, moderate or severe inflammation, according to the system described by Geboes et al [36]. Control specimens were described as normal mucosa.
Interrogation of mucin array for bacterial binding
Printing of the purified human colonic mucins on Nexterion slide H microarray slides was optimised and printed as previously described (S1 Table) [32]. Each microarray slide was printed with eight replicate subarrays, with each mucin printed in six replicates (per subarray). Microarray slides were blocked and washed as previously described [32]. Print performance and mucin glycosylation was assessed by incubating the microarray with a panel of tetramethylrhodamine-(TRITC-) labelled lectins, (see S2 Table for lectins and their incubation concentration) diluted in low salt Tris buffered saline supplemented with Ca2+ and Mg2+ ions (TBS; 20 mM Tris-HCl, 100 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, pH 7.2) with 0.05% Tween-20 (TBS-T) as previously described [32].
Prior to incubation on the microarrays, Desulfovibrio spp. were cultured overnight in iron-free modified Postgate’s medium. Bacterial strains of A. muciniphila and Desulfovibrio spp. were re-suspended to an optical density 600 nm (OD600) of 0.1. Bacteria were cultured for 2.5 hours as described above and harvested by centrifugation of 1 ml of each culture at 16,200 x g for 1 min, the supernatant discarded and the pellet re-suspended to an OD600 of 1.0 in TBS. Bacterial cultures were labelled with SYTO82 nucleic acid fluorescent dye (Life Technologies, Carlsbad, CA, U.S.A.) at a final concentration of 20 μM, protected from light and incubated for 45 min at room temperature. Bacteria were washed seven times with 1 ml of TBS for each wash to completely remove unbound dye [37]. The final pellet was re-suspended to an OD600 of 0.5 with TBS-T.
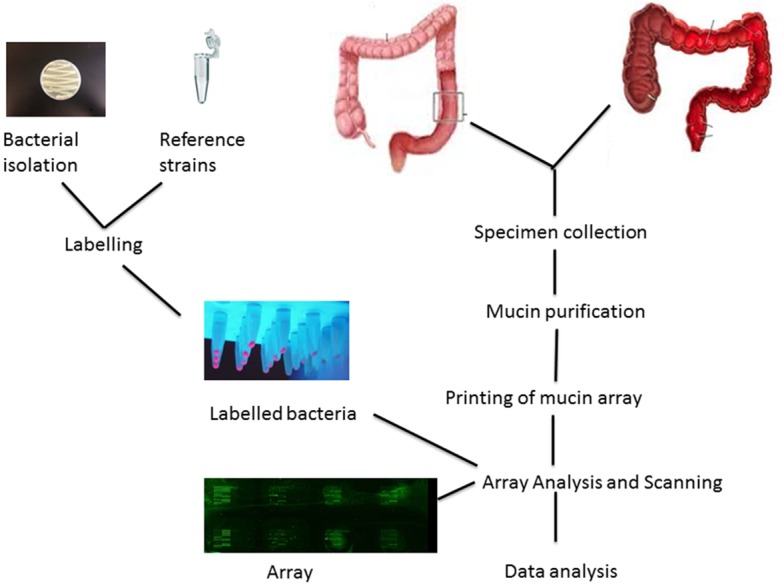
The mucin microarray slides were initially rehydrated by incubating 70 μl of TBS per subarray using an Agilent eight-well gasket slide and incubation cassette system (Agilent Technologies, Cork, Ireland) at 37°C for 45 min. The TBS was removed and the microarray slides were subsequently incubated with 70 μl of fluorescently labelled bacteria at an OD600 of O.5 per subarray. Two subarrays on each microarray slide were incubated with TRITC-labelled lectins Artocarpus integrifolia (AIA, 15 ug/mL TBS-T, final concentration) and Maackia amurensis agglutinin (MAA, 10 ug/mL TBS-T, final concentration) (EY Laboratories Ltd., San Mateo, CA, USA) to monitor print performance. The slides were incubated in a shaking incubator at 200 rpm at 37°C for 1hr followed by washing five times in TBS-T, once in TBS and once in water. Slides were dried by centrifugation at 266 x g for 5 min and scanned immediately in a GenePix 4000b microarray scanner (Molecular Devices, Wokingham, UK) with the 532 nm laser using the following settings; laser power 100%, 10μm resolution and 70% PMT [33]. Analysis of each bacterial isolate consisted of two technical replicates per microarray slide and three biological replicates on different microarray slides, resulting in a total of six data sets for each isolate. These methods are summarised in Fig 1.
Fig 1. Summary of Materials and Methods.
Data extraction and analysis
Data were extracted from the scanned images using GenePix pro software (Molecular Devices) and then exported to Excel for subsequent analysis and normalisation as previously described [33, 37]. The median of six replicate features per subarray was handled as a single data point for graphical and statistical analysis. Data intensities across the three replicate microarray slides were normalised against the ratio of total subarray fluorescence/mean total fluorescence of the six replicate subarrays to account for inter-microarray slide variability and minimise the effect of variation in mucin printing. Mucin micro-array data are available in the supplementay information (S3 and S4 Tables)
Glycosylation Profiles of Human Colonic Mucins
Data generated from lectin profiling of the mucin microarray were used to compare the glycosylation profiles of human colonic mucins in health and UC (Fig 2). Sialylation, as indicated by MAA binding, is present in the colonic mucins. Sulfation, as determined by WFA binding with low or without concomitant SBA binding, was most present in the control mucins. Varying quantities of sialylated or sulfated O-linked oligosaccharides were present throughout the samples as determined by varying intensities of AIA binding.
Fig 2. Comparison of the glycosylation profiles of human colonic mucins generated from lectin profiling of the mucin microarray.
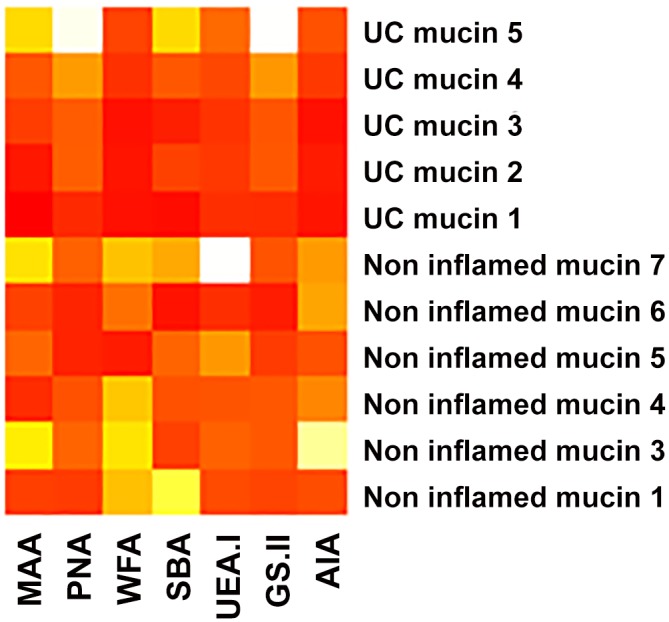
The maximum binding for colonic mucins is 8,335 RFU. The highest intensity binding is represented by red, followed by orange, yellow and white.
Statistical analysis
Normalised data were exported to SPSS statistics, version 20.0 (SPSS statistics, IBM, London, U.K.) for statistical analysis. Statistical comparisons were performed based on Mann-Whitney U test and Kruskal-Wallis comparisons.
Results
HID-AB analysis of biopsies collected from each individual indicated that UC mucin had a median percentage sulfomucin of 39.51% (IQR 32.68%) and controls a median of 57.69% (IQR 16.74%). On histological analysis of mucosal biopsies obtained from patients with UC, four were classified as severely inflamed and one as moderately inflamed according to the Geboes scoring system [36]. Control biopsies were described as normal mucosa.
Three isolates of both A. muciniphila and Desulfovibrio spp. were successfully cultured from different individuals. One isolate of A. muciniphila and Desulfovibrio spp. was isolated from a control patient, and two isolates of both species were isolated from three individuals with UC.
A. muciniphila and Desulfovibrio spp. bound to colonic mucin in health and UC
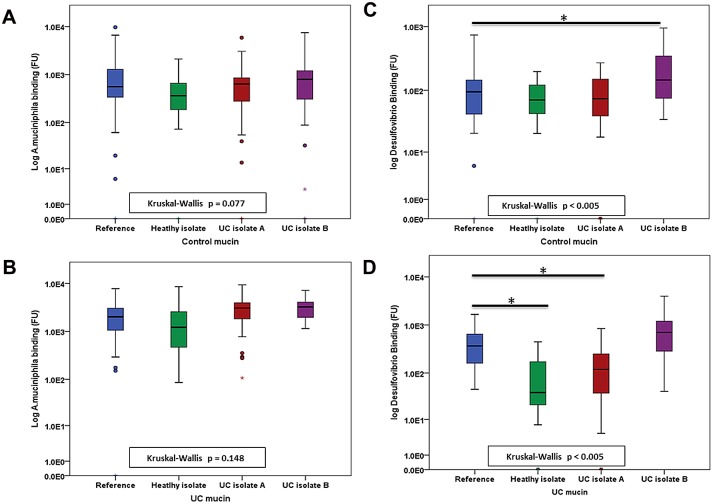
Both A. muciniphila and Desulfovibro bound to colonic mucin (Table 1). Clinical isolates of A. muciniphila did not differ from the reference strain with regard to binding to control and UC mucin (Fig 3a and 3b, Table 1). However, all clinical isolates of Desulfovibrio spp. showed differences in comparison to the reference strain D. desulfuricans. One isolate of Desulfovibrio spp., cultured from a patient with UC, (UC isolate B) displayed increased binding to mucin from controls compared to the reference strain (Fig 3c, Table 1), while the healthy isolate and one UC isolate (UC isolate A) of Desulfovibrio spp. bound to mucin isolated from UC colon with reduced affinity compared to the reference strain (Fig 3d, Table 1).
Table 1. Median binding values of each isolate to mucin from controls and the UC colon and Kruskal-Wallis tests comparing the binding of clinical isolates to that of the reference strain for A. muciniphila and Desulfovibrio spp.
| A. muciniphila | Median binding to Control mucin(FU) | IQR | Kruskal-Wallis and Wilcoxin-Mann-Whitney | Fold change in binding compared to the reference strain | Median binding to UC mucin (FU) | IQR | Kruskal-Wallis and Wilcoxin-Mann-Whitney | Fold change in binding compared to the reference strain |
|---|---|---|---|---|---|---|---|---|
| .077 | .148 | |||||||
| Reference | 559.83 | 970.89 | n/a | 2022.41 | 2076.05 | n/a | ||
| Healthy isolate | 362.58 | 493.62 | n/a | -0.65 | 1221.52 | 2176.76 | n/a | -0.60 |
| UC isolate A | 636.67 | 593.58 | n/a | +1.14 | 3075.48 | 2398.98 | n/a | +1.52 |
| UC isolate B | 792.37 | 908.92 | n/a | +1.42 | 3251.41 | 2165.19 | n/a | +1.61 |
| Desulfovibrio spp. | ||||||||
| <0.005 | <0.005 | |||||||
| Reference | 95.10 | 107.73 | n/a | 368.34 | 501.99 | n/a | ||
| Healthy isolate | 70.44 | 79.30 | 1.00 | -0.74 | 39.60 | 155.02 | <0.005 | -0.11 |
| UC isolate A | 73.94 | 113.35 | 1.00 | -0.78 | 119.74 | 238.59 | .014 | -0.32 |
| UC isolate B | 145.38 | 283.64 | .022 | +1.53 | 712.41 | 930.06 | .276 | +1.93 |
Significant values are highlighted in bold text. FU corresponds to measure of bacterial binding in fluorescent units. IQR corresponds to inter quartile range.
Fig 3. Boxplots illustrating the binding of clinical isolates and reference strains to mucin from different sources.
Median binding of isolates of A. muciniphila to control mucin (a) Median binding of isolates of A. muciniphila to UC mucin (b) Median binding of isolates of Desulfovibrio spp. to control mucin (c) Median binding of isolates of Desulfovibrio spp. to UC mucin (d).
Isolates of A. muciniphila and Desulfovibrio spp. display increased binding to mucin from the UC colon
In a direct comparison of bacterial binding to mucin from UC and controls, both the reference strain and all three clinical isolates of A. muciniphila displayed increased affinity for UC mucin compared to mucin from controls (Fig 4a, Table 2).
Fig 4. Boxplots representing the median binding of each isolate and reference strain to mucin from the UC colon compared to controls.
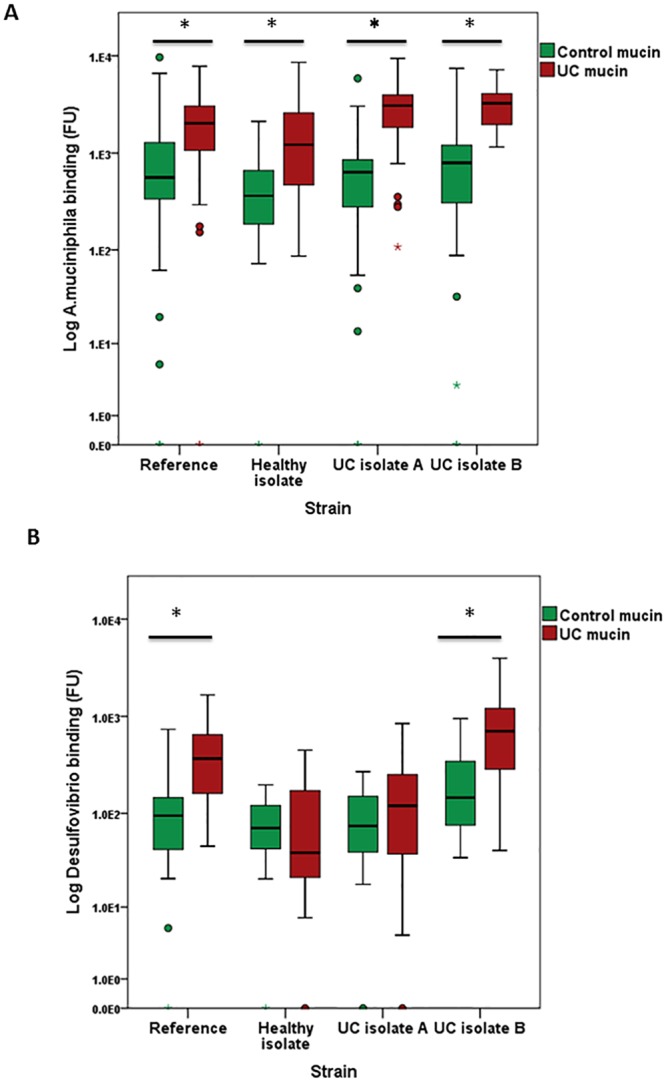
Direct comparison of the median binding of isolates of A. mucinihpila (a). Direct comparison of median binding of isolates of Desulfovibrio spp. (b).
Table 2. Comparison of bacterial binding to control and UC mucin as determined by the Mann-Whitney U test.
| Bacterial Target | Isolate | Median Binding to mucin from controls (FU) | IQR | Median Binding to UC mucin (FU) | IQR | Mann- Whitney U(p value) |
|---|---|---|---|---|---|---|
| A. muciniphila | Reference | 559.83 | 970.89 | 2022.41 | 2076.05 | .001 |
| Healthy isolate | 362.58 | 493.62 | 1221.52 | 2176.76 | .000 | |
| UC isolate A | 636.67 | 593.58 | 3075.48 | 2398.98 | .000 | |
| UC isolate B | 792.37 | 908.92 | 3251.41 | 2165.19 | .000 | |
| Desulfovibrio spp. | Reference | 95.10 | 107.73 | 368.34 | 501.99 | .000 |
| Healthy isolate | 70.44 | 79.30 | 39.59 | 155.02 | .803 | |
| UC isolate A | 73.94 | 113.35 | 119.74 | 238.59 | .456 | |
| UC isolate B | 145.38 | 283.64 | 712.41 | 930.06 | .000 |
Significant values are highlighted in bold text. FU corresponds to measure of bacterial binding in fluorescent units. IQR corresponds to inter quartile range.
In the case of Desulfovibrio spp., the reference strain and one isolate from a patient with UC (UC isolate B) bound with increased affinity to UC mucin compared to mucin from controls, while the healthy isolate and the isolate from the second patient with UC (UC isolate A) showed no difference in binding (Fig 4b, Table 2).
Discussion
UC is associated with changes in colonic mucins thought to be related to dysregulated cross-talk between the host and an altered microbiota. Mucin binding is the first point of bacterial interaction with the host and, as such, is a key mediator of this cross-talk. The present study investigated the ability of two commensals that have been implicated in the pathogenesis of UC, to bind to colonic mucin.
These preliminary data demonstrate for the first time that both A. muciniphila and Desulfovibrio spp. have the ability to bind to human colonic mucin. However, no adhesins have been identified in the currently annotated genome of either A. muciniphila or Desulfovibrio spp. [38]. Previous studies have demonstrated the ability of A. muciniphila to degrade both porcine mucin and human mucin in-vitro [19, 24] and provided putative evidence that A. muciniphila express BACON (Bacteriodetes-associated carbohydrate-binding Often N-terminal), a protein believed to mediate mucin binding [38, 39]. The present results indicate the likely production of such mucin binding proteins, or the presence of carbohydrate binding motifs (CBMs), a family of domains that bind various polysaccharides, enhancing their degradation and may interact with mucin glycan structures [40–43]. Alternatively, non-specific interactions based on hydrophobicity have been described as an adhesion mechanism in the colon [44, 45].
The observed differences in binding may be explained by strain-specific differences in the binding capacity of the microbes or alterations to mucin in UC that promote increased mucin binding. The present study focused on a number of clinical isolates and their commercially available ATCC reference strain counterparts. While the identity of all isolates was confirmed by PCR, no information regarding the strain specificities was obtained. It is known that different strains of a bacterial species may possess different adhesion molecules [46–48] and that selective pressures and natural mutations have the potential to alter these adhesion molecules [49–51]. Given these observations and the selective pressure placed upon bacteria to survive in a niche environment like the colonic MGL, it is possible that strain specific differences in adhesion molecules explain the observed difference in mucin binding.
Mucin in the UC colon shows reduced sulfation [35, 52, 53], possibly the result of bacterial sulphatase activity [54, 55], as well as changes in the degree of mucin glycosylation [13]. The data presented here indicate a reduction in the median percentage sulphation in UC mucin compared to control mucin. Longman et al. did not report significant differences in the histochemistry of UC samples compared to healthy colorectal tissue using the same staining technique. However the study did not quantify sulfomucin content and did report reduced staining of the sulfo-Lewis mucin epitope, a finding that correlated with disease severity [10]. Alterations in mucin glycosylation patterns were also observed in the present study, as outlined in Fig 2. The resultant change in the microenvironment of the MGL may well influence the binding capacity of the resident microbiota. In health, the presence of the sulphate moiety is thought to protect mucin against degradation by colonic microbes [52]. The loss of sulphation, whether enzymatically mediated or through biosynthetic reduction, could alter the mucin substrate for bacterial binding. This may occur though the loss of a sugar binding ligand, in which case a reduction in binding would be observed. Alternatively, presentation of a new or previously cryptic ligand after desulfation, possibly mediated by enhanced susceptibility to mucinase activity [56], may result in increased bacterial binding. Exposure of such motifs could explain the increased binding of A. muciniphila and Desulfovibrio spp. to UC mucin.
It has previously been reported that A. muciniphila has the ability to bind to adenocarcinoma-derived cell lines Caco2 and HT-29, but not to colonic mucus [23]. Healthy colonocytes produce a mucus layer that is rich in O-acetylated sialic acids [57]. The oligo-O-acetylation of sialic acids is lost in colorectal cancer and may be an early biomarker in the adenoma-carcinoma sequence [58, 59]., a finding that may account for the high binding of A. muciniphila to adenocarcinoma-derived cell lines. Although the degree of O-acetylation of sialic acids was not evaluated in the present study, it warrants consideration as a possible important modulator of bacterial binding.
It should also be considered that the binding process itself may influence subsequent binding events. Mucin-microbe interactions have previously been investigated by Skoog et al., who demonstrated that weak interactions between Helicobacter pylori and gastric mucin result in increased expression of H. pylori adhesion factors in an in-vitro model [60]. It is possible that similar events may be occurring in the case of these commensals and other commensals or pathogens within the colon.
While the present study has identified differences in the affinity of A. muciniphila and Desulfovibrio spp. for mucin from the inflamed and non-inflamed colon, it should be acknowledged that the purified mucin used in this study lacks many of the physiological factors and constituents of mucus such as inflammatory mediators and cytokines, that in-vivo modulate the physiology of colonic mucus and in turn may influence microbial binding. The authors also acknowledge the low number of clinical isolates investigated in this study. While the results have indicated that strain specific differences in the binding patterns of isolates of Desulfovibrio spp. exist, we are hesitant to suggest that these findings reflect how healthy and UC isolates behave in all cases. Further study involving isolates from a larger number of individuals (both healthy and UC) is required to more fully understand the mediators of mucin binding in-vivo.
However, this work does highlight the potential for use of mucin microarray technology in the investigation of microbe-mucin binding. Manipulation of the microbiota holds great potential as a treatment modality in colorectal diseases. For such biotherapies to reach full potential, a thorough understanding of the nature of the interactions between individual bacterial species and the host is required. Assays such as those described in this study, advance knowledge of microbe-mucin interactions in UC, as well as other gastrointestinal diseases.
Supporting Information
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
The authors wish to acknowledge the assistance of Dr Mary Gallagher and Ms Sarah-Louise Hassett in isolating the mucins used in the array studies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Science Foundation Ireland Grant Numbers: 09/IN.1/B2606, 08/SRC/B1393 (URL: http://www.sfi.ie). Authors receiving funding: P.R.O., L.J. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nature reviews Microbiology. 2011;9(4):265–78. 10.1038/nrmicro2538 [DOI] [PubMed] [Google Scholar]
- 2. Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 Suppl 1:4659–65. 10.1073/pnas.1006451107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hansson GC. Role of mucus layers in gut infection and inflammation. Current opinion in microbiology. 2012;15(1):57–62. 10.1016/j.mib.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science (New York, NY). 2005;307(5717):1915–20. [DOI] [PubMed] [Google Scholar]
- 5. Ouwerkerk JP, de Vos WM, Belzer C. Glycobiome: bacteria and mucus at the epithelial interface. Best practice & research Clinical gastroenterology. 2013;27(1):25–38. [DOI] [PubMed] [Google Scholar]
- 6. Backhed F. Host responses to the human microbiome. Nutrition reviews. 2012;70 Suppl 1:S14–7. 10.1111/j.1753-4887.2012.00496.x [DOI] [PubMed] [Google Scholar]
- 7. Tremaroli V, Kovatcheva-Datchary P, Backhed F. A role for the gut microbiota in energy harvesting? Gut. 2010;59(12):1589–90. 10.1136/gut.2010.223594 [DOI] [PubMed] [Google Scholar]
- 8. Lennon G, Balfe A, Earley H, Devane LA, Lavelle A, Winter DC, et al. Influences of the colonic microbiome on the mucous gel layer in ulcerative colitis. Gut microbes. 2014;5(3):277–85. 10.4161/gmic.28793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antoni L, Nuding S, Wehkamp J, Stange EF. Intestinal barrier in inflammatory bowel disease. World journal of gastroenterology: WJG. 2014;20(5):1165–79. 10.3748/wjg.v20.i5.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Longman RJ, Poulsom R, Corfield AP, Warren BF, Wright NA, Thomas MG. Alterations in the composition of the supramucosal defense barrier in relation to disease severity of ulcerative colitis. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2006;54(12):1335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35(3):353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forgue-Lafitte ME, Fabiani B, Levy PP, Maurin N, Flejou JF, Bara J. Abnormal expression of M1/MUC5AC mucin in distal colon of patients with diverticulitis, ulcerative colitis and cancer. International journal of cancer Journal international du cancer. 2007;121(7):1543–9. [DOI] [PubMed] [Google Scholar]
- 13. Larsson JM, Karlsson H, Crespo JG, Johansson ME, Eklund L, Sjovall H, et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflammatory bowel diseases. 2011;17(11):2299–307. [DOI] [PubMed] [Google Scholar]
- 14. Schultsz C, Van Den Berg FM, Ten Kate FW, Tytgat GN, Dankert J. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology. 1999;117(5):1089–97. [DOI] [PubMed] [Google Scholar]
- 15. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, et al. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut. 2011;60(5):631–7. 10.1136/gut.2010.223263 [DOI] [PubMed] [Google Scholar]
- 17. Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome biology. 2012;13(9):R79 10.1186/gb-2012-13-9-r79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Natividad JM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacological research: the official journal of the Italian Pharmacological Society. 2013;69(1):42–51. [DOI] [PubMed] [Google Scholar]
- 19. Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. International journal of systematic and evolutionary microbiology. 2004;54(Pt 5):1469–76. [DOI] [PubMed] [Google Scholar]
- 20. Derrien M, Van Baarlen P, Hooiveld G, Norin E, Muller M, de Vos WM. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Frontiers in microbiology. 2011;2:166 10.3389/fmicb.2011.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Applied and environmental microbiology. 2008;74(5):1646–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belzer C, de Vos WM. Microbes inside—from diversity to function: the case of Akkermansia. The ISME journal. 2012;6(8):1449–58. 10.1038/ismej.2012.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, et al. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Applied and environmental microbiology. 2015;81(11):3655–62. 10.1128/AEM.04050-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. The American journal of gastroenterology. 2010;105(11):2420–8. 10.1038/ajg.2010.281 [DOI] [PubMed] [Google Scholar]
- 25. James SL, Christophersen CT, Bird AR, Conlon MA, Rosella O, Gibson PR, et al. Abnormal fibre usage in UC in remission. Gut. 2015;64(4):562–70. 10.1136/gutjnl-2014-307198 [DOI] [PubMed] [Google Scholar]
- 26. Roediger WE, Duncan A, Kapaniris O, Millard S. Sulphide impairment of substrate oxidation in rat colonocytes: a biochemical basis for ulcerative colitis? Clinical science (London, England: 1979). 1993;85(5):623–7. [DOI] [PubMed] [Google Scholar]
- 27. Roediger WE, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Digestive diseases and sciences. 1997;42(8):1571–9. [DOI] [PubMed] [Google Scholar]
- 28. Pitcher MC, Beatty ER, Cummings JH. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut. 2000;46(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jia W, Whitehead RN, Griffiths L, Dawson C, Bai H, Waring RH, et al. Diversity and distribution of sulphate-reducing bacteria in human faeces from healthy subjects and patients with inflammatory bowel disease. FEMS immunology and medical microbiology. 2012;65(1):55–68. 10.1111/j.1574-695X.2012.00935.x [DOI] [PubMed] [Google Scholar]
- 30. Rowan F, Docherty NG, Murphy M, Murphy B, Calvin Coffey J, O'Connell PR. Desulfovibrio bacterial species are increased in ulcerative colitis. Diseases of the colon and rectum. 2010;53(11):1530–6. [DOI] [PubMed] [Google Scholar]
- 31. Attene-Ramos MS, Nava GM, Muellner MG, Wagner ED, Plewa MJ, Gaskins HR. DNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 Int cells. Environmental and molecular mutagenesis. 2010;51(4):304–14. 10.1002/em.20546 [DOI] [PubMed] [Google Scholar]
- 32. Kilcoyne M, Gerlach JQ, Gough R, Gallagher ME, Kane M, Carrington SD, et al. Construction of a natural mucin microarray and interrogation for biologically relevant glyco-epitopes. Analytical chemistry. 2012;84(7):3330–8. 10.1021/ac203404n [DOI] [PubMed] [Google Scholar]
- 33. Naughton JA, Marino K, Dolan B, Reid C, Gough R, Gallagher ME, et al. Divergent mechanisms of interaction of Helicobacter pylori and Campylobacter jejuni with mucus and mucins. Infection and immunity. 2013;81(8):2838–50. 10.1128/IAI.00415-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fite A, Macfarlane GT, Cummings JH, Hopkins MJ, Kong SC, Furrie E, et al. Identification and quantitation of mucosal and faecal desulfovibrios using real time polymerase chain reaction. Gut. 2004;53(4):523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lennon G, Balfe A, Bambury N, Lavelle A, Maguire A, Docherty NG, et al. Correlations between colonic crypt mucin chemotype, inflammatory grade and Desulfovibrio species in ulcerative colitis. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2014;16(5):O161–9. [DOI] [PubMed] [Google Scholar]
- 36. Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47(3):404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kilcoyne M, Twomey ME, Gerlach JQ, Kane M, Moran AP, Joshi L. Campylobacter jejuni strain discrimination and temperature-dependent glycome expression profiling by lectin microarray. Carbohydrate research. 2014;389:123–33. 10.1016/j.carres.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 38. van Passel MW, Kant R, Zoetendal EG, Plugge CM, Derrien M, Malfatti SA, et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PloS one. 2011;6(3):e16876 10.1371/journal.pone.0016876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mello LV, Chen X, Rigden DJ. Mining metagenomic data for novel domains: BACON, a new carbohydrate-binding module. FEBS letters. 2010;584(11):2421–6. 10.1016/j.febslet.2010.04.045 [DOI] [PubMed] [Google Scholar]
- 40. Boraston AB, Bolam DN, Gilbert HJ, Davies GJ. Carbohydrate-binding modules: fine-tuning polysaccharide recognition. The Biochemical journal. 2004;382(Pt 3):769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang Z, Chen H, Chen L, Liu S, Han X, Wu Q. Improving endoglucanase activity by adding the carbohydrate-binding module from Corticium rolfsii. Journal of microbiology and biotechnology. 2014;24(4):440–6. [DOI] [PubMed] [Google Scholar]
- 42. Guillen D, Sanchez S, Rodriguez-Sanoja R. Carbohydrate-binding domains: multiplicity of biological roles. Applied microbiology and biotechnology. 2010;85(5):1241–9. 10.1007/s00253-009-2331-y [DOI] [PubMed] [Google Scholar]
- 43. Gao S, You C, Renneckar S, Bao J, Zhang YH. New insights into enzymatic hydrolysis of heterogeneous cellulose by using carbohydrate-binding module 3 containing GFP and carbohydrate-binding module 17 containing CFP. Biotechnology for biofuels. 2014;7(1):24 10.1186/1754-6834-7-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ouwehand AC, Suomalainen T, Tölkkö S, Salminen S. In vitro adhesion of propionic acid bacteria to human intestinal mucus. Lait. 2002;82(1):123–30. [Google Scholar]
- 45. Del Re B, Sgorbati B, Miglioli M, Palenzona D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Letters in Applied Microbiology. 2000;31(6):438–42. [DOI] [PubMed] [Google Scholar]
- 46. McCann JR, Sheets AJ, Grass S, St Geme JW. The Haemophilus Cryptic Genospecies Cha Adhesin Has at Least Two Variants That Differ in Host Cell Binding, Bacterial Aggregation, and Biofilm Formation Properties. Journal of bacteriology. 2014;196(9):1780–8. 10.1128/JB.01409-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kline KA, Fälker S, Dahlberg S, Normark S, Henriques-Normark B. Bacterial Adhesins in Host-Microbe Interactions. Cell host & microbe. 2009;5(6):580–92. [DOI] [PubMed] [Google Scholar]
- 48. Prorok-Hamon M, Friswell MK, Alswied A, Roberts CL, Song F, Flanagan PK, et al. Colonic mucosa-associated diffusely adherent afaC plus Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut. 2014;63(5):761–70. 10.1136/gutjnl-2013-304739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hsu K-L, Pilobello KT, Mahal LK. Analyzing the dynamic bacterial glycome with a lectin microarray approach. Nat Chem Biol. 2006;2(3):153–7. [DOI] [PubMed] [Google Scholar]
- 50. Harris SL, Spears PA, Havell EA, Hamrick TS, Horton JR, Orndorff PE. Characterization of Escherichia coli Type 1 Pilus Mutants with Altered Binding Specificities. Journal of bacteriology. 2001;183(13):4099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mahdavi J, Royer P-J, Sjölinder HS, Azimi S, Self T, Stoof J, et al. Pro-inflammatory cytokines can act as intracellular modulators of commensal bacterial virulence. Open Biology. 2013;3(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raouf AH, Tsai HH, Parker N, Hoffman J, Walker RJ, Rhodes JM. Sulphation of colonic and rectal mucin in inflammatory bowel disease: reduced sulphation of rectal mucus in ulcerative colitis. Clinical science (London, England: 1979). 1992;83(5):623–6. [DOI] [PubMed] [Google Scholar]
- 53. Corfield AP, Myerscough N, Bradfield N, Corfield Cdo A, Gough M, Clamp JR, et al. Colonic mucins in ulcerative colitis: evidence for loss of sulfation. Glycoconjugate journal. 1996;13(5):809–22. [DOI] [PubMed] [Google Scholar]
- 54. Tsai HH, Dwarakanath AD, Hart CA, Milton JD, Rhodes JM. Increased faecal mucin sulphatase activity in ulcerative colitis: a potential target for treatment. Gut. 1995;36(4):570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Corfield AP, Wagner SA, Clamp JR, Kriaris MS, Hoskins LC. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infection and immunity. 1992;60(10):3971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carrington SD, Irwin JA, Liu L, Rudd PM, Matthews E, Corfield AP. Analysing mucin degradation. Methods in molecular biology (Clifton, NJ). 2012;842:191–215. [DOI] [PubMed] [Google Scholar]
- 57. Klein A, Roussel P. O-acetylation of sialic acids. Biochimie. 1998;80(1):49–57. [DOI] [PubMed] [Google Scholar]
- 58. Corfield AP, Myerscough N, Warren BF, Durdey P, Paraskeva C, Schauer R. Reduction of sialic acid O-acetylation in human colonic mucins in the adenoma-carcinoma sequence. Glycoconjugate journal. 1999;16(6):307–17. [DOI] [PubMed] [Google Scholar]
- 59. Shen Y, Tiralongo J, Kohla G, Schauer R. Regulation of sialic acid O-acetylation in human colon mucosa. Biological chemistry. 2004;385(2):145–52. [DOI] [PubMed] [Google Scholar]
- 60. Skoog EC, Sjoling A, Navabi N, Holgersson J, Lundin SB, Linden SK. Human gastric mucins differently regulate Helicobacter pylori proliferation, gene expression and interactions with host cells. PloS one. 2012;7(5):e36378 10.1371/journal.pone.0036378 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.