Abstract
The objective of this study was to determine if thermally cooled perches improve hen immunity during hot summer. White Leghorn pullets at 16 week of age were randomly assigned to 18 cages of 3 banks at 9 hens per cage. Each bank was assigned to 1 of the 3 treatments up to 32 week of age: 1) thermally cooled perches, 2) perches with ambient air, and 3) cages without perches. Hens were exposed to natural ambient temperatures from June through September 2013 in Indiana with a 4 h acute heat episode at 27.6 week of age. The packed cell volume, heterophil to lymphocyte (H/L) ratio, plasma concentrations of total IgG, and cytokines of interleukin-1β and interleukin-6, plus lipopolysaccharide-induced tumor necrosis factor-α factor were measured at both 27.6 and 32 week of age. The mRNA expressions of these cytokines, toll-like receptor-4, and inducible nitric oxide synthase were also examined in the spleen of 32 week-old hens. Except for H/L ratio, thermally cooled perches did not significantly improve currently measured immunological indicators. These results indicated that the ambient temperature of 2013 summer in Indiana (24°C, 17.1 to 33.1°C) was not high enough and the 4 h heat episode at 33.3°C (32 to 34.6°C) was insufficient in length to evoke severe heat stress in hens. However, cooled perch hens had a lower H/L ratio than both air perch hens and control hens at 27.6 week of age and it was still lower compared to control hens (P < 0.05, respectively) at 32 week of age. The lowered H/L ratio of cooled perch hens may suggest that they were able to cope with acute heat stress more effectively than control hens. Further studies are needed to evaluate the effectiveness of thermally cooled perches on hen health under higher ambient temperatures.
Introduction
During summer, high ambient temperature is one of the most important environmental stressors adversely affecting poultry health [1–2]. Hens to loss heat are limited due to feathering and the absence of sweat glands [1]. A hen can tolerate and adapt to ambient temperatures up to 25°C (77°F); temperatures above this level can lead to heat stress (HS) as a combination of hen’s physical heat production plus the environmental heat load is greater than its ability to lose heat, leading to an increase in body core temperature, and possibly death [3–4]. Especially during heat waves without acclimation [5–6], a chicken can die suddenly of heat stroke. In the Midwest, for example, the exceptionally hot summers of 1995, 2011, and 2012 were devastating to the egg industry as hen mortalities climbed to 10% due to heat [4]. For those hens which survive high temperatures, HS reduces their body weight gain [7–8], feed intake [9–10], egg production [11–12], and egg quality [13–15]. Currently, with climate change and global warming, chicken growers are struggling to fight summer heat.
It is well established that high environmental temperatures also affect immune function in hens, causing immunosuppression [16–19]. The degree of heat-induced immunosuppression depends on multiple factors including the length and intensity of the heat exposure [10, 20–21]. In hens, HS limits immunocompetence by suppressing antibody production [8] and alters the populations of immune cells, leading to an increase in the heterophil to lymphocyte (H/L) ratio which has been used as an indicator of stress [22–25]. With hens having only rudimentary lymph nodes [26–27], they rely on the spleen as a major immune organ as it is an important site for immune responses to antigens [28–29]. Hens with greater spleen weights have higher immunocompetence [30]. In addition, spleen weight decreased in laying hens [10, 31] and broilers [19, 32] after exposure to high temperatures. An additional indicator of stress besides lymphoid organ regression is the production of cytokines [33]. As molecular messengers, cytokines are involved in cell signaling and are synthesized by a variety of cells including monocytes, and B- and T-lymphocytes [34–37]. Cytokines play an important role in immunity by coordinating the humoral (B-cell) and cell mediated immune (T-cell) responses [38–42]. Examples of cytokines include the interleukin (IL) family and lipopolysaccharide-induced tumor necrosis factor-α factor (LITAF, similar to TNF-α in mammals) [43] produced by several immune cells including the CD4 T-helper cells [44]. The IL-1 has a key role in the inflammatory response and stimulates B- and T-cell development and differentiation [45–49]. The TNF-α is synthesized by monocytes and acts as a cytotoxin promoting apoptosis leading to tumor regression [50]. In addition to cytokines, toll-like receptor (TLR)-4 is a protein receptor found on the membranes of macrophages and dendritic cells [51]. These receptors recognize conserved sections of invading antigens to trigger the activation of immune cells [52]. In addition, a non-specific immune defense mechanism that hens may use during stressful conditions is nitric oxide. Nitric oxide is generated by phagocytes as part of the immune response [53–54]. Because of an unpaired electron, the nitric oxide produced by the inducible isoform of nitric oxide synthase (iNOS) acts as a free radical, attacking and destroying antigens such as viruses, bacteria, tumors, and parasites [55]. Nitric oxide also influences inflammatory responses [56]. There are many cell types that synthesize iNOS in response to cytokines [57–58]. Packed cell volume (PCV), the volume percentage of blood cells in blood, is another health indicator of humans and animals [59–61] including chickens exposed to HS [24, 62]. Attia et al. [62] reported that PCV was reduced in broiler chickens followed chronic HS, which was correlated with increased rectal temperature and respiration rate.
The majority of hens are currently housed in conventional cages in the United States; however, egg producers are updating their facilities with cages that can eventually be enriched with a nest, perches, scratch pad, and nail trim area [63–65]. Introducing perches stimulates a variety of motor patterns [66] with perching causing an increase in bone strength in laying hens [67–69]. Allowing access to cooled perches during the summer hot months may help alleviate HS for laying hens, because hens have a natural tendency to perch for resting and protection [70] and more than 25% of the heat produced by hens can be lost through their feet by modulating blood flow in hot environments [71]. Increased conductive heat transfer from the feet to a thermally controlled perch helps chickens to relieve HS as that broiler breeder hens [72] and broiler chickens [73] improved growth performance with access to cooled perches during high temperatures [73–74]. Broiler chickens subjected to HS with access to cooled perches exhibited less panting and had reduced core body temperatures compared to controls [75]. Cooled perch availability increased body weight gain and feed efficiency of broiler chickens in high ambient temperatures regardless of stocking density [76].
Currently, there are no studies on cooled perches that evaluate the immune responses of laying hens when exposed to high ambient temperatures. Therefore, the aim of the present study was to determine if cooled perches inhibit heat stress-induced immunological suppression in caged laying hens. Our hypothesis was that hens with cooled perches during hot weather will exhibit improved well-being as decreased H/L ratios, adrenal weights, and cytokines; down-regulation of splenic cytokines, TLR-4, and iNOS; and greater body weight, spleen weights, and plasma IgG concentrations as compared to hens without access to cooled perches.
Materials and Methods
Birds and Management
One-hundred and sixty-two Hy-Line W36 White Leghorn pullets, 16 week of age, were transported to the Layer Research Unit at Purdue University’s Poultry Research Farm located in West Lafayette of Indiana. The pullets were individually weighed and 162 chickens with similar body weight were leg tagged and randomly assigned to 3 banks with 18 laying cages at 9 hens per cage for 16 weeks. Within a bank, there were 3 tiers with 2 cages per tier. Feeder and floor space were 8.4 cm and 439 cm2 per hen, respectively. Two nipple drinkers were assigned to each laying cage. Dropping boards were located between tiers of cages.
A pre-lay diet with calculated values of 3,009 kcal ME/ kg, 20.0% CP, 1.0% Ca, and 0.45% non-phytate P was fed from 16 to 17 week of age. At 17 week of age, chickens consumed a layer diet with 2,890 kcal ME/kg, 18.3% CP, 4.2% Ca, and 0.30% non-phytate P until the end of the study at 32 week of age. Throughout the study, hens had free access to feed and water.
At 16 week of age, light hours were gradually stepped up from 12 h achieving a photoperiod of 16L:8D by 30 week of age, where it remained until termination of the study. Light intensity was set at 10.7 lux.
The protocol was approved by the Purdue University Animal Care and Use Committee.
Treatments
Each bank was assigned randomly to 1 of the following treatments: (1) thermally cooled perches, (2) perches with ambient air, and (3) conventional cages without perches (control). For the bank of cages assigned the cooled perch treatment, each tier had its own pump to distribute chilled deionized water (10°C) through its perch loop, round galvanized steel pipes (3.38 cm outside diameter), that ran parallel to the feeder (Fig 1). This looped arrangement provided 2 perches per cage giving each hen 16.9 cm of perch space. The cage dimensions and perch placement within the laying cages were reported previously [77]. The front perch closest to the feeder received chilled water pumped directly from a common vertical manifold. The back perch was the return loop that sent the water back to the common manifold to be re-chilled. A chiller was used to cool the water in the manifold; it had its own independent thermostat which kept the water at 10°C. A separate 4th pump continuously circulated the deionized water between the water chiller and the manifold. A sensor for monitoring air temperature was installed to the controller of each tier to activate or stop the circulation of chilled water through the perch loop when ambient temperature reached or fell below 25°C, respectively. The air perches were also arranged in a loop system as described for the cooled perches, but no water was pumped through these perches. Air temperature and relative humidity within the room, the cage, the water temperature of supply and return lines of the cooled perches, and the air temperature of the air perches were recorded using a wireless monitoring system [78].
Fig 1. The bank of cages assignment for the thermally cooled perch treatment.
Each tier had its own pump to distribute chilled deionized water (10°C) through its perch loop that ran parallel to the feeder. The front perch closest to the feeder received chilled water pumped directly from a common vertical manifold. The back perch was the return loop that sent the water back to the common manifold to be re-chilled. A chiller was used to cool the water in the manifold; it had its own independent thermostat which kept the water at 10°C. A separate 4th pump continuously circulated the deionized water between the water chiller and the manifold. A sensor for monitoring air temperature was installed to the controller of each tier to activate or stop the circulation of chilled water through the perch loop when ambient temperature reached or fell below 25°C, respectively [75].
To imitate poultry production condition and to identify the effects of thermally cooled perch system in prevent HS, evaporative cooling pads were not used at any time during the study to allow exposure of hens to naturally hot summer days. The study was conducted from June through September 2013 in Indiana of the United States. Environmental condition inside the house were not controlled throughout the study with the exception of an acute heating episode where the ambient temperature was elevated to a mean of 33.3°C for 4 h at 27.6 week of age by providing auxiliary heat. The heating episode was initiated 9 h following initiation of photo-stimulation after most hens had laid their eggs for that day.
Sampling
Two hens without an egg in their uterus as determined through palpation were randomly taken from each cage for sampling at 27.6 week of age (n = 12 per treatment). To minimize effects of circadian variation on the measured parameters, blood samples were collected from a hen per cage of each treatment by repeating the cycle of cooled perch, air perch and no perch until the end. The sample collection began at 2 h post initiation of the heating episode to ensure all samples were collected within the 4 h acute heat stress period. A 4-mL blood sample was collected from each bird via the brachial vein within 2 min of being handled. The blood was collected into EDTA-coated test tubes. A leg ring was placed on the right shank before returning the hen to its cage.
At 32 week, 2 hens per cage (n = 12 per treatment) without a leg ring were randomly removed from their cage and sampled using the order of treatments as described for the collection of blood at 27.6 week of age. Hens were sedated by injecting 30 mg of sodium pentobarbital/kg of body weight into the brachial vein. A 5-mL blood sample was collected from each hen by cardiac puncture, and the blood placed into EDTA-coated test tubes. The hen was killed by cervical dislocation immediately followed blood sampling; and its body weight was recorded. The spleen, liver, and the right adrenal gland of each sampled hen were collected and weighed. The spleen was stored at -80°C until further analysis.
All blood samples were stored on ice and transported to the laboratory to be centrifuged at 700 x g for 20 min at 4°C. The supernatant plasma was collected and stored at -80°C until analysis.
Quantitative Analysis of Blood Parameters
PCV
Heparinized glass capillary tubes were used to collect blood from a venipuncture of the brachial vein of the 2 hens that were used in blood collection at 27.6 and 32 week age. The duplicate hematocrits were spun for 15 min and the proportion of the total volume making up the cells was determined.
H/L Ratio
Immediately following the collection of hematocrits, duplicate blood smears per hen were made by generating a thin layer of cells along the slide at 27.6 and 32 week of age, respectively. After drying, the slides were stained with Wright’s staining. Through light microscopy, heterophils and lymphocytes were differentiated based on a previous counting method on the single layer cell area within the meddle 3/5 of each slide [79]. A total of 100 heterophil and lymphocyte per slide or a total 200 cells per hen per time point were counted, and then the H/L ratio was calculated [80].
ELISA
Commercially available chicken specific ELISA kits were used to measure chicken plasma IgG (Catalog No. E33-104; Bethyl Laboratories, Inc., Montgomery, TX) and cytokines of IL-1β (product code: CSB-E11230Ch. American Research Products, Inc., Waltham, MA), IL-6 (product code: CSB-E08549Ch), and LITAF (product code: CSB-E11231Ch) using the instructions from the respective companies. Briefly, plasma samples were diluted to 1:1000 with supplied sample buffer for IgG (1X Dilution Buffer B), the rest of the parameters were tested in original concentration, all samples were analyzed in duplicate with an absorbance reading of 450 nm by following the manufacture’s protocols and methods reported previously [81].
Gene Expression
Spleen mRNA expression of IL-1β, IL-6, LITAF, iNOS, and TLR-4 (Applied Biosystems, Life Technologies, Grand Island, NY) were detected by real-time PCR using the primers and probes (Table 1) developed elsewhere [10]. Results were quantitated by standard curve method, and data were log transferred and expressed as relative abundance of the interested genes to the reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) following the method described previously [10]. Briefly, after RNA extraction, reverse transcription was conducted using 61.5 μL of master mix, made of 2.5μL of Multi-Scribe reverse transcriptase, 22 μL of 25 mM MgCl, 5 μl random hexamers, 2μL RNase inhibitor, 20 μl dNTPs, and 10 μL of TaqMan reverse transcription buffer provided in the TaqMan Reverse Transcription Reagent Pack (Applied Biosystems, Foster City, CA). The 61.5 μL of master mix was then added to the quantified RNA sample and RNase-free water (Ambion Inc.) for a total of 100 μL. Reverse transcription and amplification was done using the Hybaid PCR Express thermo cycler (Midwest Scientific, St. Louis, MO). Stock primers and probes were diluted to 10 μM solutions. The conditions for PCR were a ratio of 1.625 μL of TaqMan probe, 2.25 μL of gene- specific TaqMan forward and reverse primers each, 12.5 μL of PCR Mastermix (Applied Biosystems), 3.875 μL RNase-free water (Ambion Inc.), and 2.5 μL of sample cDNA. Standards were measured in triplicates and samples in duplicates with a standard deviation of less than 2.0 and a coefficient of variation less than 2.0% for all spleen mRNA expression.
Table 1. Taqman primers and probes used.
| Gene | Primers and Probe (5'-3') | Application Efficiencies (%) | Product Length (bp) | Reference/ Accession no. |
|---|---|---|---|---|
| IL-1β | (f) TGCTGGTTTCCATCTCGTATGTAC
(r) CCCAGAGCGGCTATTCCA (p) AGTACAACCCCTGCTGCCCCGC (VIC/MGB) |
95 | 80 | NC_006096.3 |
| IL-6 | (f) CCCGCTTCTGACTGTGTTT
(r) GCCGGTTTTGAAGTTAATCTTTT (p) TGTGTTTCGGAGTGCTTT (VIC/MGB) |
86 | 139 | NC_006089.3 |
| TNF-α | (f) CCCCTACCCTGTCCCACAA
(r) ACTGCGGAGGGTTCATTCC (p) CTGGCCTCAGACCAG (VIC/MGB) |
75 | 62 | NC_006101.3 |
| iNOS | (f) GAGTGGTTTAAGGAGTTGGATCTGA
(r) TCCAGACCTCCCACCTCAAG (p) CTCTGCCTGCTGTTGCCAACATGCT (VIC/MGB) |
103 | 80 | NC_006106.3 |
| LITAF | (f) TCTGAGACCCCCAAGTCCAA
(r) CCTTAAGTTTTGCCAGAGGAGGTT (p) CCCACCACACCCACT (VIC/MGB) |
98 | 197 | NC_006104.3 |
IL-1β = interleukin 1 beta; IL-6 = interleukin 6; iNOS = inducible nitric oxide synthase; LITAF = lipopolysaccharide-induced TNF- α factor [43]; TLR-4 = toll-like receptor 4. f = forward primer; r = reverse primer; p = probe
Statistical Analysis
For each age of blood sampling, data from the randomized design were subjected to an ANOVA [82] using the MIXED model procedure of the SAS Institute [83]. The main effect of treatment was fixed. The tier (deck) of cages was the experimental unit. Subsampling error terms included cages within tiers (2 cages per tier per treatment) and hens within cages within tiers (2 hens per cage per tier per treatment). Pooling of error terms occurred when P > 0.25. The data were normally distributed and reported as least square means ± SEM. Significant treatment effects were subjected to the SLICE option [84]. Significance was set at P < 0.05 for all statistical analysis.
Results
Access of thermally cooled perches or ambient air perches did not affect body weight and weights of organs (the liver, spleen and right adrenal gland) of hens at 32 week of age followed a 16-week period of time during 2013 summer including a 4 h acute heat episode at 27.6 week of age (Table 2).
Table 2. The effect of thermally cooled perches on body and organ weights of 32-week-old White Leghorn hens 1 in 2013 summer hot months including 31 days after an acute heating episode.
| Organ weights 2 | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | BW (g) | Spleen (mg) | Relative spleen (mg/100g of BW) | Liver (g) | Relative liver (g/100g of BW) | Right adrenal (mg) | Relative right adrenal (mg/100g of BW) |
| Cooled perch | 1440 ± 31 | 1237 ± 78 | 86 ± 8 | 36 ± 2 | 2.5 ± 0.1 | 73 ± 5 | 5.0 ± 0.3 |
| Non-cooled perch | 1468 ± 31 | 1277 ± 78 | 88 ± 8 | 40 ± 2 | 2.6 ± 0.1 | 76 ± 5 | 5.1 ± 0.3 |
| No perch | 1456 ± 31 | 1178 ± 78 | 81 ± 8 | 35 ± 2 | 2.5 ± 0.1 | 73 ± 5 | 5.1 ± 0.3 |
| n 3 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| P-value | 0.81 | 0.67 | 0.86 | 0.30 | 0.99 | 0.89 | 0.96 |
1 The data were collected during 2013 summer hot months including 31 days after an acute heating episode.
2Values represent least square Mean±SEM, n = 12.
3Number of observations per least square mean.
BW = body weight
There were no thermally cooled perch effects on the levels of PCV, circulating cytokines (IL-1β, IL-6 and LITAF) immediately after a 4 h acute heat stress (at 27.6 week of age) and followed a 16-week period of the summer season (at 32 week of age) (P > 0.05, respectively; Table 3). However, plasma concentrations of IgG were reduced in hens with accessed to cooled perches or air perches compared to control hens without accessed perches at 32 week of age (P < 0.005, Table 3) but not at 27.6 week of age (P > 0.05).
Table 3. The effect of thermally cool perch availability on PCV, plasma IgG and cytokine concentrations of White Leghorn hens at 27.6 and 32 week of age 1 .
| Treatment | PCV (%) | IgG (mg/mL) | IL-1β (pg/mL) | IL-6 (mg/mL) | LITAF (pg/mL) |
|---|---|---|---|---|---|
| 27.6 week of age, during the 4 h heating episode | |||||
| Cool Perch | 28.8 | 6.92 | 1.19 | 0.93 | 144.48 |
| Air Perch | 28.2 | 6.94 | 0.92 | 1.05 | 143.40 |
| No Perch | 28.7 | 7.25 | 1.37 | 1.03 | 140.60 |
| SEM | 0.6 | 0.86 | 0.33 | 0.15 | 14.59 |
| n 2 | 12 | 12 | 12 | 12 | 12 |
| P-value | 0.76 | 0.84 | 0.63 | 0.83 | 0.50 |
| 32 week of age, after 16 weeks of summer hot months with a 4 h heating episode | |||||
| Cool Perch | 28.3 | 15.79 | 3.64 | 1.31 | 190.67 |
| Air Perch | 27.4 | 14.67 | 3.70 | 1.02 | 194.20 |
| No Perch | 28.0 | 19.35* | 3.85 | 0.89 | 188.75 |
| SEM | 0.5 | 1.57 | 0.73 | 0.25 | 4.53 |
| n 2 | 12 | 12 | 12 | 12 | 12 |
| P-value | 0.40 | 0.0053 | 0.98 | 0.50 | 0.70 |
1Data were measured using ELISA and presented as Mean±SEM.
*P < 0.05 compared with other treatments at 32 week of age.
2Average number of observations per least square mean at 27.6 and 32 week of age.
IL-1β = interleukin-1 beta; IL-6 = interleukin 6; LITAF = lipopolysaccharide-induced TNF- α factor [43]; PCV = packed cell volume.
There were no treatment effects on mRNA expression of splenic cytokines (IL-1β, IL-6 and LITAF), TLR-4, and iNOS at 32 week of age followed a 16-week period during the summer months including a 4 h acute heat episode at 27.6 week of age (P > 0.05, respectively; Table 4).
Table 4. The effect of thermally cool perch on mRNA expression of cytokines, toll-like receptors, and inducible nitric oxide synthase in the spleen of 32 week-old laying hens 1 .
| Treatment | IL-1β | IL-6 | LITAF | TLR-4 | iNOS |
|---|---|---|---|---|---|
| Cool Perch | 0.75 | 0.79 | 1.65 | 2.35 | 1.33 |
| Air Perch | 0.69 | 0.60 | 1.87 | 2.40 | 1.30 |
| No Perch | 0.68 | 0.59 | 2.10 | 2.04 | 1.04 |
| SEM | 0.11 | 0.15 | 0.57 | 0.56 | 0.27 |
| n 2 | 12 | 12 | 12 | 12 | 12 |
| P-value | 0.89 | 0.59 | 0.46 | 0.89 | 0.71 |
1Data were presented as Mean±SEM. The data were calculated by the equation: tested mRNA quantity value (mean of replicated target mRNA value/GAPDH quantity mean of replicate endogenous control value).
2Average number of observations per least square mean.
GAPDH = glyceraldehyde 3-phosphate dehydrogenase; iNOS = inducible nitric oxide synthase; IL-1β = interleukin 1 beta; IL-6 = interleukin 6; TLR-4 = toll-like receptor 4; LITAF = lipopolysaccharide-induced TNF-α factor [43].
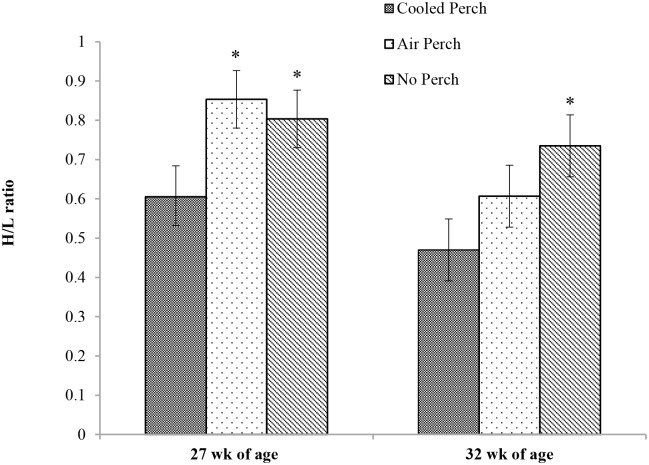
Hens with access to cooled perches had a lower H/L ratio at 27.6 week of age, followed a 4 h acute heat episode (P < 0.01) compared to hens with access to air perches or control hens without perches. At 32 week of age, after a 16-week period of the summer season, the H/L ratios were still lower in hens with access to thermally cooled perches compared to control hens without perches (P < 0.05. Fig 2), but not to hens provided with air perches (P > 0.05).
Fig 2. The effect of thermally cooled perches on heterophil/lymphocyte ratios.
The heterophil to lymphocyte (H/L) ratios of hens subjected to a heating episode at 27.6 week of age and at the end of the study at 32 week of age. *Least square means were significant differences (P < 0.05) within the same age group.
Discussion
High ambient temperature during summer months is one of the most important environmental stressors in the poultry industry. A chicken can die due to heat stress, especially hot waves, as Hegeman [6] reported that tens of thousands of turkeys and chickens in Kansas and North Carolina were killed in 2011 summer during a heat wave (over 100°F). For those hens that survive high temperatures, health profiles including immunity are detrimentally affected [1, 8, 85]. However, in the current study, compared to air perch and no perch hens, with the exception of the H/L response, thermally cooled perches during the summer months (June through September 2013) had little effect on hens’ (cooled perch hens) immune and physiological parameters as indicated by body weight; relative organ weights (the spleen, liver, and right adrenal gland); PCV; plasma levels of cytokines (IL-1ß, IL-6, and LITAF), and the mRNA expression of cytokines, TLR-4, and iNOS in the spleen. The mild summer of 2013 (a mean of 24°C ranged from 17.1 to 33.1°C) in West Lafayette, IN and the brevity of the acute heating episode (2–4 h at a mean of 33.3°C arranged from 32 to 34.6°C) were most likely the reasons for little change in these measured parameters of hens at both 27.6 and 32 week of age.
With regard to immune function, circulating catecholamines and corticosterone released by the adrenal glands that have undergone hypertrophy during stress bind to their receptors of immune cells [86] causing profound immunosuppression and lymphoid organ regression [87–89]. Heat stress reduces antibody production to specific antigens in chickens [8]. In the current study, however, cooled perch hens or air perch hens had lower natural IgG concentrations at 32 week of age but not at 27.6 week of age compared to that of control hens. Similar to the current results, Regnier et al. [18] reported that control hens had lower antibody titers than heat-stressed New Hampshire hens (36°C for 5 days). Although the cellular mechanisms of the different regulation of the hens’ IgG concentrations in the present study is unclear, it could mostly associate with perch usage as perch increasing skeletal health [77, 90] and decreasing social stress and its associated damage [91–92]. In supporting the hypothesis, Sun et al. [92] reported that the isotype titers of natural antibodies, antibodies without any previous antigen exposure, in laying hens were affected by environmental factors including row and level of the cages. Similar to the current results, a negative correlation between total IgG concentrations and productivity and survivability has been found in several previous studies, including body weight, feeding efficiency, and egg production [93–96]. In addition, Wondmeneh et al. [97] reported that chickens’ natural antibody titers were related to increased hazard in their housing environments.
The numbers of B- and T-lymphocytes, involved in antibody- and cell-mediated immunity, respectively, are reduced and their functions impaired under conditions of chronic stress [98]. Stressed-associated overexpressed glucocorticoid, as one of the reasons, induced lymphopenia, causing an increase in the H/L ratio which has been used as an indicator of stress [23, 99]. The H/L ratio was lower in cooled perch hens than both air perch and no perch hens at 27.6 week of age followed an acute heat episode; and the difference was extended to 32 week of age between cooled perch and no perch hens but not air perch hens. These results, cooled perch vs. air perch, indicate that acute heat episode (2–4 h at 33.3°C) caused a mild stress reaction but no stress response at 24°C (17.1 to 33.1°C) during the 16 wk period of 2013 summer season. The H/L ratio of hens at 32 week of age, cooled perch ≤ air perch < no perch, suggest that perches per se may have alleviated long-term stress, especially for cooled perch hens. Hens housed on a slat and littered floor with access to perches had lower H/L ratios than hens without perches suggesting that perches helped reduce stress [100], but a similar effect was not reported for caged hens with and without perches [101].
Although the results of the current study, H/L ratio, and a companion study using the same chickens, rectal temperature [102] and heat stress behaviors [103], provided some evidence that thermally cooled perches helped hens cope with acute heat stress, currently measured cytokines, IL-1β, IL-6, and LITAF, remained unaffected. Laying hens subjected to 12 d of HS (34°C) experienced an increase in serum levels of IL-1 and TNF-α [104], but the cooled perches of the current study were ineffective regulating the levels of these cytokines during a heating episode (33.3°C for 2–4 h period) even though rectal temperature was lower in the hens with perches as compared to hens without perches [102]. Similar to plasma cytokines, the splenic expression of cytokines (IL-1, IL-6, and LITAF), TLR-4, and iNOS at 32 wk of age was unaffected in hens with access to cooled perches. Other studies with chickens have reported an up-regulation of some of these immune parameters perhaps because the stressor was more severe [33]. The lack of a physiological response due to the presence of cooled perches as result of too mild of a stressful event including the acute heating episode, is further corroborated by the fact that egg production, shell quality, feed efficiency, foot health, and feather score [105], bone mineralization and muscle deposition [106] were unaffected by the cooled perch treatment.
However, a parallel behavioral observation showed that the hens exhibited heat-associated behaviors differently during the acute heating episode [103]. Specifically, perch use was significantly higher at all-time points in cooled versus air perch cages (F1,10 = 41.32, P < 0.0001) on the day of the acute heat stress event. The onset of panting and wing spreading was delayed in cooled perch cages as compared to air perch and no perch cages, and incidences of these behaviors remained lower within cooled perch cages as compared to air perch or no perch during and after the acute heating episode (P < 0.05, respectively). Furthermore, once panting and wing spreading was initiated in the cooled perch hens, the proportion of hens panting was always lower during and immediately following the 4-h heating episode compared to the air perch hens as well as the no perch hens. Interestingly, use of perches 3 d prior to the heating episode did not differ between hens provided thermally cooled as compared to air perches most likely because of mild summer weather [103]. These behavioral changes during the heating episode where hens sought out the cooled perches causing less panting and wing spreading helped them cope with the stressful heating event as exemplified by reduced rectal temperature as compared to hens with no perch but not the air perch [102]. Under HS, the method of birds to loss heat is shifted from sensible heat loss (radiation, conduction and convection) from surfaces (crown, wattles, shanks, and unfeathered areas under wings) to evaporative heat loss (panting). These results indicates that thermally cooled perch system used in this study effectively prevents acute heat episode induced stress reactions, however, 4 h heat wave at 33.3°C and natural ambient temperature of 2013 summer in Indiana were not severe enough to evoke physical and physiological changes. As suggested by Spencer [107], behavioral changes, such as panting and sweating, will occur long before physiological changes at less cost to the animal [108].
Conclusions
In summary, the most significant change of immunological biomarker that responded to the thermally cooled perches after exposure to an induced heating episode of 4 h and 16 weeks of summer hot months was the reduced H/L ratio suggesting that these hens may be able to cope with acute heat stress more effectively than hens with air perches or without perches. Further studies are needed to evaluate the effectiveness of thermally cooled perches on hen health under higher ambient temperatures.
Supporting Information
(XLSX)
(XLSX)
Acknowledgments
Funding was provided by the National Institute of Food and Agriculture, U.S. Department of Agriculture under Award No. 2013-67021-21094. The 16-wk old pullets were donated by Creighton Brothers, Atwood, IN. Perches were donated by T. L. Pollard of Big Dutchman, Holland, MI. Appreciation is extended to S. A. Enneking for her technical assistance and to F. A. Haan and B. D. Little for their contributions towards animal care and management (Purdue University, West Lafayette, IN). Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement of the USDA. The USDA is an equal opportunity provider and employer.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Institute of Food and Agriculture, United States Department of Agriculture Award No. 2013-67021-21094. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ademola SG, Shittu MD, Ayansola MO, Lawal TE, Tona GO. Effect of maxigrain supplement on growth performance, economic indices and haematological parameters of heat-stressed broilers fed three fibre sources. Online J Anim Feed Res. 2013; 3: 159–164. [Google Scholar]
- 2. Lara LJ, Rostagno MH. Impact of heat stress on poultry production. Animal. 2013; 3: 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sahin K, Sahin N, Kucuk O, Hayirli A, Prasad AS. Role of dietary zinc in heat-stressed poultry: A review. Poult Sci. 2009; 88: 2176–2183. 10.3382/ps.2008-00560 [DOI] [PubMed] [Google Scholar]
- 4.Perle, D. PETA Calls for Cruelty Charges Over Egg Farm's Negligence in Death of 300,000 Hens. 2012. Avaiable: http://www.peta.org/mediacenter/news-releases/PETA-Calls-for-Cruelty-Charges-Over-Egg-Farm-s-Negligence-in-Death-of-300-000-Hens.aspx. Accessed 27 April 2015.
- 5. Arad Z, Marder J. Comparison of the productive performances of the Sinai Bedouin fowl, the White Leghorn and their crossbreds: Study under natural desert conditions. Br Poult Sci. 1982; 23: 333–338. [DOI] [PubMed] [Google Scholar]
- 6.Hegeman R. Heat wave kills thousands of poultry. The Huffington Post. 12 September 2011. Avaiable: http://www.huffingtonpost.com/2011/07/13/heat-wave-poultry_n_896812.html. Access 27 April 2015.
- 7. Scott TA, Balnave D. Comparison between concentrated complete diets and self-selection for feeding sexually-maturing pullets at hot and cold temperatures. Br Poult Sci. 1988; 29: 613–626. [Google Scholar]
- 8. Mashaly MM, Hendricks GL 3rd, Kalama MA, Gehad AE, Abbas AO, Patterson PH. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci. 2004; 83: 889–894. [DOI] [PubMed] [Google Scholar]
- 9. Franco-Jimenez DJ, Scheideler SE, Kittok RJ, Brown-Brandl TM, Robeson LR, Taira H, Beck MM. Differential effects of heat stress in three strains of laying hens. J Appl Poult Res. 2007; 16: 628–634. [Google Scholar]
- 10. Felver-Gant JN, Mack LA, Dennis RL, Eicher SD, Cheng HW. Genetic variations alter physiological responses following heat stress in 2 strains of laying hens. Poult Sci. 2012; 91: 1542–1551. 10.3382/ps.2011-01988 [DOI] [PubMed] [Google Scholar]
- 11. Marsden A, Morris TR. Quantitative review of the effects of environmental temperature on food intake, egg output and energy balance in laying pullets. Br Poult Sci. 1987; 28: 693–704. [DOI] [PubMed] [Google Scholar]
- 12. Whitehead CC, Bollengier-Lee S, Mitchell MA, Williams PEV. Alleviation of depression in egg production in heat stressed laying hens by vitamin E. Br Poult Sci. 1998; 39: 44–46. [DOI] [PubMed] [Google Scholar]
- 13. Mahmoud KZ, Beck MM, Scheideler SE, Forman MF, Anderson KP, Kachman SD. Acute high environmental temperature and calcium-estrogen relationship in the hen. Poult. Sci. 1996; 75: 1555–1562. [DOI] [PubMed] [Google Scholar]
- 14. Yahav S. Alleviating heat stress in domestic fowl: different strategies. World’s Poult Sci J. 2009; 65: 719–732. [Google Scholar]
- 15. Ebeid TA, Suzuki T, Sugiyama T. High ambient temperature influences eggshell quality and calbindin-D28k localization of eggshell gland and all intestinal segments of laying hens. Poult Sci. 2012; 91: 2282–2287. 10.3382/ps.2011-01898 [DOI] [PubMed] [Google Scholar]
- 16. Rao DSVS, Glick B. Immunosuppressive action of heat in chickens. Proc Soc Exp Biol Med. 1970; 133: 445–448. [DOI] [PubMed] [Google Scholar]
- 17. Thaxton P, Siegel HS. Depression of secondary immunity by high environmental temperature. Poult. Sci. 1982; 51: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 18. Regnier JA, Kelley KW, Gaskins CT. Acute thermal stressors and synthesis of antibodies in chickens. Poult Sci. 1980; 59: 985–990. [DOI] [PubMed] [Google Scholar]
- 19. Niu ZY, Liu FZ, Yan QL, Li WC. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult Sci. 2009; 88: 2101–2107. 10.3382/ps.2009-00220 [DOI] [PubMed] [Google Scholar]
- 20. Henken AM, Groote Schaarsberg AMJ, Nieuwland MGB. The effect of environmental temperature on immune response and metabolism of the young chicken. 4. Effect of environmental temperature on some aspects of energy and protein metabolism. Poult Sci.1983; 62: 51–58. [DOI] [PubMed] [Google Scholar]
- 21. Kelley KW. Immunobiology of domestic animal as affected by hot and cold weather. Trans Am Soc Agric Eng. 1983; 26: 834–840. [Google Scholar]
- 22. Thaxton P, Sadler CR, Glick B. Immune response of chickens following heat exposure or injections with ACTH. Poult Sci. 1968; 47: 264–266. [DOI] [PubMed] [Google Scholar]
- 23. Gross WB, Siegel HS. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 1983; 27: 972–978. [PubMed] [Google Scholar]
- 24. McFarlane JM, Curtis SE. Multiple concurrent stressors in chicks. 3. Effects on plasma corticosterone and the heterophil: lymphocyte ratio. Poult Sci. 1983; 68: 522–527. [DOI] [PubMed] [Google Scholar]
- 25. Soleimani AF, Zulkifli I, Omar AR, Raha AR. Physiological responses of 3 chicken breeds to acute heat stress. Poult. Sci. 2011; 90: 1435–1440. 10.3382/ps.2011-01381 [DOI] [PubMed] [Google Scholar]
- 26. Biggs PM. The association of lymthoid tissues and plasma cells in paraocular and paranasal organ systems in chickens. American Journal of Pathology. 1957; 53: 735–751. [PMC free article] [PubMed] [Google Scholar]
- 27. Olab I, Vervelda L. 2008. Chapter 2, Structure of the Avian Immune System In: Avian Immunology. Eds, Davison F, Kaspers B, Schat KA, Kaiser P, editors. Academic press, 2008. pp. 13–50. [Google Scholar]
- 28. Jeurissen SHM. The role of various compartments in the chicken spleen during an antigen-speific humoral response. Immunology. 1993; 80: 29–33. [PMC free article] [PubMed] [Google Scholar]
- 29. Kita K. The spleen accumulates advanced glycation end products in the chicken: tissue comparison made with rat. Poult Sci. 2014; 93: 429–33. 10.3382/ps.2013-03576 [DOI] [PubMed] [Google Scholar]
- 30. Cheng HW, Freire R, Pajor EA. Endotoxin stress responses in chickens from different genetic sickness, behavioral, and physical responses. Poult. Sci. 2004; 83: 707–715. [DOI] [PubMed] [Google Scholar]
- 31. Ghazi SH, Habibian M, Moeini MM, Abdolmohammadi AR. Effects of different levels of organic and inorganic chromium on growth performance and immunocompetence of broilers under heat stress. Biol. Trace Elem. Res. 2002; 146: 309–317. [DOI] [PubMed] [Google Scholar]
- 32. Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sakai M, Sá LRM, Ferreira AJP, Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult Sci. 2010; 89: 1905–1914. 10.3382/ps.2010-00812 [DOI] [PubMed] [Google Scholar]
- 33. Kang SY, Ko YH, Moon YS, Sohn SH, Jang IS. Effects of the combined stress induced by stocking density and feed restriction on hematological and cytokine parameters as stress indicators in laying hens. Asian-Australas J Anim Sci. 2011; 24: 414–420. [Google Scholar]
- 34. Bao Y, Cao X. The immune potential and immunopathology of cytokine-producing B cell subsets: a comprehensive review. J Autoimmun. 2014; 55: 10–23. 10.1016/j.jaut.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 35. Kared H, Camous X, Larbi A. T cells and their cytokines in persistent stimulation of the immune system. Curr Opin Immunol. 2014; 29: 79–85. 10.1016/j.coi.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 36. Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014; 26: 552–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014; 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ashman RB, Papadimitriou JM.Production and function of cytokines in natural and acquired immunity to Candida albicans infection. Microbiol Rev. 1995; 59: 646–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol. 2003; 111(2 Suppl): S460–75. [DOI] [PubMed] [Google Scholar]
- 40. Habib T, Nelson A, Kaushansky K. IL-21: a novel IL-2-family lymphokine that modulates B, T, and natural killer cell responses. J Allergy Clin Immunol. 2003; 112: 1033–1045. [DOI] [PubMed] [Google Scholar]
- 41. Esche C, Stellato C, Beck LA. Chemokines: key players in innate and adaptive immunity. J Invest Dermatol. 2005; 125: 615–628. [DOI] [PubMed] [Google Scholar]
- 42. Osborne LC, Abraham N. Regulation of memory T cells by γc cytokines. Cytokine. 2010; 50: 105–113. 10.1016/j.cyto.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 43. Sundaresan NR, Anish D, Sasrey KVH, Saxena HK, Mohan J, Saxena M. Differential expression of lipopolysaccharide-induced TNF-α factor (LITAF) in reproductive tissue during induced molting of white leghorn hens. Animal Reproductive Science. 2007; 102: 335–342. [DOI] [PubMed] [Google Scholar]
- 44. Fujio K, Okamura T, Yamamoto K. The Family of IL-10-secreting CD4+ T cells. Adv Immunol. 2010; 105: 99–130. 10.1016/S0065-2776(10)05004-2 [DOI] [PubMed] [Google Scholar]
- 45. Blazsek I, Mathé G. Interleukins and immunosuppressive factors: a regulatory system? Biomed Pharmacother. 1983; 37: 258–265. [PubMed] [Google Scholar]
- 46. Dinarello CA. Interleukin-1. Dig Dis Sci. 1988; 33(3 Suppl): 25S–35S [DOI] [PubMed] [Google Scholar]
- 47. Billips LG, Petitte D, Landreth KS. Bone marrow stromal cell regulation of B lymphopoiesis: interleukin-1 (IL-1) and IL-4 regulate stromal cell support of pre-B cell production in vitro. Blood. 1990; 75: 611–619. [PubMed] [Google Scholar]
- 48. Manetti R, Barak V, Piccinni MP, Sampognaro S, Parronchi P, Maggi E, Dinarello CA, Romagnani S. Interleukin-1 favours the in vitro development of type 2 T helper (Th2) human T-cell clones. Res Immunol. 1994; 145: 93–100. [DOI] [PubMed] [Google Scholar]
- 49. Thomas HE, Graham KL, Chee J, Thomas R, Kay TW, Krishnamurthy B. Proinflammatory cytokines contribute to development and function of regulatory T cells in type 1 diabetes. Ann N Y Acad Sci. 2013; 1283: 81–86. 10.1111/j.1749-6632.2012.06797.x [DOI] [PubMed] [Google Scholar]
- 50. Kindler V, Sappino AP. The beneficial effects of localized tumor necrosis factor production in BCG infection. Behring Inst Mitt. 1991; 88: 120–124. [PubMed] [Google Scholar]
- 51. Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell Mol Life Sci. 2008; 65: 2964–2978. 10.1007/s00018-008-8064-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paul M, Brisbina JT, Abdul-Careemb MF, Sharif A. Immunostimulatory properties of Toll-like receptor ligands in chickens. Vet Immun and Immunopath. 2013; 152: 191–199. [DOI] [PubMed] [Google Scholar]
- 53. Green SJ, Mellouk S, Hoffman SL, Meltzer MS, Nacy CA. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol Lett. 1990; 25: 15–19. [DOI] [PubMed] [Google Scholar]
- 54. Yuan C, Zhang X, He Q, Li J, Lu J, Zou X. L-arginine stimulates CAT-1-mediated arginine uptake and regulation of inducible nitric oxide synthase for the growth of chick intestinal epithelial cells. Mol Cell Biochem. 2015; 399: 229–236. 10.1007/s11010-014-2249-2 [DOI] [PubMed] [Google Scholar]
- 55. Panaro MA, Brandonisio O, Acquafredda A, Sisto M, Mitolo V. 2003. Evidences for iNOS expression and nitric oxide production in the human macrophages. Curr Drug Targets Immune Endocr Metabol Disord. 2003; 3: 210–221. [DOI] [PubMed] [Google Scholar]
- 56. Salvemini D, Kim SF, Mollace V. 2013. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications. Am J Physio Regul Integr Com Physiol. 2013; 304: R473–R378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003; 54: 469–487. [PubMed] [Google Scholar]
- 58. Li W, Yang F, Liu Y, Gong R, Liu L. Negative feedback regulation of IL-32 production by iNOS activation in response to dsRNA or influenza virus infection. Eur J Immunol. 2009; 39: 1019–1024. 10.1002/eji.200838885 [DOI] [PubMed] [Google Scholar]
- 59. Marcotty T, Simukoko H, Berkvens D, Vercruysse J, Praet N, Van den Bossche P. Evaluating the use of packed cell volumn as an indicator of trypanosomal infections in cattle in eastern Zambia. Prev Vet Med. 2008; 87: 288–300. 10.1016/j.prevetmed.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 60. Saleh MA, Mahran OM, Bassam Al-Salahy M. Circulating oxidative stress status in dromedary camels infested with sarcoptic mange. Vet Res Commun. 2011; 35: 35–45. 10.1007/s11259-010-9450-x [DOI] [PubMed] [Google Scholar]
- 61. Hammac GK, Ku PS, Galletti MF, Noh SM, Scoles GA, Palmer GH, Brayton KA. Protective immunity induced by immunization with a live, cultured Anaplasma marginale strain. Vaccine. 2013; 31: 3617–3622. 10.1016/j.vaccine.2013.04.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Attia YA, Hassan RA, Tag El-Din AE, Abou-Shehema BM. 2011. Effect of ascorbic acid or increasing metabolizable energy level with or without supplementation of some essential amino acids on productive and physiological traits of slow-growing chicks exposed to chronic heat stress. J Anim Physiol Anim Nutr (Berl). 2011; 95: 744–755. [DOI] [PubMed] [Google Scholar]
- 63. Appleby MC, Walker AW, Nicol CJ, Lindberg AC, Freire R, Hughes BO, Elson HA. Development of furnished cages for laying hens. Br Poult Sci. 2002; 43: 489–500. [DOI] [PubMed] [Google Scholar]
- 64.United Egg Producers. 2010. United Egg Producers Animal Husbandry Guidelines for U.S. Egg Laying Flocks, 2010 Edition (Alpharetta, GA: United Egg Producers). http://www.uepcertified.com/pdf/2010-uep-animal-welfare-guidelines.pdf. Accessed 10 February 2015.
- 65.Greene JL, Cowan T. Table egg production and hen welfare: The UEP-HSUS Agreement and H.R. Congressional Research Service. 2014; 1–27. http://www.fas.org/sgp/crs/misc/R42534.pdf. Accessed 27 April 2015.
- 66. Bizeray D, Estevez I, Leterrier C, Faure JM. Effects of increasing environmental complexity on the physical activity of broiler chickens. Appl Anim Behav Sci. 2002; 79: 27–41. [Google Scholar]
- 67. Appleby MC, Hughnes BO. Cages modified with perches and nests for the improvement of bird welfare. World’s Poult. Sci. J. 1990; 46: 38–40. [Google Scholar]
- 68. Duncan ET, Appleby MC, Hughes BO. Effect of perches in laying cages on welfare and production of hens. Br Poult Sci. 1992; 33: 25–35. [Google Scholar]
- 69. Hester PY. The effect of perches installed in cages on laying hens. World’s Poult. Sci. J. 2014; 70: 247–264. [Google Scholar]
- 70. Olsson IA, Keeling LJ. Night-time roosting in laying hens and the effect of thwarting access to perches. Appl Ani Beh Sci. 2001; 68: 243–256. [DOI] [PubMed] [Google Scholar]
- 71. Hillman PE, Scott NR. Energy budget of the chicken foot. J. Thermal Biol. 1989; 4: 205–217. [Google Scholar]
- 72. Muiruri HK, Harrison PC, Gouyou HW. The use of water-cooled roosts by hens for thermoregulation. Appl Anim Behav Sci. 1991; 28: 333–339. [Google Scholar]
- 73. Reilly WM, Koelkebeck KW, Harrison PC. Performance evaluation of heat-stressed commercial broilers provided water-cooled floor perches. Poult Sci. 1991; 70: 1699–1703. [DOI] [PubMed] [Google Scholar]
- 74. Muiruri HK, Harrison PC. Effect of roost temperature on performance of chickens in hot ambient environments. Poult. Sci. 1993; 70: 2253–2258. [DOI] [PubMed] [Google Scholar]
- 75. Zhao JP, Jiao HC, Jiang YB, Song ZG, Wang XJ, Lin H. Cool perch availability improves the performance and welfare status of broiler chickens in hot weather. Poult Sci. 2012; 91: 1775–1784. 10.3382/ps.2011-02058 [DOI] [PubMed] [Google Scholar]
- 76. Zhao JP, Jiao HC, Jiang YB, Song ZG, Wang XJ, Lin H. Cool perches improve the growth performance and welfare status of broiler chickens reared at different stocking densities and high temperatures. Poult Sci. 2013; 92: 1962–1971. 10.3382/ps.2012-02933 [DOI] [PubMed] [Google Scholar]
- 77. Hester PY, Enneking SA, Jefferson-Moore KY, Einstein ME, Cheng HW, Rubin DA. The effect of perches in cages during pullet rearing and egg laying on hen performance, foot health, and plumage. Poult. Sci. 2013; 92: 310–320. 10.3382/ps.2012-02744 [DOI] [PubMed] [Google Scholar]
- 78. Gates RS, Enneking SA, Xiong Y, Hester PY, Makagon MM, Cheng HW. Design and performance of cooled perches for alternative egg laying production systems. In American Society of Agricultural and Biological Engineers; 2014; pp: 14–1901235. [Google Scholar]
- 79. Walberg J. White blood cell counting techniques in birds. Sem. Avian Exotic Pet Med. 2001; 10: 72–76. [Google Scholar]
- 80. Cheng HW, Eicher SD, Chen Y, Singleton P, and Muir WM. Effect of genetic selection for group productivity and longevity on immunological and hematological parameters of chickens. Poult Sci. 2001; 80: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 81. Jiang S, Cheng HW, Hester PY, Hou JF. Development of an ELISA for detection of chicken osteocalcin and its use in evaluation of perch effects on bone remodeling in caged White Leghorns. Poult Sci. 2013; 92: 1951–1961. 10.3382/ps.2012-02909 [DOI] [PubMed] [Google Scholar]
- 82. Steel RGD, Torrie JH, Dickey DA. Principles and Procedures of Statistics: A Biometrical Approach. 3rd ed New York: McGraw Hill Book Co. New York, NY; 1997. [Google Scholar]
- 83. SAS Institute. SAS Proprietary Software. Version 9.2. SAS Inst. Inc., Cary, NC: 2008. [Google Scholar]
- 84. Winer BJ. Statistical principles in Experimental Design, 2nd ed New York: McGraw-Hill Inc; 1997. [Google Scholar]
- 85. Torki M, Mohebbifar A, Ghasemi HA, Zardast A. Response of laying hens to feeding low-protein amino acid-supplemented diets under high ambient temperature: performance, egg quality, leukocyte profile, blood lipids, and excreta pH. Int J Biometeorol. 2015; 59: 575–584. 10.1007/s00484-014-0870-0 [DOI] [PubMed] [Google Scholar]
- 86. Plaut M. Lymphocyte hormone receptors. Annu Rev Immunol. 1997; 5: 621–669. [DOI] [PubMed] [Google Scholar]
- 87. Pope CR. Pathology of lymphoid organs with emphasis on immunosuppression. Vet Immunol Immunop. 1991; 30: 31–44. [DOI] [PubMed] [Google Scholar]
- 88. Wurtman RJ. Stress and the adrenocortical control of epinephrine synthesis. Metabolism. 2002; 51: 11–14. [DOI] [PubMed] [Google Scholar]
- 89. Yan FF, Hester PY, Cheng HW. The effect of perch access during pullet rearing and egg laying on physiological measures of stress in White Leghorns at 71 weeks of age. Poult Sci. 2014; 93: 1318–1326. 10.3382/ps.2013-03572 [DOI] [PubMed] [Google Scholar]
- 90. Enneking SA, Cheng HW, Jefferson-Moore KY, Einstein ME, Rubin DA, Hester PY. 2012. Early access to perches in caged White Leghorn pullets. Poult Sci. 2012; 91: 2114–2120. 10.3382/ps.2012-02328 [DOI] [PubMed] [Google Scholar]
- 91. Yan FF, Hester PY, Enneking SA, Cheng HW. Effects of perch access and age on physiological measures of stress in caged White Leghorn pullets. Poult Sci. 2013; 92: 2853–2859. 10.3382/ps.2013-03271 [DOI] [PubMed] [Google Scholar]
- 92. Sun Y, Ellen ED, Parmentier HK, van der Poel JJ. Genetic parameters of natural antibody isotypes and survival analysis in beak-trimmed and non-beak-trimmed crossbred laying hens. Poult Sci. 2013; 92: 2024–2033. 10.3382/ps.2013-03144 [DOI] [PubMed] [Google Scholar]
- 93. Siegel PB, Gross WB. Production and persistence of antibodies in chickens to sheep erythrocytes. 1. Directional selection. Poult Sci. 1980; 59: 1–5. [DOI] [PubMed] [Google Scholar]
- 94. Siegel PB, Gross WB, Cherry JA. Correlated responses of chickens to selection for production of antibodies to sheep erythrocytes. Anim. Blood Groups Biochem Genet. 1982; 13: 291–297. [DOI] [PubMed] [Google Scholar]
- 95. van der Zijpp AJ, Frankena K, Boneschanscher J, Nieuwland MG. Genetic analysis of primary and secondary immune responses in the chicken. Poult Sci. 1983; 62: 565–572. [DOI] [PubMed] [Google Scholar]
- 96. Gross WB, Siegel PB. Effects of early environmental stresses on chicken body weight, antibody response to RBC antigens, feed efficiency, and response to fasting. Avian Dis. 1980; 24: 569–579. [PubMed] [Google Scholar]
- 97. Wondmeneh E, Van Arendonk JAN, Van der Waaij EH, Ducro BJ, Parmentier HK. High natural antibody titers of indigenous chickens are related with increased hazard in confinement. Poultry Science. 2015; 94: 1493–1498. 10.3382/ps/pev107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Siegel HS. Stress, Strains, and resistance. Br Poult Sci. 1995; 36: 3–22. [DOI] [PubMed] [Google Scholar]
- 99. Maxwell MH. Avian blood leucocyte responses to stress. World’s Poult Sci.1983; 49: 34–43. [Google Scholar]
- 100. Campo JL, Gil MG, Dávila SG, Muñoz I. Influence of perches and footpad dermatitis on tonic immobility and heterophil to lymphocyte ratio of chickens. Poult Sci. 2005; 84: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 101. Barnett JL, Glatz PC, Newman EA, Gronin GM. Effects of modifying layer cages with perches on stress physiology, plumage, pecking and bone strength of hens. Aust. J. Exp. Agr. 1997; 37: 523–529. [Google Scholar]
- 102. Liedtke EA, Hester PY, Vezzoli G, Gates RS, Enneking SA, Cheng HW, Makagon MM. The effects of chilled perches on body surface temperature of laying hens exposed to an acute heat episode Proc. 12th Int. Soc. Appl. Ethol. (ISAE) North-American Regional Meeting. 2014. p 50. [Google Scholar]
- 103. Makagon MM, Hester PY, Vezzoli G, Gates RS, Enneking SA, Cheng HW. Access to cooling perches affects the behavioral responses of laying hens during acute heat stress Proc. 48th Congr. Int. Soc. Appl. Ethol. (ISAE). Wageningen Academic Publishers, Wageningen, the Netherlands, 2014. p 181. [Google Scholar]
- 104. Deng W, Dong XF, Tong JM, Zhang Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult Sci. 2012; 91: 575–582. 10.3382/ps.2010-01293 [DOI] [PubMed] [Google Scholar]
- 105. Cheng HW, Makagon MM, Gates RS, Hu JY, Enneking SA, Hester PY. The effect of thermally cooled perches installed in cages on White Leghorn hen performance. Poultry Science. 2014; 93 (E-Suppl.1): 92. [Google Scholar]
- 106. Hester PY, Makagon MM, Gates RS, Hu JY, Enneking SA, Cheng HW. 2014. The musculoskeletal health of caged White Leghorn hens with access to thermally cooled perches. Poultry Science. 2014; 93 (E-Suppl.1): 93. [Google Scholar]
- 107.Spencer H. Heat Abatement Programs for Midwest Dairies. Minnesota Dairy Health Conference. 2001. ST. University of Minnesota, Paul, Minnesota. Available: http://purl.umn.edu/108739. Accessed 27 April, 2015.
- 108. Lustick SI. 1983. Cost benefit of thermoregulation in birds: influences of posture, microhabitat selection, and color In: Aspey W, Lustic SI, editors. Behavioral energetics: the cost of survival in vertebrates. 1983. pp. 265–294. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.