Abstract
Background
Human papillomavirus (HPV) vaccines confer protection against the oncogenic genotypes HPV16 and HPV18 through the generation of type-specific neutralizing antibodies raised against virus-like particles (VLP) representing these genotypes. The vaccines also confer a degree of cross-protection against HPV31 and HPV45, which are genetically-related to the vaccine types HPV16 and HPV18, respectively, although the mechanism is less certain. There are a number of humoral immune measures that have been examined in relation to the HPV vaccines, including VLP binding, pseudovirus neutralization and the enumeration of memory B cells. While the specificity of responses generated against the vaccine genotypes are fairly well studied, the relationship between these measures in relation to non-vaccine genotypes is less certain.
Methods
We carried out a comparative study of these immune measures against vaccine and non-vaccine genotypes using samples collected from 12–15 year old girls following immunization with three doses of either Cervarix® or Gardasil® HPV vaccine.
Results
The relationship between neutralizing and binding antibody titers and HPV-specific memory B cell levels for the vaccine genotypes, HPV16 and HPV18, were very good. The proportion of responders approached 100% for both vaccines while the magnitude of these responses induced by Cervarix® were generally higher than those following Gardasil® immunization. A similar pattern was found for the non-vaccine genotype HPV31, albeit at a lower magnitude compared to its genetically-related vaccine genotype, HPV16. However, both the enumeration of memory B cells and VLP binding responses against HPV45 were poorly related to its neutralizing antibody responses. Purified IgG derived from memory B cells demonstrated specificities similar to those found in the serum, including the capacity to neutralize HPV pseudoviruses.
Conclusions
These data suggest that pseudovirus neutralization should be used as the preferred humoral immune measure for studying HPV vaccine responses, particularly for non-vaccine genotypes.
Introduction
The human papillomavirus (HPV) vaccines (Cervarix® and Gardasil®) contain virus-like particles (VLP) comprising the major capsid protein (L1) of HPV16 and HPV18 and are highly efficacious at preventing cervical cancer precursors associated with these two high risk genotypes in clinical trials [1–3]. Gardasil® also contains VLP representing the main gentoypes associated with the development of genital warts (HPV6 and HPV11). HPV16 and HPV18 account for ca. 70% of cervical cancers worldwide [4, 5] and a recent meta-analysis [6] of epidemiological data from Australia [7], the USA [8] and the UK [9–11] demonstrate reductions in the prevalence of these two genotypes following the introduction of national HPV vaccination programmes.
Neutralizing antibodies against vaccine genotypes can be detected in the serum and genital secretions of vaccinees [12–14] and passive transfer of neutralizing antibodies can protect animals against papillomavirus challenge [15–17] leading to the reasonable assumption that type-specific protection is mediated by neutralizing antibodies [2]. Some degree of cross-protection has been demonstrated against the non-vaccine genotypes HPV31 and HPV45 that are genetically-related to the vaccine genotypes HPV16 and HPV18, respectively [1, 3, 18, 19]. This is coincident with the detection of cross-neutralizing antibodies in the serum [14, 20–23] and cervicovaginal secretions [14] of vaccinees suggesting that such antibodies may be effectors or their detection may be useful as a correlate or surrogate of vaccine-induced cross-protection [24].
A limited number of serological assays are available for measuring vaccine-type (HPV16 and HPV18) antibody responses, including a VLP ELISA, a monoclonal antibody competitive VLP assay and a pseudovirus neutralization assay. Despite some discrepancies, overall inter-assay agreements appear to be good [25–27]. However, little is known about the relationship between these measures for non-vaccine types.
The detection of antigen-specific memory B cells may be indicative of a robust and long-lasting vaccine-induced immune response [28, 29], but relatively few studies have examined the proportion and specificity of memory B cells induced by the HPV vaccines. Early HPV studies estimated antigen-specific memory B cell frequencies induced by prototype VLP16 and/or VLP18 immunogens [30, 31] while more recent studies have assessed HPV16 and HPV18 specific memory B cell responses generated by the licensed vaccines Cervarix® and Gardasil® [12, 32]. One of these studies [12], carried out in 18–45 year old women, assessed binding and neutralizing antibody and memory B cell responses induced by both Cervarix® and Gardasil® against vaccine (HPV16 and HPV18) [12, 33, 34] and non-vaccine (HPV31 and HPV45) [22] genotypes. For all humoral immune measures, the magnitudes of the responses against HPV16 were generally greater than those responses against HPV18 with Cervarix® eliciting responses of a greater magnitude than Gardasil® [33]. For HPV31, and to a lesser extent for HPV45, Cervarix® appeared to generate neutralizing antibody responses of a greater magnitude than Gardasil®, but this was not reflected in the antibody binding or memory B cell responses [22]. Some of these observations were likely affected by the older age of the women enrolled, a parameter known to affect HPV vaccine immunogenicity [35].
We recently carried out an immunogenicity trial of Cervarix® and Gardasil® in the target age group (12–15 year old girls) for national vaccination programmes and demonstrated high levels of serum cross-neutralizing antibodies, a clear difference between the responses generated by the vaccines and an ability to detect neutralizing antibodies against vaccine and non-vaccine genotypes in the genital secretions of vaccinated girls [14]. In the present study, we examine the breadth and magnitudes of the memory B cell responses generated by both HPV vaccines against vaccine (HPV16 and HPV18) and non-vaccine (HPV31 and HPV45) HPV genotypes for which vaccine-induced protection has been consistently observed and compare these to their serum neutralizing and binding antibody responses in order to understand better these vaccine-induced immune measures in the target age group.
Materials and Methods
Study samples
The study design of the randomized, observer-blinded immunogenicity trial of Cervarix® and Gardasil® vaccines in 12–15 year old girls and the primary serum neutralizing antibody response analysis have been reported previously (Research Ethics Committee reference: 09/H0720/25) [14]. The present study examined those individuals who consented to provide an additional blood sample at month 7 (M7) following three doses of either Cervarix® or Gardasil® vaccine for evaluation of their memory B cell responses. Peripheral blood mononuclear cells (PBMC) were isolated from a 20-30mL sample of heparinized blood, processed according to standard protocols and stored in liquid nitrogen. M7 serum samples from these individuals were also included in this present study.
ELISA
L1 VLP representing HPV16, HPV18, HPV31 and HPV45 were expressed using the Bac-to-Bac® Baculovirus System (Life Technologies), as previously described [14] wherein the L1 genes shared 100% amino acid sequence identity with the L1 genes of the pseudovirus clones used for the neutralization assay [23]. Serum (M7) binding antibody responses were determined for those individuals who consented to provide a PBMC sample for memory B cell determination using an L1 VLP ELISA, as described previously [14]. L1L2 pseudoviruses (see 2.3) were also used in a binding ELISA as described above.
Neutralization assay
Serum (M7) neutralizing antibody data, previously generated [14] against HPV16, HPV18, HPV31, HPV45 pseudoviruses, were included in the present study.
ELISpot Assay
Enumeration of memory B cells by ELISpot was carried out according to the original method of Crotty et al., [29] with minor modifications including the use of cryopreserved PBMC [36]. PBMC (1x106 per mL) were rested in culture medium (RPMI 1640 with 10% fetal calf serum, 100 units/mL penicillin, 100 μg/mL streptomycin, 2mM L-Glutamine, 10mM Hepes and 1mM Sodium Pyruvate) at 37°C for one hour before being stimulated with 1μg/mL R848 (resimiquimod; MABTECH, Cincinnati, OH) and 10ng/mL recombinant human IL-2 (interleukin-2; MABTECH, Cincinnati, OH) for 5 days. The median initial PBMC viability of 93% (inter-quartile range, IQR 92–95%), as determined by trypan blue exclusion, was reduced to 86% (84–89%) following R848/IL-2 stimulation.
IgG ELISpot assays were performed using a commercially available kit (IgG ELISpot 150 plus kit (MABTECH) according to the manufacturer’s instructions. ELISpot plates were coated with 3 μg/mL HPV16, HPV18, HPV31 or HPV45 VLP (n = 3 each) [14], 15 μg/mL anti-human IgG (total IgG; MABTECH, Cincinnati, OH) (n = 3) or PBS (mock; n = 6) and incubated overnight at 4°C. The following day, 5x104 PBMC were added to the VLP and mock wells and 4x102 PBMC added to the anti-human IgG wells, then the plate was incubated at 37°C for 6 h. Cells were removed and the plate washed four times with PBS containing 0.05% Tween 20 (Sigma) and four times with PBS followed by incubation with anti-human IgG–horseradish peroxidase overnight at 4°C. The plate was washed as above, incubated for 1hr at RT with streptavidin-HRP followed by addition of TMB substrate solution for 15 min at RT. Spot-forming units (SFU) were counted using the AID ELISpot reader ELR04 (AID, Germany) and data are presented as the percentage of HPV-specific memory B cells per total IgG-secreting cells as standard [29]. The mock, no antigen control, was used to gauge the level of background staining. Overall there were a median 1.2 (IQR 0.7–2.0) apparent SFU per well in the mock wells resulting in an effective median background level compared to total IgG SFU of 0.025% (IQR 0.014–0.036%). For a well to be considered positive it had to have at least 5 SFU per well, equivalent to 100 SFU per 106 PBMC or ca. 0.1% of IgG-bearing cells, and be at least 3 times higher than the mock SFU level for that individual. This is a similar approach to that taken by Walsh et al., [37] as part of a methodological evaluation for The NIAID HIV Vaccine Trials Network.
Memory B cell derived IgG
Memory B cell derived IgG were purified (Protein G GraviTrap and Ab buffer kit; GE Healthcare Life Sciences, UK) from culture supernatant collected following R848/IL-2 stimulation. The eluted IgG were concentrated by centrifugation using an Amicon® Ultra-4 Centrifugal Filter Unit (10 kDa cutoff; Millipore, UK) and human IgG levels estimated using an indirect ELISA as previously described [14]. The linearity of the standards was good with an average r 2 of 0.990 (s.d. 0.004; n = 3). The median level of recovered IgG was 51.3 (IQR 40.6–66.8) μg/mL.
Data analysis
Fisher’s Exact test was used to test for differences in the proportion of individuals positive in a particular test. The Mann Whitney U test was used to test for differences in the magnitude of responses in a particular test and for any differences in the age range in the vaccinees. A non-parametric trend analysis was used to test for an association between measures for one target in one test against the neutralizing antibody responses by the HPV16 or HPV18 vaccine-genotype, as appropriate. All analyses were 2-tailed where appropriate and performed using Stata 13.1 (Statacorp, College Station, TX).
Results
Study participants
PBMC from eighty four consenting individuals were available. Four were excluded due to low viability resulting in PBMC from eighty (Cervarix® n = 36; Gardasil® n = 44) individuals being used. The ages of the individuals who received Cervarix® (median 14, range 12–15 years) were similar to those who received Gardasil® (14, 12–15 years; p = 0.461 Mann Whitney U test).
Proportion of responders
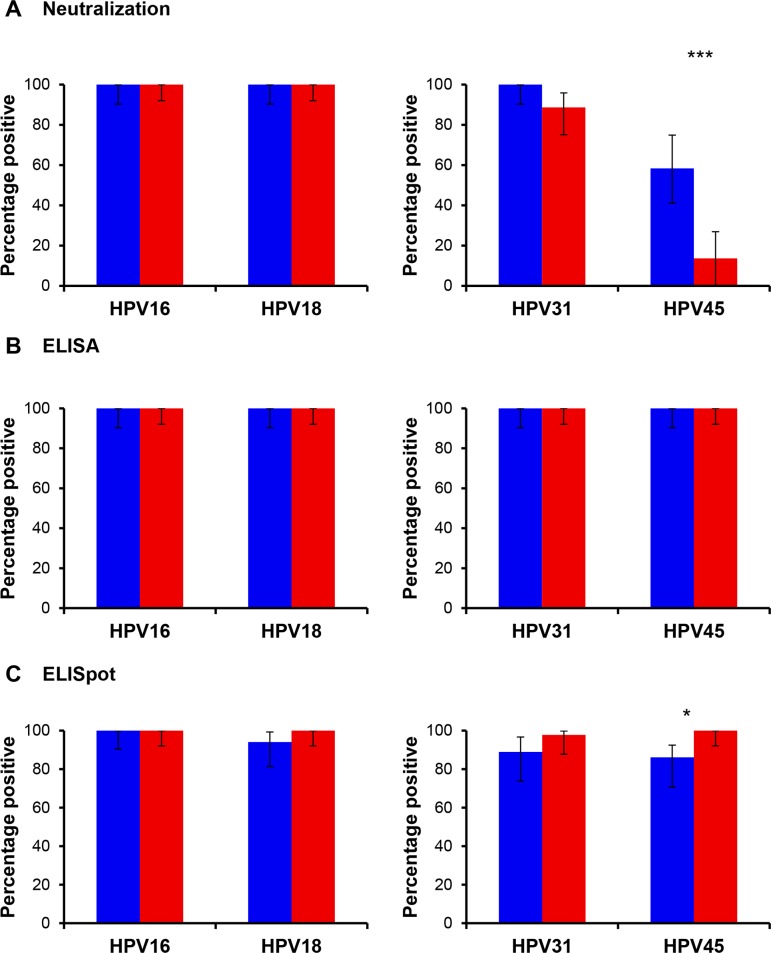
Seropositivity rates for vaccine-type (HPV16 and HPV18) neutralizing and binding antibodies were 100% for individuals receiving either Cervarix® or Gardasil® vaccines, as expected (Fig 1). These were similar to the proportion of individuals with detectable memory B cell responses against HPV16 (Cervarix® 100% and Gardasil® 100%) and HPV18 (Cervarix® 94% and Gardasil® 100%). A high proportion of individuals immunized with Cervarix® (100%) or Gardasil® (89%) elicited a neutralizing antibody response against HPV31, with 100% of individuals generating binding antibody response against this non-vaccine genotype and almost all (Cervarix®, 89% and Gardasil® 98%) had detectable HPV31-specific memory B cells. For HPV45, however, the relatively low and differential proportion of individuals with a measurable neutralizing antibody response following Cervarix® (58%) or Gardasil® (14%) vaccination was in contrast to the 100% seropositivity rates for binding antibodies and the similarly high proportion of individuals with detectable HPV45-specific memory B cells (Cervarix®, 86% and Gardasil® 100%).
Fig 1. Percentage of responders to each humoral immune measure.
Percentage of Cervarix® (Blue) or Gardasil® (Red) responders in the (A) neutralization assay, (B) binding assay or (C) B cell ELISpot assay against the indicated vaccine (HPV16, HPV18) and non-vaccine (HPV31, HPV45) genotypes. Error bars, 95% CI. * p<0.05; ** p<0.01; *** p<0.001.
Magnitude of humoral immune response measures
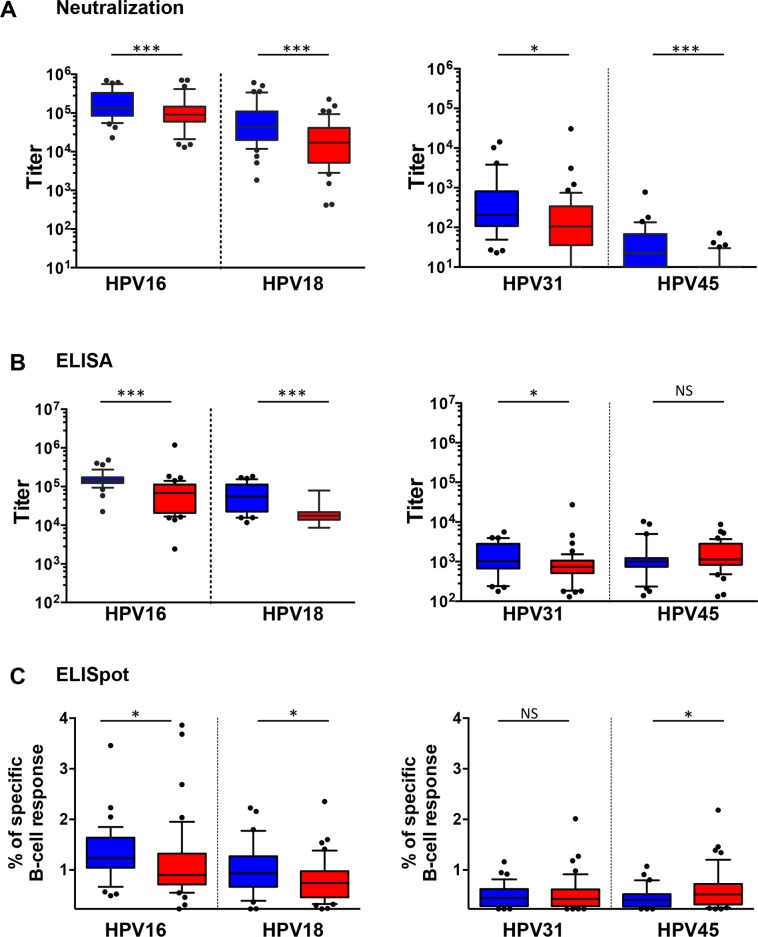
Serum HPV16 neutralizing antibody titers for Cervarix® vaccinees (median 135,617, IQR 85,137–328,767) were higher than for those who received the Gardasil® vaccine (44,048, 20,696–105,982; p<0.001) (Fig 2 and S1 Table) similar to the differential HPV18 titers for Cervarix® (90,693, 63,222–142,890) and Gardasil® (17,011, 5,544–40,707; p<0.001). Similar differences between the vaccines were seen for HPV16 (Cervarix: 140,040, 123,850–172,575 and Gardasil: 68,423, 21,355–112,534; p<0.001) and HPV18 (Cervarix: 55,394, 23,657–109,494 and Gardasil: 17,559, 13,792–21,814; p<0.001) serum binding antibody responses. The median percentage of HPV16-specific memory B cell responses for Cervarix® vaccinees was 1.23% (IQR 1.07–1.61%) which was higher than the median 0.91% (0.72–1.31%; p = 0.034) seen in Gardasil® vaccinees. HPV18-specific memory B cells were lower than for HPV16, with individuals receiving Cervarix® (0.93%, 0.69–1.24%) having higher levels than Gardasil® (0.75%, 0.47–0.97%; p = 0.021) vaccinees.
Fig 2. Magnitude of humoral immune responses.
Box (median and IQR) and whisker (10th - 90th percentiles) plots of the magnitude of humoral immune responses elicited by Cervarix® (Blue) and Gardasil® (Red) vaccinees in the (A) neutralization assay, (B) binding assay or (C) B cell ELISpot assay against the indicated vaccine (HPV16, HPV18) and non-vaccine (HPV31, HPV45) genotypes. * p<0.05; ** p<0.01; *** p<0.001.
HPV31 neutralizing antibody titers were slightly higher in Cervarix® (204, IQR 121–686) compared to Gardasil® (112, 41–379) vaccinees (p = 0.019). Similarly, HPV31 binding titers were slightly higher in Cervarix® (1,032, 680–2,743) compared to Gardasil® (739, 533–1,060) vaccinees (p = 0.024). Estimates of circulating HPV31-specific memory B cells for individuals receiving Cervarix® (0.44%, 0.30–0.62%) were similar to those who received Gardasil® (0.43%, 0.28–0.59%; p = 0.954) vaccine.
HPV45 neutralizing antibody titers were higher in Cervarix® (23, 10–59) compared to Gardasil® (10, 10–10; p<0.001) vaccinees but this was not reflected in the binding titers which were similar between Cervarix® (998, 754–1,230) and Gardasil® (1,131, 866–2,803; p = 0.171) vaccinees. Estimates of circulating HPV45-specific memory B cells for individuals receiving Cervarix® (0.41%, 0.28–0.51%) were slightly lower than those who received Gardasil® (0.52%, 0.33–0.71%; p = 0.020) vaccine.
Association with vaccine-type neutralizing antibody responses
To elucidate further any potential relationship(s) between neutralizing and binding antibody titers and the levels of memory B cells specific for vaccine and non-vaccine genotypes, we compared whether a measure increased in a stepwise manner according to the low, middle or high tertiles of the corresponding vaccine-type neutralization titers which would be suggestive of a relationship between the magnitudes of these responses. In this respect, vaccine-type specific serum binding antibody (HPV16, p<0.001; HPV18, p<0.001) and memory B cell (HPV16, p<0.001; HPV18, p<0.009) responses demonstrated close associations with their respective neutralizing antibody tertiles (Table 1).
Table 1. Comparison of non-vaccine type and vaccine-type immune measures.
| Immune responses against vaccine and non-vaccine types from indicated species groups a | ||||||
|---|---|---|---|---|---|---|
| Target | Immune measure | Tertile | Alpha-9 (HPV16 and HPV31) | p value | Alpha-7 (HPV18 and HPV45) | p value |
| Vaccine types | Neutralizing antibody | T1 | 22,960 (17,690–31,289) | 11,028 (4,259–13,361) | ||
| T2 | 83,869 (71,796–104,323) | 51,451 (30,028–66,105) | ||||
| T3 | 329,411 (157,076–473,509) | N/A | 123,701 (105,933–200,388) | N/A | ||
| Binding antibody | T1 | 22,418 (18,937–59,497) | 15,306 (12,257–18,448) | |||
| T2 | 112,452 (90,889–130,872) | 21,387 (16,246–36,373) | ||||
| T3 | 168,576 (139,593–221,501) | <0.001 | 101,046 (61,129–139,189) | <0.001 | ||
| Memory B cells | T1 | 0.80% (0.71–1.00%) | 0.61% (0.48–0.97%) | |||
| T2 | 1.25% (0.99–1.64%) | 0.84% (0.58–0.94%) | ||||
| T3 | 1.39% (1.16–1.71%) | <0.001 | 1.07% (0.83–1.30%) | <0.009 | ||
| Non-vaccine types | Neutralizing antibody | T1 | 93 (31–159) | 10 (10–10) | ||
| T2 | 157 (80–340) | 10 (10–36) | ||||
| T3 | 511 (138–2,270) | <0.001 | 21 (10–49) | <0.001 | ||
| Binding antibody | T1 | 569 (258–821) | 943 (749–1,234) | |||
| T2 | 974 (662–1,073) | 1,057 (779–1,884) | ||||
| T3 | 1,558 (798–3,352) | <0.001 | 1,188 (859–4,085) | 0.062 | ||
| Memory B cells | T1 | 0.36% (0.26–0.47%) | 0.49% (0.33–0.70%) | |||
| T2 | 0.44% (0.32–0.58%) | 0.49% (0.28–0.59%) | ||||
| T3 | 0.54% (0.32–0.75%) | 0.022 | 0.44% (0.28–0.69%) | 0.318 | ||
a Median (inter-quartile range, IQR) for immune measures against vaccine (HPV16, HPV18) and non-vaccine (HPV31, HPV45) genotypes from the Alpha-9 (HPV16, HPV31) and Alpha-7 (HPV18, HPV45) species groups. p value, test for trend for each measure following separation of responses into tertiles (T1-T3) based upon vaccine-type neutralizing antibody responses. N/A, not applicable.
HPV31 neutralizing (p<0.001) and binding (p<0.001) antibody titers, and to a lesser extent memory B cell responses (p = 0.022), demonstrated close associations with the magnitude of the neutralizing antibody titers generated against the vaccine genotype, HPV16. For HPV45, however, although neutralizing antibody titers were generally related to the magnitude of the responses against the vaccine genotype HPV18 (p<0.001), binding antibody titers (p = 0.062) and memory B cell responses (p = 0.318) were poorly related.
Functionality of memory B cell-derived IgG
To demonstrate that memory B cell derived IgG retained neutralizing antibody capability we purified total IgG from the culture supernatant of R848/IL-2 stimulated PBMC (n = 10) and used these in neutralization and binding assays (Table 2). Memory B cell derived IgG from all individuals were able to neutralize pseudoviruses representing both vaccine genotypes (HPV16 and HPV18) and this was similarly reflected in their binding capability. Memory B cell derived IgG samples were also able to neutralize non-vaccine genotypes, HPV31 and HPV45, although the inhibitory concentration required to do so was 1–2 Log10 higher. Nine of ten samples were able to neutralize the non-vaccine genotype HPV31 while only four of ten purified IgG samples were able to neutralize HPV45. Almost all samples were able to bind L1 VLP by ELISA in keeping with the antibody specificities seen using serum. However, when L1L2 pseudoviruses were used as the target antigen in binding assays, fewer samples were able to bind the antigen representing HPV45. Quantitative differences between all three datasets suggest that the target antigen and its context impact on apparent vaccine antibody specificity.
Table 2. Purified memory B-cell derived IgG neutralize L1L2 PSV and bind L1L2 PSV and L1 VLP.
| Concentration of purified IgG (μg/mL) a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSV Neutralization | PSV Binding | VLP Binding | ||||||||||||
| Vaccine | ID | Purified IgG (μg/mL) | PSV16 | PSV18 | PSV31 | PSV45 | PSV16 | PSV18 | PSV 31 | PSV45 | VLP16 | VLP18 | VLP31 | VLP 45 |
| Cervarix | 5 | 74.3 | 0.02 | 0.05 | 1.38 | 4.03 | 0.19 | 2.39 | 8.51 | - | 0.15 | 0.20 | 1.54 | 3.23 |
| 17 | 58.4 | 0.03 | 0.06 | 1.34 | - | 0.14 | 0.65 | 1.32 | - | 0.11 | 0.20 | 1.01 | 3.12 | |
| 20 | 51.3 | 0.01 | 0.04 | 6.20 | 2.32 | 0.11 | 0.28 | 6.00 | 3.37 | 0.07 | 0.09 | 0.65 | 0.52 | |
| 25 | 51.2 | 0.03 | 0.07 | 2.30 | - | 0.32 | 1.03 | 6.59 | - | 0.17 | 0.50 | 3.69 | 10.06 | |
| 30 | 35.6 | 0.06 | 0.06 | 4.04 | 1.19 | 0.12 | 0.17 | 4.66 | 3.08 | 0.08 | 0.09 | 3.73 | 1.89 | |
| 34 | 43.2 | 0.10 | 0.06 | 1.64 | 5.78 | 0.50 | - | - | - | 0.30 | 0.47 | 2.38 | 2.97 | |
| Gardasil | 7 | 111.9 | 0.10 | 0.40 | 5.47 | - | 0.44 | 1.27 | 10.17 | - | 0.25 | 0.36 | 1.93 | 1.41 |
| 17 | 38.3 | 0.07 | 0.59 | 5.39 | - | 0.33 | 1.56 | - | - | 0.18 | 0.43 | 2.00 | 1.61 | |
| 37 | 69.6 | 0.03 | 0.12 | 3.32 | - | 0.15 | 0.96 | 5.19 | - | 0.13 | 0.45 | 1.76 | 3.39 | |
| 43 | 39.7 | 0.11 | 0.75 | - | - | 0.63 | - | - | - | 0.43 | - | - | - | |
a Midpoint neutralizing or binding antibody concentration of memory B cell derived IgG (μg/mL) or not achievable (-) with maximum amount of purified IgG tested. PSV, pseudovirus.
Discussion
This study evaluated three humoral immune measures (neutralizing antibody, binding antibody and memory B cell responses) raised against vaccine (HPV16 and HPV18) and non-vaccine (HPV31 and HPV45) genotypes in order to provide possible insights into the differential protection afforded by the current HPV vaccines, Cervarix® and Gardasil®. The study was carried out in the target age group included in national vaccination programmes and would be expected to represent the optimum responses generated by the current generation of HPV vaccines.
As expected, 100% of individuals were seropositive for binding and neutralizing antibodies against the vaccine genotypes following three vaccine doses. The greater magnitude of serum antibody responses against HPV16 compared to HPV18 [12–14, 21, 23, 25, 26], and the increased immunogenicity of the Cervarix® vaccine compared to Gardasil® for these two vaccine genotypes [12, 14], are consistent with published studies. The proportion of individuals positive for detection of vaccine-type (HPV16 and HPV18) specific memory B cells and the magnitude of their responses were higher than those reported in a study of 18–45 year old women examining responses generated by Cervarix® and Gardasil® [12]. This is probably due to the lower age group recruited to this present study, as suggested by memory B cell responses following Gardasil® vaccination of 9–13 year old girls and 16–26 year old women [32]. Vaccine-type specific memory B cell responses tracked well with the magnitude of serum immune measures.
The proportion of responders and magnitude of their responses against non-vaccine HPV31 and HPV45 were higher than those reported from older women [22], allowing a more robust comparison between these immune measures for non-vaccine genotypes. We previously reported that HPV31 and HPV45 neutralizing antibody responses were quantitatively related to their respective vaccine-type response, that Cervarix® responses were higher than those elicited in Gardasil® vaccinees and that the differential cross-protection against non-vaccine genotypes bestowed by the current vaccines [1, 3, 18, 19] was, in part at least, reflected in the differential neutralizing antibody responses against these genotypes [14]. In the present sub-study, all individuals were positive for binding antibodies and >80% of individuals had measurable memory B cell responses against both HPV31 and HPV45, suggesting a robust response to vaccination irrespective of vaccine received. While the magnitude of the binding antibody response was generally in line with the magnitude of the vaccine-type neutralizing antibody response, for each non-vaccine genotype, the magnitude of the memory B cell response was poorly related, particularly for HPV45.
In order to address this discrepancy in a little more detail, we evaluated the purified IgG derived from memory B cells in both antibody neutralization and binding assays. That in vitro stimulated memory B cells from vaccinees elicited both L1 VLP binding and neutralizing antibodies of similar specificities to those found in the serum suggests that stimulation of resting memory B cells did not introduce a bias in the derived specificities. We used L1 VLP in this study as they are the immunogens used in the HPV vaccines [2] and the antigen widely used in serological studies of vaccine immunogenicity [13, 22, 25, 26, 32, 33]. However, when we used L1L2 pseudoviruses as the target antigens in antibody binding assays there were differences in the antibody specifiities derived. These data demonstrate that the apparent impact on L1 topography induced by incorporation of the L2 protein, previously shown for murine monoclonal antibodies [38], also applies to those antibodies elicited by the HPV vaccines, particularly for those antibodies with specificity against non-vaccine genotypes.
Taken together these data corroborate observations that the measurement of binding antibodies [25, 26], and possibly enumeration of memory B cells [12], may be useful measures for the evaluation of an HPV vaccine genotype-specific immune response. The detection of neutralizing antibodies against non-vaccine genotypes in the serum and cervicovaginal secretions of vaccinated individuals [14, 20–23], protection against HPV31 pseudovirus transduction in the murine challenge model [39] and a reduced risk of HPV31 infection in vaccinated individuals who generate HPV31 neutralizing antibodies [40] do appear to suggest that such cross-neutralizing antibodies are functionally relevant. However, discrepancies between the assay systems and target antigens suggest that binding antibodies and the enumeration of memory B cells were poorly correlated with the cross-neutralizing antibody response and therefore we consider these measures are unlikely to be useful for evaluating the immune response to non-vaccine genotypes.
A next generation HPV vaccine comprising an extended range of VLP [41] should provide greater coverage than the current bivalent (Cervarix®) and quadrivalent (Gardasil®) vaccines [42]. The significant cost implications of multivalent vaccines may be mitigated by observations that genotype-specific antibody titers in reduced dosing schedules of the current HPV vaccines are non-inferior to those generated under the standard three dose schedule [43–45]. Although next generation HPV vaccines with increased valency will likely be introduced into national programmes over the coming years these are likely to be prohibitively expensive for many low and middle income countries, at least initially. Furthermore, even in countries that adopt such vaccines in the near future several birth cohorts of girls (and in some cases, boys) have already received the current generation of vaccines. An improved understanding of the immune responses elicited following immunization with the current generation of vaccines, and in particular the relationship between immune measures for both vaccine and non-vaccine genotypes, should improve our ability to track such responses in vaccinated individuals and perhaps anticipate any decline in a protective immune response.
Supporting Information
(XLSX)
Acknowledgments
We are grateful to the vaccine research nurses Lynne Joslin, Norah Ashwood, Diane Webb, Anne Maher and Wendy Nedoma for the exceptional execution of this study including recruitment, vaccine administration and support given to the participants. Special thanks go to the study participants for their interest in this study and without whom this study would not have been possible. We are indebted to Teresa Gibbs, Deborah Cohen and Elizabeth Sheasby for the excellent administration of this study and Dr Anu Ohrling and Dr Chee Yung for their clinical expertise. We thank Eve Draper and Kavita Panwar for their contributions to this study. We are indebted to Prof. John T. Schiller and Dr. Chris Buck (National Cancer Institute, Bethesda, U.S.A.) for access to the pseudovirus clones used in this study. The views expressed in this publication are those of the author(s) and not necessarily those of the Department of Health.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The laboratory work was supported by the UK Medical Research Council (grant number G0701217) while the vaccine research nurses were supported by a grant from the UK Department of Health Policy Research Programme (grant number 039/0031). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nature reviews Clinical oncology. 2013;10(7):400–10. Epub 2013/06/06. 10.1038/nrclinonc.2013.84 . [DOI] [PubMed] [Google Scholar]
- 2. Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol. 2012;10(10):681–92. 10.1038/nrmicro2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herrero R, Gonzalez P, Markowitz LE. Present status of human papillomavirus vaccine development and implementation. Lancet Oncol. 2015;16(5):e206–e16. Epub 2015/05/07. 10.1016/s1470-2045(14)70481-4 . [DOI] [PubMed] [Google Scholar]
- 4. de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. 10.1016/S1470-2045(10)70230-8 [DOI] [PubMed] [Google Scholar]
- 5. Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2010;128(4):927–35. . [DOI] [PubMed] [Google Scholar]
- 6. Drolet M, Benard E, Boily MC, Ali H, Baandrup L, Bauer H, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015. Epub 2015/03/07. 10.1016/s1473-3099(14)71073-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206(11):1645–51. 10.1093/infdis/jis590 [DOI] [PubMed] [Google Scholar]
- 8. Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208(3):385–93. 10.1093/infdis/jit192 [DOI] [PubMed] [Google Scholar]
- 9. Mesher D, Soldan K, Howell-Jones R, Panwar K, Manyenga P, Jit M, et al. Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine. 2013;32(1):26–32. Epub 2013/11/12. 10.1016/j.vaccine.2013.10.085 ; PubMed Central PMCID: PMCPmc3898718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sonnenberg P, Clifton S, Beddows S, Field N, Soldan K, Tanton C, et al. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet. 2013;382(9907):1795–806. Epub 2013/11/30. 10.1016/s0140-6736(13)61947-9 ; PubMed Central PMCID: PMCPmc3899025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kavanagh K, Pollock KG, Potts A, Love J, Cuschieri K, Cubie H, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer. 2014;110(11):2804–11. Epub 2014/04/17. 10.1038/bjc.2014.198 ; PubMed Central PMCID: PMCPmc4037824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5(10):705–19. . [DOI] [PubMed] [Google Scholar]
- 13. Kemp TJ, Garcia-Pineres A, Falk RT, Poncelet S, Dessy F, Giannini SL, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008;26(29–30):3608–16. 10.1016/j.vaccine.2008.04.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, et al. A randomized, observer-blinded immunogenicity trial of Cervarix((R)) and Gardasil((R)) Human Papillomavirus vaccines in 12–15 year old girls. PLoS One. 2013;8(5):e61825 Epub 2013/05/08. 10.1371/journal.pone.0061825 ; PubMed Central PMCID: PMCPmc3641072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92(25):11553–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69(6):3959–63. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011;85(24):13253–9. 10.1128/JVI.06093-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):100–10. 10.1016/S1470-2045(11)70287-X [DOI] [PubMed] [Google Scholar]
- 19. Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199(7):926–35. 10.1086/597307 [DOI] [PubMed] [Google Scholar]
- 20. Smith JF, Brownlow M, Brown M, Kowalski R, Esser MT, Ruiz W, et al. Antibodies from Women Immunized with Gardasil ((R)) Cross-Neutralize HPV 45 Pseudovirions. Human Vaccines. 2007;3(4):109–16. [DOI] [PubMed] [Google Scholar]
- 21. Kemp TJ, Hildesheim A, Safaeian M, Dauner JG, Pan Y, Porras C, et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011;29(11):2011–4. 10.1016/j.vaccine.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18–45 years. Hum Vaccin. 2011;7(12):1359–73. Epub 2011/11/04. 10.4161/hv.7.12.18282 ; PubMed Central PMCID: PMCPmc3338933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Draper E, Bissett SL, Howell-Jones R, Edwards D, Munslow G, Soldan K, et al. Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera. Vaccine. 2011;29(47):8585–90. Epub 2011/09/24. 10.1016/j.vaccine.2011.09.021 ; PubMed Central PMCID: PMCPmc3359499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47(3):401–9. 10.1086/589862 [DOI] [PubMed] [Google Scholar]
- 25. Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4(6):425–34. . [DOI] [PubMed] [Google Scholar]
- 26. Krajden M, Cook D, Yu A, Chow R, Su Q, Mei W, et al. Assessment of HPV 16 and HPV 18 antibody responses by pseudovirus neutralization, Merck cLIA and Merck total IgG LIA immunoassays in a reduced dosage quadrivalent HPV vaccine trial. Vaccine. 2014;32(5):624–30. Epub 2013/09/24. 10.1016/j.vaccine.2013.09.007 . [DOI] [PubMed] [Google Scholar]
- 27. Brown D, Muller M, Sehr P, Pawlita M, Seitz H, Rubio I, et al. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomaviruse types 16 and 18. Vaccine. 2014;32(44):5880–7. Epub 2014/08/26. 10.1016/j.vaccine.2014.08.004 . [DOI] [PubMed] [Google Scholar]
- 28. Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298(5601):2199–202. Epub 2002/12/14. 10.1126/science.1076071 . [DOI] [PubMed] [Google Scholar]
- 29. Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286(1–2):111–22. Epub 2004/04/17. 10.1016/j.jim.2003.12.015 . [DOI] [PubMed] [Google Scholar]
- 30. Dauner JG, Pan Y, Hildesheim A, Harro C, Pinto LA. Characterization of the HPV-specific memory B cell and systemic antibody responses in women receiving an unadjuvanted HPV16 L1 VLP vaccine. Vaccine. 2010;28(33):5407–13. Epub 2010/07/02. 10.1016/j.vaccine.2010.06.018 ; PubMed Central PMCID: PMCPmc2913111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24(33–34):5937–49. . [DOI] [PubMed] [Google Scholar]
- 32. Smolen KK, Gelinas L, Franzen L, Dobson S, Dawar M, Ogilvie G, et al. Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine. 2012;30(24):3572–9. Epub 2012/04/04. 10.1016/j.vaccine.2012.03.051 . [DOI] [PubMed] [Google Scholar]
- 33. Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12–24 in a Phase III randomized study of healthy women aged 18–45 years. Hum Vaccin. 2011;7(12):1343–58. Epub 2011/11/04. 10.4161/hv.7.12.18281 ; PubMed Central PMCID: PMCPmc3338932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Einstein MH, Levin MJ, Chatterjee A, Chakhtoura N, Takacs P, Catteau G, et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: Follow-up through Month 48 in a Phase III randomized study. Hum Vaccin Immunother. 2014;10(12):3455–65. Epub 2014/12/09. 10.4161/hv.36117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petaja T, Keranen H, Karppa T, Kawa A, Lantela S, Siitari-Mattila M, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health. 2009;44(1):33–40. 10.1016/j.jadohealth.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 36. Truck J, Mitchell R, Thompson AJ, Morales-Aza B, Clutterbuck EA, Kelly DF, et al. Effect of cryopreservation of peripheral blood mononuclear cells (PBMCs) on the variability of an antigen-specific memory B cell ELISpot. Hum Vaccin Immunother. 2014;10(8):2490–6. Epub 2014/11/27. 10.4161/hv.29318 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walsh PN, Friedrich DP, Williams JA, Smith RJ, Stewart TL, Carter DK, et al. Optimization and qualification of a memory B-cell ELISpot for the detection of vaccine-induced memory responses in HIV vaccine trials. J Immunol Methods. 2013;394(1–2):84–93. Epub 2013/05/28. 10.1016/j.jim.2013.05.007 ; PubMed Central PMCID: PMCPmc3736720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Culp TD, Spatz CM, Reed CA, Christensen ND. Binding and neutralization efficiencies of monoclonal antibodies, Fab fragments, and scFv specific for L1 epitopes on the capsid of infectious HPV particles. Virology. 2007;361(2):435–46. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8(3):260–70. 10.1016/j.chom.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Safaeian M, Kemp TJ, Pan DY, Porras C, Rodriguez AC, Schiffman M, et al. Cross-protective vaccine efficacy of the bivalent HPV vaccine against HPV31 is associated with humoral immune responses: results from the Costa Rica Vaccine Trial. Hum Vaccin Immunother. 2013;9(7):1399–406. Epub 2013/04/11. 10.4161/hv.24340 ; PubMed Central PMCID: PMCPmc3974884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–23. Epub 2015/02/19. 10.1056/NEJMoa1405044 . [DOI] [PubMed] [Google Scholar]
- 42. Van de Velde N, Boily MC, Drolet M, Franco EL, Mayrand MH, Kliewer EV, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104(22):1712–23. 10.1093/jnci/djs395 [DOI] [PubMed] [Google Scholar]
- 43. Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. Jama. 2013;309(17):1793–802. 10.1001/jama.2013.1625 [DOI] [PubMed] [Google Scholar]
- 44. Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103(19):1444–51. 10.1093/jnci/djr319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila). 2013;6(11):1242–50. Epub 2013/11/06. 10.1158/1940-6207.capr-13-0203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.