Abstract
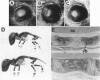
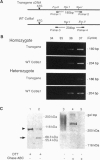
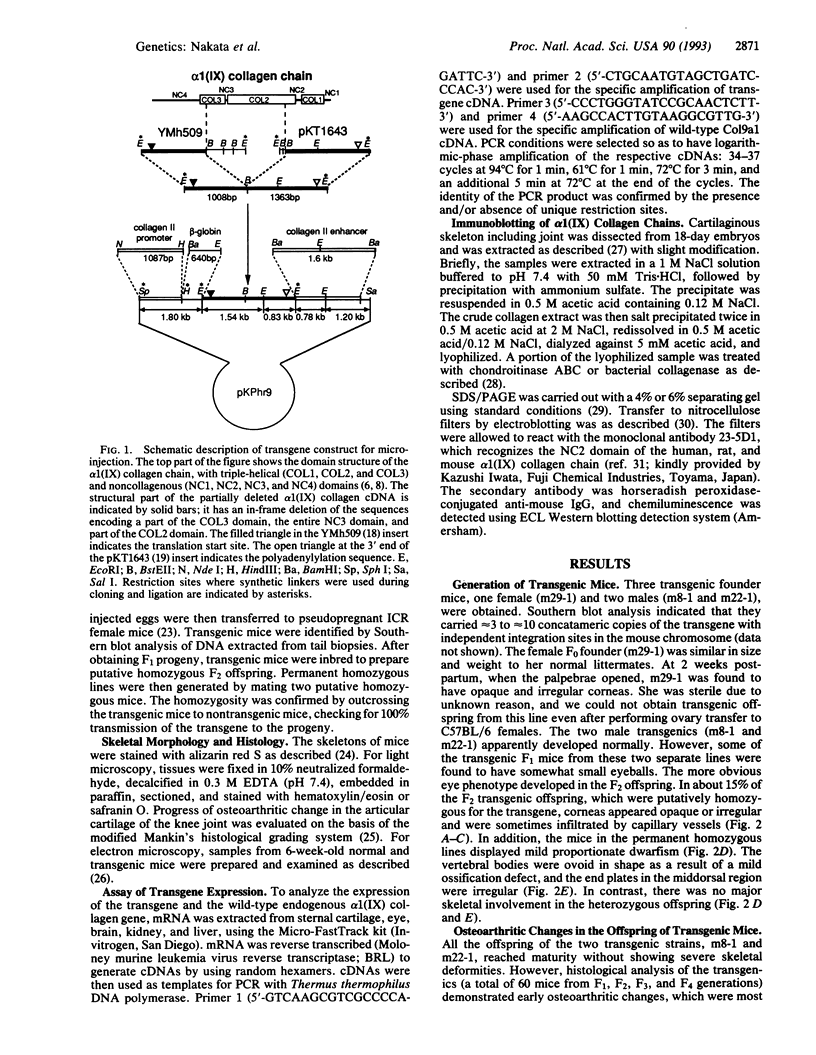
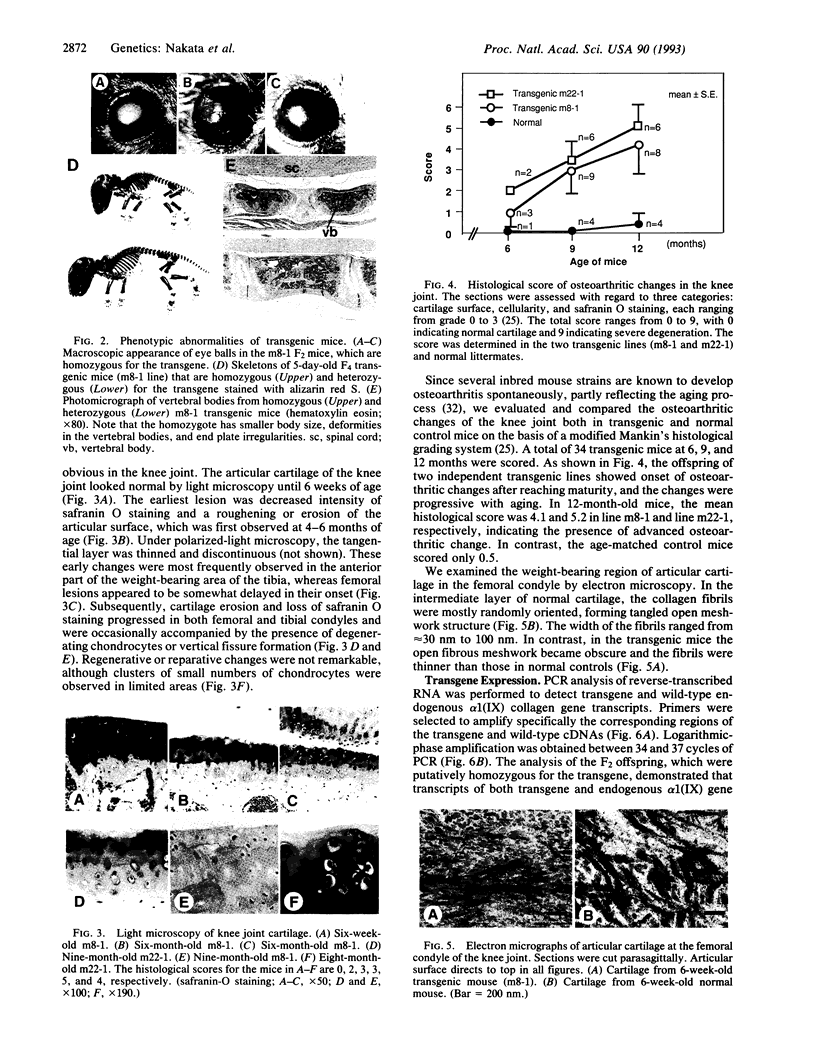
Type IX collagen, containing molecules of the three distinct polypeptides alpha 1(IX), alpha 2(IX), and alpha 3(IX), is an interesting hybrid extracellular matrix component in cartilage and eye tissues, with the properties of both a proteoglycan and a collagen. The alpha 1 (IX) chain has two forms, as a result of the tissue-specific utilization of two alternative promoters; the alpha 2(IX) chain carries a covalently attached glycosaminoglycan side chain. We have introduced a gene construct controlled by a tissue-specific promoter/enhancer and expressing a truncated alpha 1(IX) chain into mice. Examination of the offspring of two different founders revealed pathological changes similar to osteoarthritis in the articular cartilage of knee joints. In addition, mice homozygous for the transgene developed mild chondrodysplasia (i.e., mild dwarfism, anterior tonguing in the vertebral bodies, and ophthalmopathy). The relative ratio of transgene product to the endogenous alpha 1(IX) chain was approximately one in homozygotes and less than one in heterozygotes. Therefore, the phenotypic severity correlated well with the level of transgene expression. These findings suggest that mutations in type IX collagen genes may cause certain forms of osteoarthritis and chondrodysplasia in humans.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N. N., Ala-Kokko L., Knowlton R. G., Jimenez S. A., Weaver E. J., Maguire J. I., Tasman W., Prockop D. J. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6624–6627. doi: 10.1073/pnas.88.15.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Kokko L., Baldwin C. T., Moskowitz R. W., Prockop D. J. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Garofalo S., Vuorio E., Metsaranta M., Rosati R., Toman D., Vaughan J., Lozano G., Mayne R., Ellard J., Horton W. Reduced amounts of cartilage collagen fibrils and growth plate anomalies in transgenic mice harboring a glycine-to-cysteine mutation in the mouse type II procollagen alpha 1-chain gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9648–9652. doi: 10.1073/pnas.88.21.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Kimura J. H. Proteoglycans: isolation and characterization. Methods Enzymol. 1982;82(Pt A):769–800. doi: 10.1016/0076-6879(82)82102-2. [DOI] [PubMed] [Google Scholar]
- Horton W., Miyashita T., Kohno K., Hassell J. R., Yamada Y. Identification of a phenotype-specific enhancer in the first intron of the rat collagen II gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8864–8868. doi: 10.1073/pnas.84.24.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S., van der Rest M., Bruckner P., Rodriguez E., Winterhalter K. H., Vaughan L. Identification of the type IX collagen polypeptide chains. The alpha 2(IX) polypeptide carries the chondroitin sulfate chain(s). J Biol Chem. 1986 May 5;261(13):5965–5968. [PubMed] [Google Scholar]
- Irwin M. H., Mayne R. Use of monoclonal antibodies to locate the chondroitin sulfate chain(s) in type IX collagen. J Biol Chem. 1986 Dec 15;261(35):16281–16283. [PubMed] [Google Scholar]
- Kim H. K., Moran M. E., Salter R. B. The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion. An experimental investigation in rabbits. J Bone Joint Surg Am. 1991 Oct;73(9):1301–1315. [PubMed] [Google Scholar]
- Kimura T., Mattei M. G., Stevens J. W., Goldring M. B., Ninomiya Y., Olsen B. R. Molecular cloning of rat and human type IX collagen cDNA and localization of the alpha 1(IX) gene on the human chromosome 6. Eur J Biochem. 1989 Jan 15;179(1):71–78. doi: 10.1111/j.1432-1033.1989.tb14522.x. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kohno K., Sullivan M., Yamada Y. Structure of the promoter of the rat type II procollagen gene. J Biol Chem. 1985 Apr 10;260(7):4441–4447. [PubMed] [Google Scholar]
- Konomi H., Seyer J. M., Ninomiya Y., Olsen B. R. Peptide-specific antibodies identify the alpha 2 chain as the proteoglycan subunit of type IX collagen. J Biol Chem. 1986 May 25;261(15):6742–6746. [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Lee B., Vissing H., Ramirez F., Rogers D., Rimoin D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science. 1989 May 26;244(4907):978–980. doi: 10.1126/science.2543071. [DOI] [PubMed] [Google Scholar]
- Lozano G., Ninomiya Y., Thompson H., Olsen B. R. A distinct class of vertebrate collagen genes encodes chicken type IX collagen polypeptides. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4050–4054. doi: 10.1073/pnas.82.12.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow V. C., Mak A. F., Lai W. M., Rosenberg L. C., Tang L. H. Viscoelastic properties of proteoglycan subunits and aggregates in varying solution concentrations. J Biomech. 1984;17(5):325–338. doi: 10.1016/0021-9290(84)90027-7. [DOI] [PubMed] [Google Scholar]
- Muragaki Y., Kimura T., Ninomiya Y., Olsen B. R. The complete primary structure of two distinct forms of human alpha 1 (IX) collagen chains. Eur J Biochem. 1990 Sep 24;192(3):703–708. doi: 10.1111/j.1432-1033.1990.tb19279.x. [DOI] [PubMed] [Google Scholar]
- Muragaki Y., Nishimura I., Henney A., Ninomiya Y., Olsen B. R. The alpha 1 (IX) collagen gene gives rise to two different transcripts in both mouse embryonic and human fetal RNA. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2400–2404. doi: 10.1073/pnas.87.7.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y., Olsen B. R. Synthesis and characterization of cDNA encoding a cartilage-specific short collagen. Proc Natl Acad Sci U S A. 1984 May;81(10):3014–3018. doi: 10.1073/pnas.81.10.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura I., Muragaki Y., Olsen B. R. Tissue-specific forms of type IX collagen-proteoglycan arise from the use of two widely separated promoters. J Biol Chem. 1989 Nov 25;264(33):20033–20041. [PubMed] [Google Scholar]
- O'Hare K., Benoist C., Breathnach R. Transformation of mouse fibroblasts to methotrexate resistance by a recombinant plasmid expressing a prokaryotic dihydrofolate reductase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1527–1531. doi: 10.1073/pnas.78.3.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J Biol Chem. 1990 Sep 15;265(26):15349–15352. [PubMed] [Google Scholar]
- Roth V., Mow V. C. The intrinsic tensile behavior of the matrix of bovine articular cartilage and its variation with age. J Bone Joint Surg Am. 1980 Oct;62(7):1102–1117. [PubMed] [Google Scholar]
- Steinmann B., Rao V. H., Vogel A., Bruckner P., Gitzelmann R., Byers P. H. Cysteine in the triple-helical domain of one allelic product of the alpha 1(I) gene of type I collagen produces a lethal form of osteogenesis imperfecta. J Biol Chem. 1984 Sep 10;259(17):11129–11138. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg P., Khillan J. S., Prockop D. J., Helminen H., Kontusaari S., Ala-Kokko L. Expression of a partially deleted gene of human type II procollagen (COL2A1) in transgenic mice produces a chondrodysplasia. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7640–7644. doi: 10.1073/pnas.88.17.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasios G., Nishimura I., Konomi H., van der Rest M., Ninomiya Y., Olsen B. R. Cartilage type IX collagen-proteoglycan contains a large amino-terminal globular domain encoded by multiple exons. J Biol Chem. 1988 Feb 15;263(5):2324–2329. [PubMed] [Google Scholar]
- Vaughan L., Mendler M., Huber S., Bruckner P., Winterhalter K. H., Irwin M. I., Mayne R. D-periodic distribution of collagen type IX along cartilage fibrils. J Cell Biol. 1988 Mar;106(3):991–997. doi: 10.1083/jcb.106.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Godfrey M., Hollister D. W. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989 Nov 5;264(31):18265–18267. [PubMed] [Google Scholar]
- Wright D. W., Mayne R. Vitreous humor of chicken contains two fibrillar systems: an analysis of their structure. J Ultrastruct Mol Struct Res. 1988 Sep;100(3):224–234. doi: 10.1016/0889-1605(88)90039-0. [DOI] [PubMed] [Google Scholar]
- Yada T., Suzuki S., Kobayashi K., Kobayashi M., Hoshino T., Horie K., Kimata K. Occurrence in chick embryo vitreous humor of a type IX collagen proteoglycan with an extraordinarily large chondroitin sulfate chain and short alpha 1 polypeptide. J Biol Chem. 1990 Apr 25;265(12):6992–6999. [PubMed] [Google Scholar]
- van der Rest M., Mayne R., Ninomiya Y., Seidah N. G., Chretien M., Olsen B. R. The structure of type IX collagen. J Biol Chem. 1985 Jan 10;260(1):220–225. [PubMed] [Google Scholar]
- van der Rest M., Mayne R. Type IX collagen proteoglycan from cartilage is covalently cross-linked to type II collagen. J Biol Chem. 1988 Feb 5;263(4):1615–1618. [PubMed] [Google Scholar]