Abstract
IMPORTANCE
Cortical lesions (CLs) contribute to physical and cognitive disability in multiple sclerosis (MS). Accurate methods for visualization of CLs are necessary for future clinical studies and therapeutic trials in MS.
OBJECTIVE
To evaluate the clinical relevance of measures of CL burden derived from high-field magnetic resonance imaging (MRI) in MS.
DESIGN, SETTING, AND PARTICIPANTS
An observational clinical imaging study was conducted at an academic MS center. Participants included 36 individuals with MS (30 relapsing-remitting, 6 secondary or primary progressive) and 15 healthy individuals serving as controls. The study was conducted from March 10, 2010, to November 23, 2012, and analysis was performed from June 1, 2011, to September 30, 2014. Seven-Tesla MRI of the brain was performed with 0.5-mm isotropic resolution magnetization-prepared rapid acquisition gradient echo (MPRAGE) and whole-brain, 3-dimensional, 1.0-mm isotropic resolution magnetization–prepared, fluid-attenuated inversion recovery (MPFLAIR). Cortical lesions, seen as hypointensities on MPRAGE, were manually segmented. Lesions were classified as leukocortical, intracortical, or subpial. Images were segmented using the Lesion-TOADS (Topology-Preserving Anatomical Segmentation) algorithm, and brain structure volumes and white matter (WM) lesion volume were reported. Volumes were normalized to intracranial volume.
MAIN OUTCOMES AND MEASURES
Physical disability was measured by the Expanded Disability Status Scale (EDSS). Cognitive disability was measured with the Minimal Assessment of Cognitive Function in MS battery.
RESULTS
Cortical lesions were noted in 35 of 36 participants (97%), with a median of 16 lesions per participant (range, 0-99). Leukocortical lesion volume correlated with WM lesion volume (ρ = 0.50; P = .003) but not with cortical volume; subpial lesion volume inversely correlated with cortical volume (ρ = −0.36; P = .04) but not with WM lesion volume. Total CL count and volume, measured as median (range), were significantly increased in participants with EDSS scores of 5.0 or more vs those with scores less than 5.0 (count: 29 [11-99] vs 13 [0-51]; volume: 2.81 × 10−4 [1.30 × 10−4 to 7.90 × 10−4] vs 1.50 × 10−4 [0 to 1.01 × 10−3]) and in cognitively impaired vs unim-paired individuals (count: 21 [0-99] vs 13 [1-54]; volume: 3.51 × 10−4 [0 to 1.01 × 10−4] vs 1.19 × 10−4 [0 to 7.17 × 10−4]). Cortical lesion volume correlated with EDSS scores more robustly than did WM lesion volume (ρ = 0.59 vs 0.36). Increasing log[CL volume] conferred a 3-fold increase in the odds of cognitive impairment (odds ratio [OR], 3.36; 95% CI, 1.07-10.59; P = .04) after adjustment for age and sex and a 14-fold increase in odds after adjustment for WM lesion volume and atrophy (OR, 14.26; 95% CI, 1.06-192.37; P = .045). Leukocortical lesions had the greatest effect on cognition (OR for log [leukocortical lesion volume], 9.65; 95% CI, 1.70-54.59, P = .01).
CONCLUSIONS AND RELEVANCE
This study provides in vivo evidence that CLs are associated with cognitive and physical disability in MS and that leukocortical and subpial lesion subtypes have differing clinical relevance. Quantitative assessments of CL burden on high-field MRI may further our understanding of the development of disability and progression in MS and lead to more effective treatments.
Cortical demyelination was described in the earliest pathologic studies1,2 of multiple sclerosis (MS). In addition to lesions occurring in the white matter (WM), autopsy studies3,4 consistently demonstrate considerable cortical pathology, including cortical atrophy, discrete cortical lesions (CLs), and diffuse subpial (SP) demyelination. Despite knowledge of the existence of cortical pathology in MS, in vivo visualization remains technically challenging.
Novel acquisition techniques, such as double-inversion recovery and phase-sensitive inversion recovery, have been used in studies of cortical pathology in MS.5,6 Unfortunately, the resolution of double-inversion recovery is limited by a poor signal to noise ratio, allowing for misclassification of juxtacortical WM lesions and small cortical vessels as CLs.7,8 Although the resolution of phase-sensitive inversion recovery leads to more accurate lesion classification, it may not adequately visualize SPCLs.6
Another approach to delineate CLs in MS is to take advantage of the improved signal to noise ratio possible at higher magnetic fields, such as 7 T.9 Although standard methods for CL identification on 7-T magnetic resonance imaging (MRI) have yet to be agreed on, several studies8,10 have demonstrated superior detection of both CLs and WM lesions on 7-T MRI compared with lower fields.
Accurate visualization and quantification of CLs are necessary in MS research, since cortical pathology contributes to greater levels of physical and cognitive disability.11-14 Although there are suggestions that MS immunomodulatory therapies may prevent cortical pathology,15 this hypothesis will remain unclear until protocols for accurate imaging of CLs are validated. In this study, we aimed to visualize CLs in MS on 7-T MRI and to evaluate the ability of quantified CL burden (ie, lesion count, volume, or both) to help explain physical disability, cognitive impairment, and a progressive phenotype.
Methods
Standard Protocol Approvals and Patient Consent
Protocols were approved by the institutional review boards at the Johns Hopkins University School of Medicine and the Kennedy Krieger Institute. Written informed consent was obtained from all participants. The healthy volunteers serving as controls received financial compensation. The study was conducted from March 10, 2010, to November 23, 2012, and analysis was performed from June 1, 2011, to September 30, 2014.
Participants
Volunteers with diagnoses of relapsing-remitting, secondary progressive, and primary progressive MS were recruited from the Johns Hopkins Multiple Sclerosis Center. Exclusion criteria were an MS relapse in the prior 30 days or symptoms of a major depressive episode. Age and educational level–matched healthy volunteers serving as controls were also studied.
MRI Protocol and Image Analysis
Magnetic resonance imaging was performed on a 7-T scanner (Achieva; Philips) with a volume transmit/32-channel receive head coil (Nova Medical Products) and with dielectric padding. Whole-brain, 3-dimensional (3-D), magnetization-prepared rapid acquisition of gradient echoes (MPRAGE) was acquired with 0.5-mm isotropic resolution (repetition time, 5.2 milliseconds; delay time, 4500 milliseconds; echo time, 2.3, milliseconds, flip angle, 7°; parallel imaging factor, 2.5 [anterior-posterior] × 2 [right-left] for 13 minutes, 12 seconds). This sequence was chosen given data16 indicating the advantages of MPRAGE over double-inversion recovery and phase-sensitive inversion recovery for CL identification. Whole-brain, 3-D, magnetization-prepared, fluid-attenuated inversion recovery (MPFLAIR)17 was acquired with 1.0-mm isotropic resolution (repetition time, 8107 milliseconds, inversion time, 2175 milliseconds; echo time, 293 milliseconds; flip angle, 90°; turbo spin echo factor, 115; parallel imaging factor 2 [anterior-posterior] × 3 [right-left] for 8 minutes, 14 seconds).
Images were processed in MIPAV, version 5.3 (http://mipav.cit.nih.gov). The MPRAGE images were smoothed with an anisotropic diffusion filter, and the MPFLAIR was rigidly registered to the MPRAGE (Figure 1A and B). Linked image sections were viewed and lesions were manually demarcated by a neurologist (D.M.H.) blinded to participants’ identity and diagnostic category, with oversight from a trained neuroradiologist (I.I.). Methodologic reliability was assessed by having a second rater (J.O.) identify CLs on 10 sample cases (inter-rater), and both raters reviewed 2 cases twice (intrarater). Given the demonstrated advantages of MPRAGE over T2-weighted imaging for CL identification at high field,18 CLs were primarily identified on MPRAGE, with MPFLAIR used for additional visual guidance. Similar to the identification criteria of Sinnecker et al,18 CLs were required to be a minimum of 15% hypointense on gray scale measurement relative to the adjacent normal-appearing cortex. Hypointensities with a linear or tubular appearance or less than 1.0 mm in width were rejected to eliminate cortical blood vessels. Semiautomated region-of-interest tools in MIPAV were used to draw borders around CLs and derive raw CL volumes.
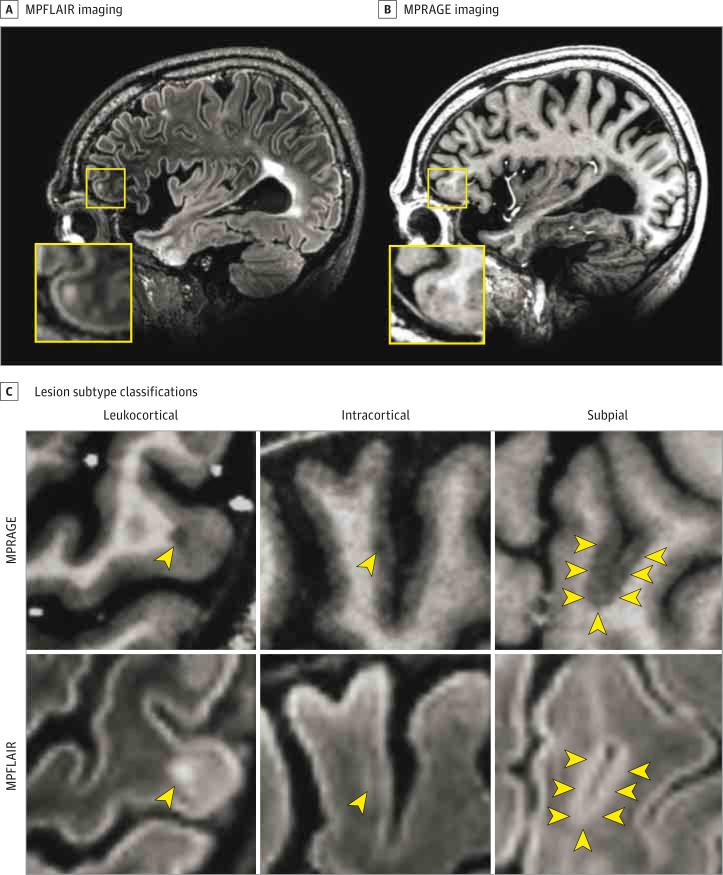
Figure 1. Identification and Classification of Cortical Lesions on 7-T Magnetic Resonance Imaging.
Sample images from the imaging protocol used in the present study. A and B, Coregistered image sections from magnetization-prepared, fluid-attenuated inversion recovery (MPFLAIR) and magnetization-prepared rapid acquisition of gradient echoes (MPRAGE) obtained from a participant with multiple sclerosis. The yellow boxes show an area of higher magnification, which is centered on an anterior frontal lobe leukocortical lesion seen on both MPFLAIR and MPRAGE. C, Samples of lesion subtype classification. Lesions are identified by yellow arrowheads. The top row shows lesions on MPRAGE and the bottom row shows the same lesion on MPFLAIR. Three lesion subtypes were identified: leukocortical (traversing white and gray matter), intracortical (located exclusively within cortex), and subpial (more widespread areas of cortical signal abnormality, arising at the subpial surface, usually in deep sulci and/or traversing multiple sulci or gyri).
Cortical lesions were divided into subtypes according to pathologic definitions (Figure 1C)4: (1) leukocortical (LC), borders traversing both WM and gray matter (GM); (2) intracortical (IC), located exclusively in GM; and (3) SP, widespread areas of signal abnormality extending inward from the pial surface, usually located in deep sulci.
The Lesion-TOADS (Topology-Preserving Anatomical Segmentation) algorithm19 was modified for 7-T images to obtain WM lesions and structural volumes. Images underwent nonparametric, nonuniform intensity normalization (generation N4) inhomogeneity correction20 and skull stripping before segmentation. Segmentation errors were corrected manually. Raw volumes were normalized to intracranial volume, and brain parenchymal fraction (BPF) was calculated as total brain volume divided by intracranial volume.
A CL subtype ratio was calculated as (LC lesion count – IC lesion count)/SP lesion count, as described by Nielsen et al.21 A CL subtype ratio was similarly calculated for lesion volumes. A novel metric, termed CL–WM lesion burden ratio, was calculated as total CL volume/total WM lesion volume.
Disability Measures
Neurologic examinations were performed to determine Expanded Disability Status Scale (EDSS) scores, which were split into tertiles of severity. Participants were stratified into a highly disabled group if in the highest tertile (EDSS score, ≥5.0) or low/moderately disabled group if in the lower 2 ter-tiles (EDSS score, <5.0). The Timed 25-Foot Walk, 9-Hole Peg Test, and 3-second delay Paced Auditory Serial Addition Test were administered to determine the MS Functional Composite score, using the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force data set for normalization.22,23
The Minimal Assessment of Cognitive Function in MS (MACFIMS) neuropsychological battery was used to assess cognitive function.24 The components of this battery are listed in Table 1. Methods used for quantitative analyses of individual tests scores as well as dichotomization of participants into those who were cognitively impaired vs cognitively intact were performed according to the recommendations of the MACFIMS consensus panel.24
Table 1.
Demographics and Disability Scores
| Characteristic | Mean (SD) | |||
|---|---|---|---|---|
| HV (n = 15) | All MS (n = 36) | RRMS (n = 30) | SPMS/PPMS (n = 6) | |
| Age, y | 40.9 (9.8) | 42.6 (10.0) | 41.8 (10.1) | 46.3 (9.2) |
| Female sex, No. (%) | 10 (67) | 20 (56) | 18 (60) | 2 (33) |
| Years of education | 17.9 (3.2) | 16.7 (2.2) | 16.7 (2.0) | 16.3 (3.0) |
| Disease duration, y | NA | 9.8 (7.5) | 9.1 (7.0) | 13.2 (9.6) |
| MS treatment, No. (%) | NA | 28 (78) | 26 (87) | 2 (33)a |
| BDI-FS score | 4.7 (6.0) | 9.2 (9.9) | 9.2 (10.6) | 9.2 (6.0) |
| MFIS score | 11.7 (11.0) | 35.5 (23.1)b | 34.3 (23.7) | 41.5 (20.4) |
| AMNART score | 34.8 (6.5) | 36.6 (5.5) | 36.4 (5.6) | 37.5 (5.4) |
| EDSS score, median (range) | NA | 3 (1 to 6.5) | 2.5 (1 to 6) | 5.5 (2 to 6.5)a |
| MSSS score | NA | 4.7 (2.3) | 4.4 (2.4) | 6.1 (1.2) |
| MSFC z score | 0.77 (0.41) | 0.12 (0.60)b | 0.24 (0.44) | –0.51 (0.89)a |
| 9-Hole Peg Test time, s | ||||
| Dominant hand | 17.4 (2.1) | 24.2 (12.2)b | 22.0 (5.4) | 35.2 (26.4)a |
| Nondominant hand | 18.4 (2.5) | 25.0 (7.8)b | 24.4 (7.7) | 28.3 (8.0) |
| Timed 25-Foot Walk, s | 3.8 (0.5) | 6.4 (4.9)b | 5.4 (2.6) | 11.4 (9.7)a |
| PASAT-3, No. correctc | 51.2 (7.9) | 46.6 (10.7) | 47.0 (10.6) | 44.3 (12.8) |
| COWAT scorec | 39.3 (10.3) | 38.2 (12.6) | 38.4 (11.3) | 37.5 (19.0) |
| JLO, No. correctc | 24.9 (4.3) | 24.7 (4.6) | 25.5 (3.9) | 21.0 (6.4)a |
| CVLTc | ||||
| Total learning score | 56.5 (7.3) | 50.9 (10.8) | 52.3 (9.4) | 43.8 (15.1) |
| Delayed recall score | 13.4 (2.1) | 10.3 (3.8)b | 10.7 (3.8) | 8.7 (3.4) |
| BVMTc | ||||
| Total recall score | 26.0 (7.4) | 22.6 (6.5) | 23.3 (5.8) | 19.0 (9.1) |
| Delayed recall score | 9.5 (2.6) | 8.3 (2.6) | 8.5 (2.5) | 7.5 (3.3) |
| SDMT, total correctc | 63.0 (10.0) | 50.8 (11.2)b | 52.0 (10.2) | 44.8 (15.1) |
| DKEFSc | ||||
| No. of sorts | 10.4 (1.5) | 9.9 (2.1) | 10.0 (2.1) | 9.5 (2.1) |
| Description score | 40.0 (6.8) | 38.8 (8.5) | 39.1 (8.6) | 36.8 (8.2) |
| MACFIMS-defined cognitive impairment, No. (%) | 4(27) | 19 (53) | 15 (50) | 4 (67) |
Abbreviations: AMNART, American National Adult Reading Test (test of premorbid intelligence/reading ability); BDI-FS, Beck Depression Inventory–Fast Screen; BVMT, Brief Visuospatial Memory Test; COWAT, Controlled Oral Word Association Test; CVLT, California Verbal Learning Test; DKEFS, Delis-Kaplan Executive Function System; EDSS, Expanded Disability Status Scale; HV, healthy volunteer; JLO, Judgment of Line Orientation; MACFIMS, Minimal Assessment of Cognitive Function in MS; MFIS, Modified Fatigue Impact Scale; MS, multiple sclerosis; MSFC, Multiple Sclerosis Functional Composite; MSSS, Multiple Sclerosis Severity Score; NA, not applicable; PASAT-3, Paced Auditory Serial Addition Test–3-second delay; PPMS, primary progressive multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SDMT, Symbol Digit Modalities Test; SPMS, secondary progressive multiple sclerosis.
P < .05 for difference from healthy volunteers.
P < .05 for difference from RRMS.
MACFIMS Component Test.
Statistical Analysis
Group differences were assessed by Wilcoxon rank sum test and correlations by Spearman rank correlation testing. Healthy volunteer data were included only in group comparisons as indicated in the tables and figures and were not included in correlations with disability. Logistic regression was used to test whether MRI values predicted dichotomized clinical outcomes. A multiple logistic regression model was used with age and sex as covariates. To aid with interpretability, normalized lesion volumes were log-transformed, and ratios, such as BPF, were converted to a percent value. This process resulted in odds ratios (ORs) representing the increase in odds for each percentage point increase in the MRI quantity for ratios and natural log base (standard statistical constant e, approximately 2.718) increase for lesion volumes. Significant predictors were assessed for independence from the effects of atrophy and WM lesions in a multivariate model including cortical GM volume, WM volume, WM lesion volume, age, and sex. Lin's25 concordance coefficient was used to assess interrater and intrarater reliability. Statistical analysis was performed in Stata, version 10.1 IC (StataCorp).
Results
Thirty-six participants with MS (30 relapsing-remitting, 6 secondary or primary progressive) and 15 healthy volunteers were recruited. There were no significant differences between those with MS and the control participants for age, years of education, or premorbid intelligence (Table 1). Nineteen (53%) of the participants with MS met the MACFIMS definition of cognitive impairment. Those with MS fared poorly on most MACFIMS component tests, with significantly worse scores on the Symbol Digit Modalities Test and the delayed recall portion of the California Verbal Learning Test.
Cortical lesions were found in 35 of 36 participants with MS (97%), with a median of 16 lesions per participant (range, 0-99). Almost no CLs were seen in the controls, indicating that the identified CLs were not due to image artifact. Interrater reliability was modest (eTable 1 in the Supplement) but similar to that in previously reported 7-T methods and far superior to prior 3-T double-inversion recovery studies.8 Intrarater concordance was excellent (concordance coefficient, 0.96). Intra-cortical lesion count was significantly increased in individuals with secondary progressive and primary progressive MS, and there were nonsignificant trends toward greater CL count and volume in individuals with secondary progressive and primary progressive MS for total CLs, LC and SP lesion subtypes, and the CL subtype ratio for volume (Table 2).
Table 2.
Cortical Lesion Burden Stratified by Clinical Categories
| Clinical Phenotype, Median (Range) |
Disability Severity, Median (Range) |
Cognitive Statusa |
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | HV (n = 15) | All MS (n = 36) | RRMS (n = 30) | SPMS/PPMS (n = 6) | EDSS Score <5.0 (n = 27) | EDSS Score ≥5.0 (n = 9) | Intact (n = 17) | Impaired (n = 19) |
| Total cortical | ||||||||
| Lesion count | 0 (0 to 11) | 16 (0 to 99)b | 14.5 (0 to 54) | 31.5 (11 to 99) | 13 (0 to 51) | 29 (11 to 99)b | 13 (1 to 54) | 21 (0 to 99) |
| Lesion volumec | 0 (0 to 6.57 × 10–5) | 1.85 × 10–4 (0 to 1.01 × 10–3)b | 1.66 × 10–4 (0 to 1.01 × 10–3) | 2.72 × 10–4 (1.19 × 10–4 to 7.54 × 10–4) | 1.50 × 10–4 (0 to 1.01 × 10–3) | 2.81 × 10–4 (1.30 × 10–4 to 7.90 × 10–4)b | 1.19 × 10–4 (0 to 7.17 × 10–4) | 3.51 × 10–4 (0 to 1.01 × 10–4)b |
| Leukocortical | ||||||||
| Lesion count | 0 (0 to 6)b | 9 (0 to 38) | 8 (0 to 38) | 18 (6 to 30) | 8 (0 to 38) | 15 (8 to 30) | 6 (0 to 30) | 15 (0 to 38)b |
| Lesion volumec | 0 (0 to 5.29 × 10–5) | 9.62 × 10–5 (0 to 6.43 × 10–4)b | 7.60 × 10–5 (0 to 6.43 × 10–4) | 1.46 × 10–4 (5.20 × 10–5 to 4.65 × 10–4) | 7.46 × 10–5 (0 to 6.43 × 10–4) | 1.18 × 10–4 (4.70 × 10–5 to 4.65 × 10–4) | 4.57 × 10–5 (0 to 2.43 × 10–4) | 1.75 × 10–4 (0 to 6.43 × 10–4)b |
| Intracortical | ||||||||
| Lesion count | 0 (0 to 5) | 3 (0 to 39)b | 2 (0 to 18) | 6 (2 to 39)d | 3 (0 to 18) | 3 (2 to 39) | 3 (0 to 16) | 2 (0 to 39) |
| Lesion volumec | 0 (0 to 1.28 × 10–5) | 7.54 × 10–6 (0 to 8.91 × 10–5)b | 6.87 × 10–6 (0 to 8.34 × 10–5) | 1.63 × 10–5 (3.75 × 10–6 to 8.91 × 10–5) | 7.09 × 10–6 (0 to 3.84 × 10–5) | 1.37 × 10–4 (3.45 × 10–6 to 8.91 × 10–5) | 7.09 × 10–6 (0 to 8.34 × 10–5) | 8.00 × 10–6 (0 to 8.91 × 10–5) |
| Subpial | ||||||||
| Lesion count | 0 (0 to 1) | 3 (0 to 30)b | 3 (0 to 30) | 6 (2 to 30) | 3 (0 to 19) | 9 (0 to 30)b | 3 (0 to 30) | 3 (0 to 30) |
| Lesion volumec | 0 (0 to 2.71 × 10–5) | 7.92 × 10–5 (0 to 5.46 × 10–4)b | 7.01 × 10–5 (0 to 5.46 × 10–4) | 1.20 × 10–4 (3.27 × 10–5 to 5.05 × 10–4) | 6.35 × 10–5 (0 to 5.05 × 10–4) | 1.59 × 10–4 (0 to 5.46 × 10–4) | 9.54 × 10–5 (0 to 5.46 × 10–4) | 7.66 × 10–5 (0 to 5.05 × 10–4) |
| BPF | 0.78 (0.73 to 0.82) | 0.76 (0.65 to 0.83) | 0.76 (0.65 to 0.83) | 0.74 (0.67 to 0.80) | 0.78 (0.66 to 0.83) | 0.74 (0.65 to 0.79)b | 0.79 (0.65 to 0.82) | 0.76 (0.66 to 0.83) |
| Volumec | ||||||||
| WM | 0.33 (0.29 to 0.36) | 0.35 (0.21 to 0.41)b | 0.35 (0.21 to 0.41) | 0.34 (0.24 to 0.40) | 0.35 (0.21 to 0.41) | 0.34 (0.24 to 0.38) | 0.37 (0.30 to 0.40) | 0.34 (0.21 to 0.41) |
| Cortical GM | 0.32 (0.30 to 0.35) | 0.29 (0.23 to 0.37)b | 0.29 (0.23 to 0.36) | 0.28 (0.27 to 0.37) | 0.29 (0.24 to 0.36) | 0.28 (0.23 to 0.37) | 0.29 (0.23 to 0.34) | 0.29 (0.24 to 0.37) |
| WM lesion | 0 (0 to 6.87 × 10–3) | 8.97 × 10–3 (6.96 × 10–4 to 0.02)b | 8.97 × 10–3 (6.96 × 10–4 to 0.02) | 4.16 × 10–3 (1.16 × 10–3 to 0.02) | 8.39 × 10–3 (6.96 × 10–4 to 1.79 × 10–2) | 9.76 × 10–3 (4.16 × 10–3 to 2.37 × 10–2) | 7.80 × 10–3 (1.16 × 10–3 to 1.64 × 10–2) | 9.53 × 10–3 (6.96 × 10–4 to 2.37 × 10–2) |
| Cortical lesion subtype ratio | ||||||||
| Count | NA | 3.38 (0.77 to 14.00) | 3.38 (0.77 to 14.00) | 3.40 (1.26 to 10.33) | 4.18 (1.14 to 14.00) | 2.26 (0.77 to 10.33) | 2.30 (0.80 to 14.00) | 4.25 (0.77 to 10.33) |
| Volumec | NA | 0.89 (0.15 to 47.8) | 0.86 (0.15 to 47.84) | 1.26 (0.38 to 7.04) | 0.85 (0.15 to 47.84) | 0.92 (0.31 to 7.04) | 0.59 (0.15 to 9.28) | 1.64 (0.38 to 47.84)b |
| Cortical lesion to WM lesion burden ratio | NA | 0.03 (0 to 0.26) | 0.03 (0 to 0.12) | 0.05 (0.02 to 0.26) | 0.03 (0 to 0.26) | 0.05 (0.01 to 0.12) | 0.03 (0 to 0.12) | 0.03 (0 to 0.26) |
Abbreviations: BPF, brain parenchymal fraction; EDSS, Expanded Disability Status Scale; GM, gray matter; HV, healthy volunteer; MS, multiple sclerosis; NA, not applicable; PPMS, primary progressive MS; RRMS, relapsing-remitting MS; SPMS, secondary progressive MS; WM, white matter.
Cognitive impairment was defined as per recommendations of the Minimal Assessment of Cognitive Function in MS consensus panel. Group differences were tested by the Wilcoxon rank sum test.
P < .05 for difference from HV.
All volumes were normalized to intracranial volume and therefore are expressed as ratios (thus, without units).
P < .05 for difference from RRMS.
The count and volume of total CLs and each subtype significantly correlated with disability as measured by the EDSS score (Table 3). The magnitude of correlation between EDSS scores and the degree of cortical involvement was similar to that for EDSS and BPF (ρ = −0.64; P < .001) and twice the magnitude of the EDSS score and WM lesion volume (ρ = 0.36; P = .04). Cortical lesion measures and BPF both correlated with the MSFC results, whereas WM lesion volume did not. Total CL volume correlated with most MACFIMS cognitive test scores, which was driven by LC lesion volume for all except the Judgment of Line Orientation test, which was driven by a correlation with SP lesion count.
Table 3.
Correlation of Magnetic Resonance Imaging Indices With Disability Measuresa
| Cortical Lesion Count |
Cortical Lesion Volume |
Volume |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total | LC | IC | SP | Total | LC | IC | SP | BPF | WM | Cortical GM | WM Lesion |
| EDSS | 0.63b | 0.65b | 0.44b | 0.44b | 0.59b | 0.63b | 0.41c | 0.34c | –0.64b | –0.51b | –0.13 | 0.36c |
| MSFC z score | –0.34c | –0.43b | –0.19 | –0.17 | –0.29 | –0.35c | –0.18 | –0.05 | 0.49b | 0.55b | –0.04 | –0.14 |
| 9-Hole Peg Test time | ||||||||||||
| Dominant hand | 0.40c | 0.36c | 0.38c | 0.27 | 0.36c | 0.31 | 0.40c | 0.15 | –0.46b | –0.37c | –0.18 | 0.25 |
| Nondominant hand | 0.56b | 0.58c | 0.41c | 0.41c | 0.59b | 0.59b | 0.36c | 0.26 | –0.41c | –0.32 | –0.17 | 0.32 |
| Timed 25-Foot Walk | 0.28 | 0.33 | 0.25 | 0.10 | 0.22 | 0.29 | 0.30 | 0.08 | –0.42c | –0.40c | –0.06 | 0.19 |
| PASAT-3 | –0.03 | –0.13 | 0.00 | 0.10 | 0.00 | –0.099 | –0.05 | 0.10 | 0.27 | 0.53b | –0.27 | 0.03 |
| COWAT | –0.34c | –0.32 | –0.24 | –0.17 | –0.20 | –0.30 | –0.19 | 0.04 | 0.35c | 0.50b | –0.10 | –0.27 |
| JLO | –0.29 | –0.28 | –0.19 | –0.27 | –0.35c | –0.31 | –0.25 | –0.38c | 0.11 | 0.12 | 0.07 | 0.02 |
| CVLT, mean | ||||||||||||
| Total Learning score | –0.28 | –0.37c | –0.19 | –0.09 | –0.34c | –0.46b | –0.16 | 0.05 | 0.43b | 0.47b | 0.06 | –0.22 |
| Delayed Recall score | –0.28 | –0.37c | –0.04 | –0.20 | –0.42c | –0.46b | –0.04 | –0.07 | 0.41c | 0.38 | 0.14 | –0.22 |
| BVMT, mean | ||||||||||||
| Total recall score | –0.25 | –0.44b | 0.51b | –0.06 | –0.40c | –0.51b | –0.13 | –0.12 | 0.36c | 0.34 | 0.03 | –0.23 |
| Delayed recall score | –0.29 | –0.48b | –0.16 | –0.08 | –0.44b | –0.56b | –0.18 | –0.14 | 0.29 | 0.26 | 0.05 | –0.27 |
| SDMT, total correct, mean | –0.33c | –0.48b | –0.23 | –0.09 | –0.35c | –0.50b | –0.28 | 0.02 | 0.58b | 0.58b | –0.06 | –0.55b |
| DKEFS, mean | ||||||||||||
| No. of sorts | –0.24 | –0.42c | 0.01 | –0.09 | –0.34c | –0.47c | 0.02 | 0.04 | 0.28 | 0.22 | 0.06 | –0.27 |
| Description score | –0.18 | –0.38c | 0.04 | –0.02 | –0.26 | –0.42c | 0.04 | 0.13 | 0.33 | 0.29 | 0.05 | –0.31 |
Abbreviations: BPF, brain parenchymal fraction; BVMT, Brief Visuospatial Memory Test; COWAT, Controlled Oral Word Association Test; CVLT, California Verbal Learning Test; DKEFS, Delis-Kaplan Executive Function System; GM, gray matter; IC, intracortical; JLO, Judgment of Line Orientation; LC, leukocortical; MSFC, Multiple Sclerosis Functional Composite; PASAT-3, Paced Auditory Serial Addition Test–3-second delay; SDMT, Symbol Digit Modalities Test; SP, subpial; WM, white matter.
Spearman rank correlation was determined between variables; ρ values are shown.
P < .01.
P < .05.
Participants with more severe disability (EDSS score, ≥5.0) had approximately twice the CL burden of those with less disability (EDSSS score, <5.0) (Table 2). Subpial lesion count was higher in those with EDSS scores of 5.0 or more (median, 9; range, 0-30) compared with EDSS scores of less than 5.0 (median, 3; range, 0-19) (P = .03). The remainder of the CL sub-types had nonsignificant trends toward greater severity in participants with EDSS scores of 5.0 or more. Although BPF was lower in individuals with EDSS scores of 5.0 or more, no significant differences based on disability stratification were seen for WM volume, cortical GM volume, and WM lesion volume, or for any of the CL subtype ratios or CL-WM lesion ratio.
Leukocortical lesion count and total CL and LC lesion volumes were greater in participants with cognitive impairment compared with those without cognitive impairment (Table 2). The CL subtype ratio for volume was also increased in participants with cognitive impairment (median, 1.64; range, 0.38-47.84) compared with those with normal cognition (median, 0.59; range, 0.15-9.28) (P = .02).
Logistic regression for prediction of clinical outcomes by MRI values, adjusted for age and sex, found no significant relationship between MRI outcomes and EDSS score or progressive phenotype (eTable 2 in the Supplement). However, the odds of cognitive impairment were increased by more than 3-fold and for each natural log increase (approximately double) in CL volume (OR, 3.36; 95% CI, 1.07-10.59; P = .04) and more than 9-fold for approximate doubling of LC lesion volume (OR for log[leukocortical lesion volume], 9.65; 95% CI, 1.70-54.59; P = .01). An increase in the CL subtype ratio for volume was also predictive of cognitive impairment (0.048). Cortical lesion volume and LC lesion volume were also found to be independent predictors of cognitive impairment in a multivariate model that included cortical GM volume, WM volume, WM lesion volume, age, and sex (eTable 3 in the Supplement). In this multivariate model, an approximate doubling of the CL volume resulted in a 14.26 (95% CI, 1.06-192.37; P = .045) increase in the odds of cognitive impairment, and an approximate doubling of LC lesion volume resulted in a 40.96 (95% CI, 1.26-1369.23; P = .04) increase in the odds of cognitive impairment. Evaluation of individual variables in the multivariate model found that the increased odds were primarily driven by the addition of cortical GM volume to the model.
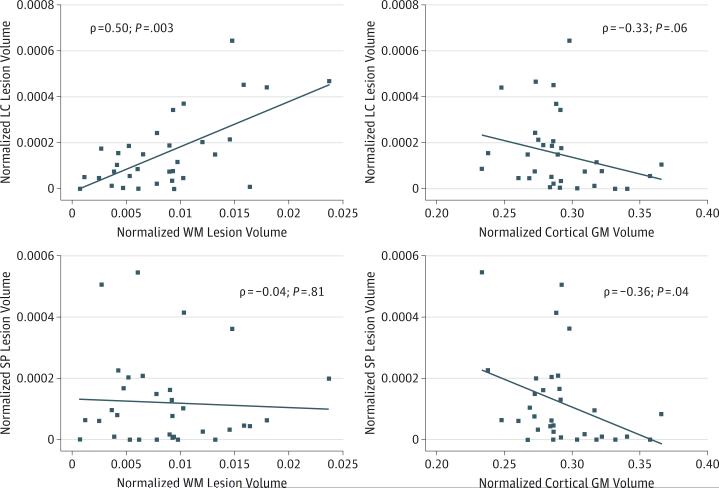
The association between CL volume and standard MRI outcomes was also investigated (Figure 2 and eTable 4 in the Supplement). Total CL volume and LC and IC lesion volume correlated with BPF, but SP lesion volume did not. The only CL subtype that correlated with WM lesion volume was LC lesions (ρ = 0.50; P = .003). The only CL subtype that correlated with cortical GM volume was SP lesions (ρ = −0.36; P = .04).
Figure 2. Correlation of Leukocortical (LC) and Subpial (SP) Cortical Lesions With Cortical Gray Matter (GM) Volume and White Matter (WM) Lesion Volume.
Correlations were tested using Spearman rank correlation testing, with ρ values (magnitude of correlation) and P values shown. The diagonal lines indicate line of best fit through data points. Volumes are normalized to intracranial volume, and are thus unitless.
Discussion
In this study, we found CLs to be associated with disability scores, a progressive phenotype, and cognitive impairment. These findings are in keeping with previous imaging and histopathology studies in MS.11,13,14,26,27 Our finding that CL volume predicts cognitive impairment independent of WM lesion volume or atrophy supports the notion that assessments of inflammatory WM pathology alone provide an insufficient appraisal of the pathology responsible for disability in MS.28 Given the established low sensitivity of 1.5-T and 3-T MRI techniques for identification of CLs,8,10 consideration should be given to integration of high-field MRI into future MS research and clinical trials. Although the availability of 7-T MRI at present limits this approach, our data show that it is feasible to perform clinical-quality, whole-brain imaging in reasonable scan times and to quantify clinically relevant WM and GM pathology.
Although associations were seen in this study between CLs and disability scores and a progressive phenotype, we found that these associations were not independent of age and sex. Since the age of an individual with MS has an established association with disability scores (also seen in our data) and the likelihood of progression,29 this finding is likely indicative of the coupling of the accumulation of CLs to the general long-term disease processes of MS. The longitudinal accumulation of CLs and its association with disability have been demonstrated at lower field strengths5,14 but have yet to be confirmed with high-field imaging.
The association between cognitive impairment and CL volume was independent of age and sex in this cohort. This striking finding may indicate that disproportionate or earlier CL accumulation may be a predisposing factor for cognitive impairment.30 The association between cognitive impairment and CL volume was also independent of atrophy and WM lesion volume, which may explain why some patients with MS can exhibit apparent disease stability with conventional MRI while continuing to accrue the “silent” symptoms of MS (eg, cognitive deficits, fatigue). Although the odds of cognitive impairment predicted by CL volume in our multivariate model was high, this estimate may be inflated by the contribution of cortical GM atrophy, which is a risk factor for cognitive impairment,11 vs false inflation owing to widening confidence intervals.
This study also furthers our understanding of the differing clinical impact and pathologic sources of CL subtypes. We found that SP lesion volume correlated with cortical GM atrophy but not with WM lesion volume. On the other hand, LC lesions strongly correlated with WM lesions without a significant correlation with cortical GM atrophy. This finding implies that LC lesions may be triggered by the same processes as WM lesions, whereas SP demyelination is not. Our in vivo findings coincide with pathologic evidence for a link between diffuse, SP cortical demyelination and local meningeal inflammation, as well as atrophic and degenerative processes in both the cortex and normal-appearing WM, all of which is independent of WM lesion burden.26,27,31
Of the 3 CL subtypes, LC lesions were most clinically relevant, potentially indicating a pathologic difference between these lesions and other CL subtypes. The LC lesions are more likely to show histopathologic signs of active or chronic inflammation and have a high density of transected cortical neurites, whereas nearly one-half of SP lesions appear to be chronically inactive, and the overall density of transected neurites is less than other CL subtypes.4 Substantial glial cell loss and reduction in synaptic density in LC lesions has also been described.32 The involvement of both GM and WM in LC lesions may also explain their relationship to cognitive impairment, not only resulting in local cortical GM damage but also causing interruption of critical cortical-cortical and subcortical pathways, both of which are linked with cognitive impairment in MS.11,14,33,34
The weaker correlation of SP lesions and disability seen in the present study could also be the result of poor visualization of SP demyelination on MPRAGE. With the use of a 7-T fast low-angle shot (FLASH-T2*) imaging technique, a recent study at the Beth Israel Deaconess MS center21 had comparable findings for LC lesions but found a greater proportion of SP lesions and significant correlations between SP lesions and disability. Direct comparisons with the present study are difficult, as their imaging protocol used a 2-D acquisition with prolonged scanning of 2 designated brain slabs at fine resolution, and we used a 3-D acquisition at lower resolution across the entire brain. The difference in SP lesion identification could thus be biased by the brain location chosen for analysis by the Beth Israel Deaconess study, differences in resolution, or an advantage of the FLASH-T2* imaging technique over MPRAGE for identification of SP lesions. The poor interrater reliability of SP lesion identification in our study (eTable 1 in the Supplement) may indicate the differences in the sensitivity of these techniques, although, to our knowledge, a similar analysis for independent raters by subtype has not been reported by other authors for their techniques. Although the 2-D approach used by the Beth Israel Deaconess study allows for greater in-plane resolution, section thickness is large and has implications for potential overcounting of individual SP lesions as multiple lesions since SP lesions can be amorphous and traverse large areas of cortex. Our 3-D acquisition allows for full brain coverage and for determination of the effect of the volume of CLs on disability, which, to our knowledge, have not been previously reported with high-field imaging.
There is currently no standard method for CL identification, especially at high-field imaging. Although retrospective review of 7-T T2*-weighted images after reviewing corresponding pathologic brain sections shows high sensitivity for identification of CLs, prospective CL identification using this sequence and others is still poor.35,36 In reality, 7-T FLASH-T2*, MPRAGE, MPFLAIR, and other sequence types are all viewing only the “tip of the iceberg,” with a large percentage of true cortical pathology being difficult to visualize.37 Although the improved signal to noise ratio of 7-T MRI allows for the resolution necessary to visualize smaller CLs, the smaller amount of inflammatory and gliotic change in CLs compared with WM lesions4,38 likely results in less profound signal change on MRI.
The data presented here are limited by a small sample size. Consequently, our comparisons between relapsing-remitting and progressive MS should be taken as preliminary. Adjustment of significance for multiple comparisons was not undertaken; therefore, the strength of the conclusions must be tempered in turn. However, a full accounting for tests performed as well as specific P values and levels of significance were included in our report to further the interpretability of the results with respect to multiplicity. Because the comparisons made were interrelated and the findings of this study are biologically plausible and internally consistent, the face validity of the results is high.39 In addition to a larger sample, future work should evaluate the long-term implications of these cross-sectional findings with longitudinal follow-up.
Conclusions
Despite limitations, our data support the use of 7-T MRI as a tool for quantification of cortical pathology in MS. Furthermore, our findings of CL volume as an independent predictor of cognitive impairment highlights the need to determine whether current disease-modifying drugs reduce CL formation. If this drug class is not effective in CL prevention, this line of research may spur the development of novel therapeutics capable of reducing CLs and their associated disability.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported in part by National Institutes of Health supplemental grants 5P41 RR15241-09S1 and 5P41EB15909 (Dr van Zijl) and Bayer Neurosciences (Dr Calabresi). Time for data analysis was supported by National Institutes of Health–mentored grant K23NS072366 (Dr Harrison).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Harrison had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Harrison, Calabresi. Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Harrison. Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Harrison, Oh, Caffo.
Obtained funding: Harrison, van Zijl, Calabresi. Administrative, technical, or material support: Harrison, Roy, Jones, van Zijl, Calabresi.
Study supervision: Izbudak, van Zijl, Calabresi.
Additional Contributions: Janelle Aquino, BA, Michaela Seigo, BA, Stephanie Syc, BA, and Anna Whetstone, BA (Department of Neurology, School of Medicine, The Johns Hopkins University), helped with participant recruitment and study visit logistics; they received no financial compensation. We are also grateful to the dedicated Kirby Center staff, who make studies of this type possible.
Conflict of Interest Disclosures: Dr Harrison has received research support from Bayer Schering Pharma and EMD Serono and has received consulting fees from EMD Serono, Genzyme, Mallinckrodt Pharmaceuticals, and MedImmune. Dr Oh has received personal compensation for consulting or speaking from Biogen-Idec, EMD Serono, Genzyme, and Novartis. She also has received an unrestricted research grant from Genzyme. Drs Jones and van Zijl receive research support from Philips Medical Systems. Dr Calabresi has received research funding from Bayer, Biogen Idec, MedImmune, Novartis, and Vertex and honoraria for consulting from Abbott, Merck, Prothena, Vaccinex, and Vertex. No other disclosures were reported.
Supplemental content at jamaneurology.com
REFERENCES
- 1.Dawson J. The histology of multiple sclerosis. Trans R Soc Edinb. 1916;50:517–740. [Google Scholar]
- 2.Brownell B, Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1962;25:315–320. doi: 10.1136/jnnp.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bø L, Vedeler CA, Nyland HI, Trapp BD, Mørk SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62(7):723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- 4.Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50(3):389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese M, Filippi M, Rovaris M, et al. Morphology and evolution of cortical lesions in multiple sclerosis: a longitudinal MRI study. Neuroimage. 2008;42(4):1324–1328. doi: 10.1016/j.neuroimage.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Sethi V, Yousry TA, Muhlert N, et al. Improved detection of cortical MS lesions with phase-sensitive inversion recovery MRI. J Neurol Neurosurg Psychiatry. 2012;83(9):877–882. doi: 10.1136/jnnp-2012-303023. [DOI] [PubMed] [Google Scholar]
- 7.Sethi V, Muhlert N, Ron M, et al. MS cortical lesions on DIR: not quite what they seem? PLoS One. 2013;8(11):e78879. doi: 10.1371/journal.pone.0078879. doi:10.1371/journal.pone .0078879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen AS, Kinkel RP, Tinelli E, Benner T, Cohen-Adad J, Mainero C. Focal cortical lesion detection in multiple sclerosis: 3 tesla DIR versus 7 Tesla FLASH-T2. J Magn Reson Imaging. 2012;35(3):537–542. doi: 10.1002/jmri.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalf M, Xu D, Okuda DT, et al. High-resolution phased-array MRI of the human brain at 7 tesla: initial experience in multiple sclerosis patients. J Neuroimaging. 2010;20(2):141–147. doi: 10.1111/j.1552-6569.2008.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollia K, Maderwald S, Putzki N, et al. First clinical study on ultra-high-field MR imaging in patients with multiple sclerosis: comparison of 1.5T and 7T. AJNR Am J Neuroradiol. 2009;30(4):699–702. doi: 10.3174/ajnr.A1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66(9):1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese M, Filippi M, Rovaris M, et al. Evidence for relative cortical sparing in benign multiple sclerosis: a longitudinal magnetic resonance imaging study. Mult Scler. 2009;15(1):36–41. doi: 10.1177/1352458508096686. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese M, Poretto V, Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain. 2012;135(pt 10):2952–2961. doi: 10.1093/brain/aws246. [DOI] [PubMed] [Google Scholar]
- 14.Roosendaal SD, Moraal B, Pouwels PJ, et al. Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult Scler. 2009;15(6):708–714. doi: 10.1177/1352458509102907. [DOI] [PubMed] [Google Scholar]
- 15.Calabrese M, Bernardi V, Atzori M, et al. Effect of disease-modifying drugs on cortical lesions and atrophy in relapsing-remitting multiple sclerosis. Mult Scler. 2012;18(4):418–424. doi: 10.1177/1352458510394702. [DOI] [PubMed] [Google Scholar]
- 16.Nelson F, Poonawalla A, Hou P, Wolinsky JS, Narayana PA. 3D MPRAGE improves classification of cortical lesions in multiple sclerosis. Mult Scler. 2008;14(9):1214–1219. doi: 10.1177/1352458508094644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Graaf WL, Zwanenburg JJ, Visser F, et al. Lesion detection at seven Tesla in multiple sclerosis using magnetisation prepared 3D-FLAIR and 3D-DIR. Eur Radiol. 2012;22(1):221–231. doi: 10.1007/s00330-011-2242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79(7):708–714. doi: 10.1212/WNL.0b013e3182648bc8. [DOI] [PubMed] [Google Scholar]
- 19.Shiee N, Bazin PL, Ozturk A, Reich DS, Calabresi PA, Pham DL. A topology-preserving approach to the segmentation of brain images with multiple sclerosis lesions. Neuroimage. 2010;49(2):1524–1535. doi: 10.1016/j.neuroimage.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen AS, Kinkel RP, Madigan N, Tinelli E, Benner T, Mainero C. Contribution of cortical lesion subtypes at 7T MRI to physical and cognitive performance in MS. Neurology. 2013;81(7):641–649. doi: 10.1212/WNL.0b013e3182a08ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer JS, Rudick RA, Cutter GR, Reingold SC. National MS Society Clinical Outcomes Assessment Task Force. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. Mult Scler. 1999;5(4):244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 23.Rudick R, Antel J, Confavreux C, et al. Recommendations from the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force. Ann Neurol. 1997;42(3):379–382. doi: 10.1002/ana.410420318. [DOI] [PubMed] [Google Scholar]
- 24.Benedict RH, Fischer JS, Archibald CJ, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol. 2002;16(3):381–397. doi: 10.1076/clin.16.3.381.13859. [DOI] [PubMed] [Google Scholar]
- 25.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–268. [PubMed] [Google Scholar]
- 26.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128(pt 11):2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 27.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(pt 9):2755–2771. doi: 10.1093/brain/awr182. [DOI] [PubMed] [Google Scholar]
- 28.Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol. 2002;15(3):239–245. doi: 10.1097/00019052-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Scalfari A, Neuhaus A, Daumer M, Ebers GC, Muraro PA. Age and disability accumulation in multiple sclerosis. Neurology. 2011;77(13):1246–1252. doi: 10.1212/WNL.0b013e318230a17d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calabrese M, Rinaldi F, Grossi P, Gallo P. Cortical pathology and cognitive impairment in multiple sclerosis. Expert Rev Neurother. 2011;11(3):425–432. doi: 10.1586/ern.10.155. [DOI] [PubMed] [Google Scholar]
- 31.Magliozzi R, Howell OW, Reeves C, et al. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68(4):477–493. doi: 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- 32.Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology. 2006;67(6):960–967. doi: 10.1212/01.wnl.0000237551.26858.39. [DOI] [PubMed] [Google Scholar]
- 33.Hulst HE, Steenwijk MD, Versteeg A, et al. Cognitive impairment in MS: impact of white matter integrity, gray matter volume, and lesions. Neurology. 2013;80(11):1025–1032. doi: 10.1212/WNL.0b013e31828726cc. [DOI] [PubMed] [Google Scholar]
- 34.Walsh M, Montojo CA, Sheu YS, et al. Object working memory performance depends on microstructure of the frontal-occipital fasciculus. Brain Connect. 2011;1(4):317–329. doi: 10.1089/brain.2011.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67(7):812–818. doi: 10.1001/archneurol.2010.148. [DOI] [PubMed] [Google Scholar]
- 36.Yao B, Hametner S, van Gelderen P, et al. 7 Tesla magnetic resonance imaging to detect cortical pathology in multiple sclerosis. PLoS One. 2014;9(10):e108863. doi: 10.1371/journal.pone.0108863. doi:10.1371/journal.pone .010886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geurts JJ, Blezer EL, Vrenken H, et al. Does high-field MR imaging improve cortical lesion detection in multiple sclerosis? J Neurol. 2008;255(2):183–191. doi: 10.1007/s00415-008-0620-5. [DOI] [PubMed] [Google Scholar]
- 38.Bø L, Vedeler CA, Nyland H, Trapp BD, Mørk SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9(4):323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 39.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.