Abstract
BACKGROUND
Ideal triage uses simple criteria to identify severely injured patients. Glasgow Coma Scale motor (GCSm) may be easier for field use and was considered for the National Trauma Triage Protocol (NTTP). This study evaluated performance of the NTTP if GCSm is substituted for the current GCS score ≤ 13 criterion.
METHODS
Subjects in the National Trauma Data Bank undergoing scene transport were included. Presence of NTTP physiologic (Step 1) and anatomic (Step 2) criteria was determined. GCSm score ≤ 5 was defined as a positive criterion. Trauma center need (TCN) was defined as Injury Severity Score (ISS) > 15, intensive care unit admission, urgent operation, or emergency department death. Test characteristics were calculated to predict TCN. Area under the curve was compared between GCSm and GCS scores, individually and within the NTTP. Logistic regression was used to determine the association of GCSm score ≤ 5 and GCS score ≤ 13 with TCN after adjusting for other triage criteria. Predicted versus actual TCN was compared.
RESULTS
There were 811,143 subjects. Sensitivity was lower (26.7% vs. 30.3%), specificity was higher (95.1% vs. 93.1%), and accuracy was similar (66.1% vs. 66.3%) for GCSm score ≤ 5 compared with GCS score ≤ 13. Incorporated into the NTTP Steps 1 + 2, GCSm score ≤ 5 traded sensitivity (60.4% vs. 62.1%) for specificity (67.1% vs. 65.7%) with similar accuracy (64.2% vs. 64.2%) to GCS score ≤ 13. There was no difference in the area under the curve between GCSm score ≤ 5 and GCS score ≤ 13 when incorporated into the NTTP Steps 1 + 2 (p = 0.10). GCSm score ≤ 5 had a stronger association with TCN (odds ratio, 3.37; 95% confidence interval, 3.27–3.48; p < 0.01) than GCS score ≤ 13 (odds ratio, 3.03; 95% confidence interval, 2.94–3.13; p < 0.01). GCSm had a better fit of predicted versus actual TCN than GCS at the lower end of the scales.
CONCLUSION
GCSm score ≤ 5 increases specificity at the expense of sensitivity compared with GCS score ≤ 13. When applied within the NTTP, there is no difference in discrimination between GCSm and GCS. GCSm score ≤ 5 is more strongly associated with TCN and better calibrated to predict TCN. Further study is warranted to explore replacing GCS score ≤ 13 with GCSm score ≤ 5 in the NTTP.
Keywords: Prehospital, triage, Glasgow Coma Scale, motor, trauma
Field triage is one of the most important aspects of a trauma system, as prehospital providers using limited data must decide whether an injured patient requires transport to a trauma center for specialized care. Ideal triage uses simple criteria to identify severely injured patients; however, operationalizing this concept remains challenging.
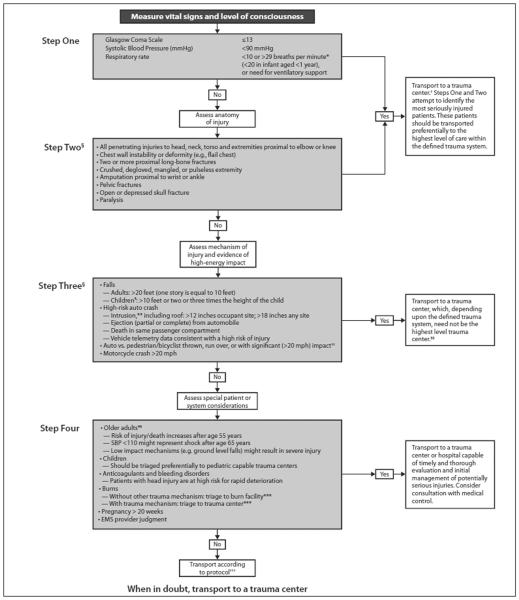
The American College of Surgeons’ Committee on Trauma (ACS COT) and the Centers for Disease Control jointly developed the National Trauma Triage Protocol (NTTP), which is based on the stepwise identification of four aspects of clinical presentation that should be readily identifiable to prehospital providers at the scene of injury (Fig. 1).1 These include physiologic (PHY) criteria, anatomic (ANA) criteria, mechanism of injury criteria, and special considerations criteria, which are evaluated in a sequential fashion to identify patients who should be transported to a trauma center.
Figure 1.
ACS COT and Centers for Disease Control NTTP, 2011. Reproduced from Sasser et al.1 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
The Glasgow Coma Scale (GCS) has long been used to quantify head injury and has been incorporated into a variety of trauma scoring systems and triage algorithms.1–4 The GCS consists of the eye opening, verbal, and motor subscales, which are summed to give the total GCS. However, the full GCS has several limitations in terms of complexity, interrater reliability, and the inability to be calculated in some conditions.5–8 Previous work has demonstrated that the GCS motor subscale (GCSm) performs similarly or better than GCS as a predictor of severe head injury and mortality.9–12
In the context of trauma triage, GCSm may be easier for field use and was considered at the last revision of the NTTP; however, evidence to institute a change was lacking.1 The simplicity and consistency of the GCSm may offer benefits over using the GCS if the characteristics and predictive power of the GCSm are not worse than those of the GCS for field triage to a trauma center.
The objective of this study was to evaluate the performance of the NTTP if GCSm score ≤ 5 was substituted for the current GCS score ≤ 13 criterion. Comparisons of these criteria were performed both as independent criteria and within the stepwise context of the NTTP as would be applied in the field. The study hypothesis was that substitution of a GCSm criterion for the current GCS criterion within the NTTP would result in similar triage characteristics and predictive power for field triage.
PATIENTS AND METHODS
All subjects age 3 years or older13 transported from the scene of injury during 2007 to 2008 were identified in the National Trauma Data Bank (NTDB). Subjects undergoing interfacility transfer were excluded. Demographics, injury characteristics and severity, prehospital and admission vital signs, DRG International Classification of Diseases—9th Rev. diagnosis codes, intensive care unit (ICU) admission, emergency department (ED) disposition, and hospital disposition were collected for each subject.
The primary outcome of trauma center need (TCN) was defined as a composite of Injury Severity Score (ISS) > 15, ICU admission 24 hours or greater, need for urgent surgery defined as ED disposition to the operating room, or death in the ED to identify patients who would potentially benefit from trauma center care. The presence of PHY Step 1 criteria (GCS score ≤ 13, systolic blood pressure [SBP] < 90, respiratory rate [RR] < 10 or >29) were determined using prehospital vital signs. The presence of ANA Step 2 criteria (penetrating injury, flail chest, open skull fracture, ≥2 proximal long bone fractures, pelvic fracture, crush injury, amputation, paralysis) were determined using DRG International Classification of Diseases—9th Rev. diagnosis codes. Subjects were classified as to the presence or absence of each of the 11 available Step 1 and Step 2 triage criteria from the NTTP. To compare with the current GCS score ≤ 13 criterion, a prehospital GCSm score ≤ 5 was defined as a positive triage criterion for transport to a trauma center.
To address missing data, multiple imputation was performed for prehospital SBP, prehospital RR, prehospital GCS, prehospital GCSm, and ISS. Multiple imputation using a fully conditional specification model based on available demographics, prehospital and admission physiology, and injury characteristics was performed using five imputation steps to develop a complete data set. Data analysis was performed on the pooled data. Sensitivity analysis was performed using complete cases to assess the success of the multiple imputation procedure.
To compare accuracy of the current GCS triage criterion to the GCSm triage criterion, standard diagnostic test characteristics including sensitivity, specificity, and accuracy were calculated for GCS score ≤ 13 and GCSm score ≤ 5 to predict TCN both individually and within the first two steps of the stepwise NTTP. Receiver operating characteristic (ROC) curves were constructed for the GCS and GCSm triage criteria to predict TCN. The area under the ROC curve (AUC) for GCS score ≤ 13 and GCSm score ≤ 5 were compared using methodology described by Hanley and McNeil14 to compare discrimination performance for each in predicting TCN. The sequential application of the PHYand ANA steps was analyzed to replicate the way in which emergency medical service personnel would apply the algorithm.
Two forward stepwise logistic regression models were then constructed to determine the independent association of TCN with either GCS score ≤ 13 or GCSm score ≤ 5 after adjusted for the presence or absence of the other triage criteria in the first two steps of the NTTP. A p < 0.2 for the association of a covariate with TCN was used for entry into the models. The adjusted odds ratios (ORs) for GCS and GCSm were compared for the strength of their association with TCN. These models were also used to generate the predicted probability of TCN for each subject. This predicted probability of TCN was then plotted against the actual proportion of subjects meeting the definition of TCN within each discrete GCS (3–15) or GCSm (1–6) category to graphically assess the calibration of each triage criterion. A straight diagonal line represents perfect calibration. Akaike’s information criterion was also assessed to compare goodness-of-fit for each model. Linear regression was performed with residual plots and calculation of r2 to determine the extent of the linear relationship between predicted versus actual TCN in GCS and GCSm.
Data analysis was conducted using SPSS version 19 (IBM, Chicago, IL). Continuous data are presented as median (interquartile range) or mean (SD). For univariate analyses, χ2 tests were used to compare categorical variables and Mann-Whitney U-tests to compare continuous variables. A p ≤ 0.05 was considered significant.
RESULTS
There were 811,143 subjects included in the study. Table 1 illustrates the characteristics and triage criteria for the study population. Overall, prehospital vital signs were present in 63% of the subjects. Prehospital GCS was present in 59% and prehospital GCSm was present in 58% of the subjects.
TABLE 1.
Study Population Characteristics
| N | 811,143 |
| Age, y | 39 (23–57) |
| Sex, male, % | 66.1 |
| Prehospital time, min | 42 (31–55) |
| ISS | 9 (4–13) |
| Trauma center, Level I or II, % | 74.3 |
| Survival, % | 95.7 |
| TCN, % | 38.7 |
| Step 1 physiologic triage criteria | |
| GCS score ≤ 13, % | 16.8 |
| GCSm score ≤ 5, % | 14.2 |
| SBP < 90, % | 5.2 |
| RR < 10 or >29, % | 6.3 |
| Any PHY criterion, % | 23.2 |
| Any PHY new criterion, %* | 20.6 |
| Step 2 anatomic triage criteria | |
| Penetrating injury, % | 11.6 |
| Flail chest, % | 0.4 |
| Open skull fracture, % | <0.1 |
| ≥2 long bone fractures, % | 1.3 |
| Pelvic fracture, % | 6.3 |
| Crush injury, % | 0.5 |
| Amputation, % | 0.2 |
| Paralysis, % | 0.4 |
| ANA criterion, % | 19.9 |
| Any PHYor ANA criterion, % | 46.5 |
| Any PHY new or ANA criterion, %* | 44.9 |
Physiologic criteria using GCSm score ≤ 5.
Using a triage criterion of GCSm score ≤ 5 resulted in increased specificity and reduced sensitivity compared with the current criterion of GCS score ≤ 13; however, overall accuracy was similar for both criteria (Table 2). When comparing individual criteria, the AUC for the GCSm criteria was statistically lower than for the current GCS criteria (0.609 vs. 0.617, p < 0.01). When assessing the first two steps of the NTTP as would be applied in the field for triage, incorporating GCSm score ≤ 5 resulted in a similar trade-off of sensitivity for specificity; however, overall accuracy for the first two steps of the NTTP was identical whether using GCSm or the current GCS criterion. There was no difference in AUC between GCSm and GCS when assessed within the first two steps of the NTTP (0.637 vs. 0.639 p = 0.10).
TABLE 2.
Triage Characteristics of GCS Score ≤ 13 and GCSm Score ≤ 5
| Sensitivity, % | Specificity, % | Accuracy, % | ROC AUC | |
|---|---|---|---|---|
| GCSm score ≤ 5 | 26.7 | 95.1 | 66.1 | 0.609 |
| GCS score ≤ 13 | 30.3 | 93.1 | 66.3 | 0.617 |
| NTTP Step 1 and 2 using GCSm score ≤ 5 | 60.4 | 67.1 | 64.2 | 0.637 |
| NTTP Step 1 and 2 using GCS score ≤ 13 | 62.1 | 65.7 | 64.2 | 0.639 |
Stepwise logistic regression demonstrated that GCSm score ≤ 5 had a stronger association with TCN (OR, 3.37; 95% confidence interval, [CI], 3.27–3.48; p < 0.01) than GCS score ≤ 13 (OR, 3.03; 95% CI, 2.94–3.13; p < 0.01) after adjusting for the presence of other triage criteria (Tables 3 and 4). When graphically assessing the calibration of these models, GCSm demonstrated a more linear plot of predicted TCN versus actual TCN than GCS over the discrete categories of each, particularly at the lower end of the scales (Fig. 2). The Akaike’s information criterion was lower (better goodness-of-fit) for the model using GCSm compared with GCS (1,301.4 vs. 1,497.2). Linear regression revealed a higher correlation of predicted and actual TCN in GCSm (r2 = 0.964) than in GCS (r2 = 0.882). Residual plots demonstrated less deviation from the regression line in GCSm than in GCS (Fig. 3). This indicates that GCSm has better calibration for predicting TCN, with GCS categories less than 9 poorly calibrated.
TABLE 3.
Stepwise Logistic Regression Model of Step 1 and 2 NTTP Triage Criteria Using GCSm Score ≤ 5
| Step | Criterion | OR | 95% CI | p |
|---|---|---|---|---|
| 1 | GCSm score ≤ 5 | 3.37 | 3.27–3.48 | <0.01 |
| 2 | RR < 10 or >29 | 1.68 | 1.64–1.72 | <0.01 |
| 3 | Pelvic fracture | 2.35 | 2.30–2.39 | <0.01 |
| 4 | Penetrating injury | 1.80 | 1.77–1.83 | <0.01 |
| 5 | SBP < 90 | 1.76 | 1.71–1.81 | <0.01 |
| 6 | Paralysis | 88.54 | 66.13–118.54 | <0.01 |
| 7 | Flail chest | 37.60 | 31.16–45.37 | <0.01 |
| 8 | ≥2 proximal long bone fractures | 2.03 | 1.95–2.12 | <0.01 |
| 9 | Amputation | 14.39 | 12.04–17.21 | <0.01 |
| 10 | Crush injury | 2.26 | 2.11–2.42 | <0.01 |
| 11 | Open skull fracture | 6.25 | 2.34–16.70 | <0.01 |
TABLE 4.
Stepwise Logistic Regression Model of Step 1 and 2 NTTP Triage Criteria Using GCS Score ≤ 13
| Step | Criterion | OR | 95% CI | p |
|---|---|---|---|---|
| 1 | GCS score ≤ 13 | 3.03 | 2.94 – 3.13 | <0.01 |
| 2 | RR < 10 or >29 | 1.73 | 1.69–1.77 | <0.01 |
| 3 | Pelvic fracture | 2.36 | 2.31–2.40 | <0.01 |
| 4 | Penetrating injury | 1.82 | 1.79–1.84 | <0.01 |
| 5 | SBP < 90 | 1.78 | 1.73–1.83 | <0.01 |
| 6 | Paralysis | 90.62 | 67.70–121.31 | <0.01 |
| 7 | Flail chest | 37.86 | 31.37–45.71 | <0.01 |
| 8 | ≥2 proximal long bone fractures | 2.05 | 1.96–2.14 | <0.01 |
| 9 | Amputation | 14.53 | 12.16–17.35 | <0.01 |
| 10 | Crush injury | 2.29 | 2.14–2.45 | <0.01 |
| 11 | Open skull fracture | 6.12 | 2.27–16.52 | <0.01 |
Figure 2.
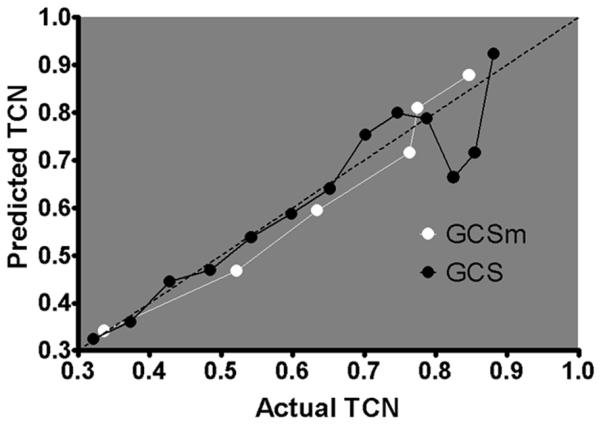
Predicted TCN plotted versus actual TCN through the range of scores for GCS (3–15) and GCSm (1–6). The dotted diagonal line represents perfect calibration. The more linear line of the GCSm model indicates calibration better than that of the GCS model through the range of scores to predict TCN, particularly at the lower end of the scales.
Figure 3.
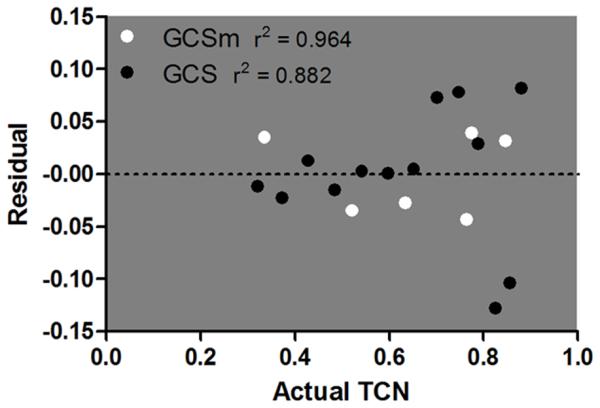
Residual plot over the range of scores for GCS (3–15) and GCSm (1–6) versus actual TCN. The y-axis displays the residuals or deviation from the regression line in linear regression. The dotted line at zero represents perfect linear relationship in linear regression. GCSm demonstrates lower residuals and higher r2 compared with GCS, indicating a more linear relationship between predicted and actual TCN in GCSm.
When examining the effect of changing from the GCS score ≤ 13 criterion to the GCSm score ≤ 5 criterion within the first two steps of the NTTP based on TCN, an additional 1.1% of the subjects would have been undertriaged, while 1.7% of overtriage would have been prevented. Furthermore, changing to GCSm would have captured an additional 0.2% of the subjects who would have been undertriaged by GCS and would overtriage only 0.4% of the subjects. Thus, when considering the net effect on overtriage and undertriage rates if GCSm is used instead of GCS, a net benefit of 0.4% is seen by using GCSm over GCS [(prevented overtriage 1.7% + prevented undertriage 0.2%) j (overtriage 0.4% + undertriage 1.1%) = net benefit 0.4%]. Furthermore, 0.5% of subjects that would have been undertriaged by using GCSm instead of GCS would have been captured by other elements in NTTP resulting in the appropriate triage decision regardless. Sensitivity analysis using complete cases only (n = 509,194) demonstrated that subjects with missing prehospital GCS or GCSm score had similar admission GCS score (mean 14.2 vs. 13.7), admission GCSm score (mean, 5.7 vs. 5.5), and age (median, 38 vs. 39). ISS, TCN, and mortality were lower among those with missing prehospital GCS or GCSm score (median, 5 vs. 9, 33% vs. 42%, 2.8 vs. 5.2%, respectively). After imputation, original and pooled means were similar for prehospital SBP (131.1 vs. 130.8), prehospital RR (18.7 vs. 18.7), prehospital GCS score (13.7 vs. 13.6), prehospital GCSm score (5.6 vs. 5.6), and ISS (10.0 vs. 10.1).
When complete cases were compared with the imputation data set, sensitivity, specificity, and overall accuracy for GCSm score ≤ 5 and GCS score ≤ 13 were identical. AUCs between GCSm and GCS as individual criterion were also identical. When assessing the first two steps of the NTTP using GCSm score ≤ 5 or GCS score ≤ 13, sensitivity was slightly lower (55.4%, 57.5%), specificity was higher (74.6%, 72.9%), and accuracy was slightly higher (66.3%, 66.2%) in the complete cases, although the relationship between GCSm and GCS was the same. There was also no difference in the AUC between the first two steps of the NTTP using GCSm score ≤ 5 or GCS score ≤ 13 (0.650 vs. 0.652, p = 0.08). Regression models of GCSm score ≤ 5 and GCS score ≤ 13 adjusted for other triage criteria demonstrated slightly higher ORs for TCN (OR, 4.87; 95% CI, 4.70–4.97; p < 0.01; and OR, 4.84; 95% CI, 4.40–4.57; p < 0.01) in the complete cases; however, the same relationship between GCSm and GCS was present.
Since missing GCS and GCSm data were likely less injured patients based on lower ISS, TCN, and mortality, a best case sensitivity analysis was performed, imputing the median values for prehospital vital signs, resulting in subjects with missing data not meeting any PHY criteria. This analysis similarly demonstrated no significant difference in accuracy or AUC between the NTTP with GCSm or GCS incorporated in the first two steps. Regression model results were similar to those seen in the complete case analysis.
DISCUSSION
The goal of field triage is to get the right patient to the right hospital at the right time. Prospectively identifying patients that would benefit from trauma care is essential to the success of trauma systems. However, this remains an ongoing challenge as prehospital providers have limited data to make this decision.15 Moreover, this decision has been clearly shown to have implications in patient outcomes.16,17 The NTTP was structured into a stepwise approach to evaluate patient and injury characteristics available in the field. This triage scheme is regularly reviewed by the National Expert Panel on Field Triage and updated based on available evidence.1
The specific criteria in this algorithm have evolved over time, and the last revision of the NTTP in 2011 considered the addition of the GCSm as an alternative to the GCS. The authors cited evidence that GCSm predicts lifesaving prehospital interventions;18,19 however, it was felt that there was limited confirmatory evidence in the context of field triage, and the long-standing familiarity among prehospital providers with GCS led to no change in the criterion.
The rationale for using GCSm over GCS is that the GCSm overcomes many limitations of the full GCS while retaining similar or superior predictive power. Notably, the GCS cannot be assessed in certain circumstances. Intubated patients cannot undergo assessment of the verbal subscale, and some have attempted to predict verbal subscales in these patients.7 In addition, the GCS does not accurately assess consciousness or predict outcome in patients who are intoxicated, have undergone pharmacologic sedation, or have facial injuries that alter ability to obtain eye opening or verbal subscales.5,8,10,20 There has been significant variation in the way these situations are handled between centers, preventing accurate comparisons among studies and patients.21,22
The GCS is a complex scale that takes more than a few seconds to assess.5 The three subscales each have a different number of possible responses, which increases the difficulty for providers to memorize for rapid assessment in the field. Some authors have shown error rates up to 20% in GCS assessments, even among clinicians who underwent formal training.23 Furthermore, several studies have demonstrated poor interrater reliability of the GCS in both prehospital and in-hospital settings.24–26 Finally, various combinations of the subscales that sum to the same total GCS have markedly different outcomes.10,27
In light of these limitations, several authors have suggested the use of GCSm. Meredith et al.11 evaluated the predictive value of a trauma score ≤ 12 compared with GCSm score ≤ 5. The authors found that the highest discriminatory power was seen using GCSm score ≤ 5 for risk of mortality; however, the authors did not evaluate the full GCS. Healey et al.10 examined the relationship between GCS and GCSm with survival in the NTDB from 1994 to 2001. Using regression and fractional polynomial analyses, they demonstrated in more than 200,000 patients that the GCSm was linearly related to mortality, contained almost all the predictive ability of the GCS, and was better calibrated to predict mortality. The authors concluded that the superior properties of the GCSm warrant its use over the GCS for mortality prediction.
Others have investigated the role of GCSm to predict traumatic brain injury (TBI). Ross et al.12 found that a prehospital GCSm score ≤ 5 had a similar sensitivity and specificity as GCS score ≤ 13 to predict a head Abbreviated Injury Scale (AIS) score > 3. The authors also noted that GCSm and GCS had similar performance for identifying patients undergoing craniotomy. They concluded that the simplicity of GCSm should be favored over GCS for field triage. Gill et al.5 reported that the admission GCSm AUC was only 1.3% to 4.5% less than the GCS AUC when predicting intubation, neurosurgical intervention, TBI, or mortality, and GCSm was the best predictor among the GCS subscales. Caterino and Raubenolt28 compared the simplified motor score, a three-point scale based on prehospital GCSm, with GCS to predict mortality, TBI, neurosurgical intervention, and intubation in more than 50,000 patients. The authors demonstrated similar sensitivity, specificity, and AUC for all outcomes between the motor score and GCS.
In a prospective study, Al-Salamah29 evaluated admission GCS and GCS subscales in 795 blunt trauma patients. It was found that only GCSm had a similar predictive ability as GCS for mortality, and both significantly predicted ICU admission. The authors concluded that admission GCSm was valid for in-hospital triage of blunt trauma patients.
Although these previous studies illustrate the promise of the GCSm, they are variously limited by lack of direct comparison between GCSm and GCS, use of in-hospital GCSm and GCS, or only considering narrow outcomes of mortality or TBI. No study to date has directly compared prehospital GCSm and GCS in the context of a field triage algorithm such as the NTTP.
The current study demonstrates that when considering TCN, GCSm score ≤ 5 increases specificity at the expense of sensitivity when compared with GCS score ≤ 13. The trade-off of sensitivity for specificity will likely benefit the overall NTTP because the physiologic and anatomic criteria typically have lower sensitivity and higher specificity, while mechanism of injury and special consideration criteria have higher sensitivity in the algorithm.30 Overall accuracy is similar, and although the AUC is statistically lower, the difference of 0.8% is of questionable clinical significance and similar to previous work.
More importantly, this is the first study to evaluate GCSm and GCS in the context of the NTTP as would be applied during field triage. These data illustrate a similar trade-off of sensitivity for specificity, but identical accuracy and no difference in AUC when comparing the first two steps of the NTTP using GCSm score ≤ 5 or GCS score ≤ 13. This suggests that once incorporated into the stepwise NTTP, the first two steps of the algorithm have the same predictive power to determine TCN, whether GCSm score ≤ 5 or GCS score ≤ 13 is used. Furthermore, GCSm score ≤ 5 had stronger association with the odds of TCN, and the lower 95% confidence bound for GCSm score ≤ 5 does not cross the upper 95% confidence bound for GCS score ≤ 13, suggesting a significant difference in the association with TCN between the two criteria. The GCSm is better calibrated to predict TCN over the scales when compared with GCS, which has poor calibration when less than a score of 9.
Finally, a potential net benefit in overall triage of 0.4% was seen by substituting GCSm for GCS in the NTTP. This, however, assumes equal benefit and harm between overtriage and undertriage, which may not hold true. Despite this, the increase in undertriage using GCSm in the first two steps of the NTTP may not actually result in an increase in overall undertriage given the stepwise nature of the protocol. For instance, as noted earlier, 0.5% of the subjects that would have been undertriaged by using GCSm instead of GCS would be captured by other elements in the first two steps of the NTTP resulting in the appropriate triage decision regardless. This reduces the actual net undertriage rate from 0.9% (net undertriage for GCSm = undertriage 1.1% j prevented undertriage 0.2% = 0.9%) to 0.4%.
In addition, the physiologic and anatomic criteria representing the first two steps of the NTTP have been shown to be highly specific, while the mechanism of injury criteria and special considerations representing Steps 3 and 4 are more sensitive and necessary to prevent undertriage in the overall NTTP.30 It is very likely that the remaining patients that would be undertriaged by using GCSm instead of GCS in the NTTP would be captured by the remaining Step 3 or 4 criteria, resulting in no actual increase in undertriage. However, we were unable to assess Step 3 or 4 criteria in the NTDB, and this requires further study to confirm.
This study has several limitations. First are those inherent to a retrospective design. Second are those outlined by ACS COT for the use of the NTDB.31 Despite a large national sample, there are limited variables for analysis. This limited our ability to directly evaluate mechanism of injury or special consideration criteria. Furthermore, data are restricted to centers that submit to the NTDB and are skewed toward trauma centers, introducing potential selection bias. A substantial amount of prehospital physiologic data was missing; however, multiple imputation was used to mitigate this limitation. We felt that it was important to use prehospital vital signs rather than admission vital signs because we were interested in field triage. Furthermore, imputation did not skew the physiologic data and has been shown effective for physiologic data in the NTDB,32 and our sensitivity analyses demonstrate similar results, which engenders confidence in the results presented here. The outcome of TCN was a composite outcome we defined and has been published elsewhere;30,33 however, a consistent outcome measure for identifying patients that need trauma center care is lacking.34 Finally, there are situations in which even the GCSm cannot be assessed, such as pharmacologic or traumatic paralysis; however these patients will meet other NTTP criteria and thus should not hinder appropriate triage.
In conclusion, this represents the first study to compare triage performance using GCSm score ≤ 5 or the current GCS score ≤ 13 criterion within the NTTP. GCSm score ≤ 5 increases specificity at the expense of sensitivity compared with GCS score ≤ 13. When applied within the NTTP, there is no difference in discrimination between GCSm and GCS. GCSm score ≤ 5 is more strongly associated with TCN and better calibrated to predict TCN. Because of the similar predictive power and less complex use for field triage, further study is warranted to explore replacing the current GCS score ≤ 13 criterion with GCSm score ≤ 5 in the NTTP.
Footnotes
LEVEL OF EVIDENCE: Prognostic study, level III.
This study was presented at the 27th Annual Scientific Assembly of the Eastern Association for the Surgery of Trauma, January 14–18, 2014, in Naples, Florida.
AUTHORSHIP
J.B.B. designed the study and performed the literature search, data collection, and data analysis. J.B.B., M.L.G., and J.L.S. participated in the initial manuscript preparation. All authors contributed to the data interpretation and critical revision of the manuscript.
DISCLOSURE
The authors declare no conflicts of interest.
Contributor Information
Joshua B. Brown, Division of General Surgery and Trauma, Department of Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
Raquel M. Forsythe, Division of General Surgery and Trauma, Department of Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
Nicole A. Stassen, Division of Acute Care Surgery, Department of Surgery, University of Rochester Medical Center, Rochester, New York.
Andrew B. Peitzman, Division of General Surgery and Trauma, Department of Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
Timothy R. Billiar, Division of General Surgery and Trauma, Department of Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
Jason L. Sperry, Division of General Surgery and Trauma, Department of Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
Mark L. Gestring, Division of Acute Care Surgery, Department of Surgery, University of Rochester Medical Center, Rochester, New York.
REFERENCES
- 1.Sasser SM, Hunt RC, Faul M, Sugerman D, Pearson WS, Dulski T, Wald MM, Jurkovich GJ, Newgard CD, Lerner EB, et al. Guidelines for field triage of injured patients: recommendations of the National Expert Panel on Field Triage, 2011. MMWR Recomm Rep. 2012;61:1–20. [PubMed] [Google Scholar]
- 2.Boyd CR, Tolson MA, Copes WS. Evaluating trauma care: the TRISS method. Trauma Score and the Injury Severity Score. J Trauma. 1987;27:370–378. [PubMed] [Google Scholar]
- 3.Champion HR, Sacco WJ, Copes WS, Gann DS, Gennarelli TA, Flanagan ME. A revision of the Trauma Score. J Trauma. 1989;29:623–629. doi: 10.1097/00005373-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Teasdale G, Murray G, Parker L, Jennett B. Adding up the Glasgow Coma Score. Acta Neurochir Suppl (Wien) 1979;28:13–16. doi: 10.1007/978-3-7091-4088-8_2. [DOI] [PubMed] [Google Scholar]
- 5.Gill M, Windemuth R, Steele R, Green SM. A comparison of the Glasgow Coma Scale score to simplified alternative scores for the prediction of traumatic brain injury outcomes. Ann Emerg Med. 2005;45:37–42. doi: 10.1016/j.annemergmed.2004.07.429. [DOI] [PubMed] [Google Scholar]
- 6.Marshall LF, Becker DP, Bowers SA, Cayard C, Eisenberg H, Gross CR, Grossman RG, Jane JA, Kunitz SC, Rimel R, et al. The National Traumatic Coma Data Bank. Part 1: design, purpose, goals, and results. J Neurosurg. 1983;59:276–284. doi: 10.3171/jns.1983.59.2.0276. [DOI] [PubMed] [Google Scholar]
- 7.Meredith W, Rutledge R, Fakhry SM, Emery S, Kromhout-Schiro S. The conundrum of the Glasgow Coma Scale in intubated patients: a linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J Trauma. 1998;44:839–845. doi: 10.1097/00005373-199805000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Segatore M, Way C. The Glasgow Coma Scale: time for change. Heart Lung. 1992;21:548–557. [PubMed] [Google Scholar]
- 9.Baxt WG, Jones G, Fortlage D. The trauma triage rule: a new, resource-based approach to the prehospital identification of major trauma victims. Ann Emerg Med. 1990;19:1401–1406. doi: 10.1016/s0196-0644(05)82608-3. [DOI] [PubMed] [Google Scholar]
- 10.Healey C, Osler TM, Rogers FB, Healey MA, Glance LG, Kilgo PD, Shackford SR, Meredith JW. Improving the Glasgow Coma Scale score: motor score alone is a better predictor. J Trauma. 2003;54:671–678. doi: 10.1097/01.TA.0000058130.30490.5D. discussion 678–680. [DOI] [PubMed] [Google Scholar]
- 11.Meredith W, Rutledge R, Hansen AR, Oller DW, Thomason M, Cunningham P, Baker CC. Field triage of trauma patients based upon the ability to follow commands: a study in 29,573 injured patients. J Trauma. 1995;38:129–135. doi: 10.1097/00005373-199501000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Ross SE, Leipold C, Terregino C, O’Malley KF. Efficacy of the motor component of the Glasgow Coma Scale in trauma triage. J Trauma. 1998;45:42–44. doi: 10.1097/00005373-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Holmes JF, Palchak MJ, MacFarlane T, Kuppermann N. Performance of the pediatric Glasgow Coma Scale in children with blunt head trauma. Acad Emerg Med. 2005;12:814–819. doi: 10.1197/j.aem.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 15.Henry MC, Alicandro JM, Hollander JE, Moldashel JG, Cassara G, Thode HC., Jr Evaluation of American College of Surgeons trauma triage criteria in a suburban and rural setting. Am J Emerg Med. 1996;14:124–129. doi: 10.1016/S0735-6757(96)90117-5. [DOI] [PubMed] [Google Scholar]
- 16.MacKenzie EJ, Rivara FP, Jurkovich GJ, Nathens AB, Frey KP, Egleston BL, Salkever DS, Scharfstein DO. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354:366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 17.Nirula R, Maier R, Moore E, Sperry J, Gentilello L. Scoop and run to the trauma center or stay and play at the local hospital: hospital transfer’s effect on mortality. J Trauma. 2010;69:595–601. doi: 10.1097/TA.0b013e3181ee6e32. [DOI] [PubMed] [Google Scholar]
- 18.Holcomb JB, Niles SE, Miller CC, Hinds D, Duke JH, Moore FA. Prehospital physiologic data and lifesaving interventions in trauma patients. Mil Med. 2005;170:7–13. doi: 10.7205/milmed.170.1.7. [DOI] [PubMed] [Google Scholar]
- 19.Holcomb JB, Salinas J, McManus JM, Miller CC, Cooke WH, Convertino VA. Manual vital signs reliably predict need for life-saving interventions in trauma patients. J Trauma. 2005;59:821–828. doi: 10.1097/01.ta.0000188125.44129.7c. discussion 828–829. [DOI] [PubMed] [Google Scholar]
- 20.Walther SM, Jonasson U, Gill H. Comparison of the Glasgow Coma Scale and the Reaction Level Scale for assessment of cerebral responsiveness in the critically ill. Intensive Care Med. 2003;29:933–938. doi: 10.1007/s00134-003-1757-4. [DOI] [PubMed] [Google Scholar]
- 21.Buechler CM, Blostein PA, Koestner A, Hurt K, Schaars M, McKernan J. Variation among trauma centers’ calculation of Glasgow Coma Scale score: results of a national survey. J Trauma. 1998;45:429–432. doi: 10.1097/00005373-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Marion DW, Carlier PM. Problems with initial Glasgow Coma Scale assessment caused by prehospital treatment of patients with head injuries: results of a national survey. J Trauma. 1994;36:89–95. doi: 10.1097/00005373-199401000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Holt AW, Bury LK, Bersten AD, Skowronski GA, Vedig AE. Prospective evaluation of residents and nurses as severity score data collectors. Crit Care Med. 1992;20:1688–1691. doi: 10.1097/00003246-199212000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Bazarian JJ, Eirich MA, Salhanick SD. The relationship between pre-hospital and emergency department Glasgow Coma Scale scores. Brain Inj. 2003;17:553–560. doi: 10.1080/0269905031000070260. [DOI] [PubMed] [Google Scholar]
- 25.Gill MR, Reiley DG, Green SM. Interrater reliability of Glasgow Coma Scale scores in the emergency department. Ann Emerg Med. 2004;43:215–223. doi: 10.1016/s0196-0644(03)00814-x. [DOI] [PubMed] [Google Scholar]
- 26.Kerby JD, MacLennan PA, Burton JN, McGwin G, Jr, Rue LW., 3rd Agreement between prehospital and emergency department glasgow coma scores. J Trauma. 2007;63:1026–1031. doi: 10.1097/TA.0b013e318157d9e8. [DOI] [PubMed] [Google Scholar]
- 27.Teoh LS, Gowardman JR, Larsen PD, Green R, Galletly DC. Glasgow Coma Scale: variation in mortality among permutations of specific total scores. Intensive Care Med. 2000;26:157–161. doi: 10.1007/s001340050039. [DOI] [PubMed] [Google Scholar]
- 28.Caterino JM, Raubenolt A. The prehospital simplified motor score is as accurate as the prehospital Glasgow Coma Scale: analysis of a statewide trauma registry. Emerg Med J. 2012;29:492–496. doi: 10.1136/emj.2010.110437. [DOI] [PubMed] [Google Scholar]
- 29.Al-Salamah MA. Initial emergency department trauma scores from the OPALS study: the case for the motor score in blunt trauma. Acad Emerg Med. 2004;11:834–842. doi: 10.1111/j.1553-2712.2004.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 30.Brown JB, Stassen NA, Bankey PE, Sangosanya AT, Cheng JD, Gestring ML. Mechanism of injury and special consideration criteria still matter: an evaluation of the National Trauma Triage Protocol. J Trauma. 2011;70:38–44. doi: 10.1097/TA.0b013e3182077ea8. discussion 44–45. [DOI] [PubMed] [Google Scholar]
- 31.American Colleger of Surgeons Committee on Trauma National Trauma Databank User Manual: NTDB Research Data Set Admision Year 2008. Available at: http://www.facs.org/trauma/ntdb/pdf/ntdbmanual2008.pdf. Accessed November 2, 2013.
- 32.Moore L, Hanley JA, Turgeon AF, Lavoie A, Emond M. A multiple imputation model for imputing missing physiologic data in the National Trauma Data Bank. J Am Coll Surg. 2009;209:572–579. doi: 10.1016/j.jamcollsurg.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Brown JB, Forsythe RM, Stassen NA, Gestring ML. The National Trauma Triage Protocol: can this tool predict which patients with trauma will benefit from helicopter transport? J Trauma Acute Care Surg. 2012;73:319–325. doi: 10.1097/TA.0b013e3182572bee. [DOI] [PubMed] [Google Scholar]
- 34.Practive Management Guidelines for the Appropriate Triage of the Victim of Trauma. Available at: http://www.east.org/resources/treatment-guidelines/triage-of-the-trauma-patient. Accessed November 2, 2013.