Abstract
In animal models of stroke, spinal cord injury, and subarachnoid hemorrhage, transient receptor potential melastatin 4 (Trpm4), a non-selective monovalent cation channel, is transcriptionally upregulated in neural and vascular cells. In these contexts, Trpm4 has been shown to co-associate with sulfonylurea receptor 1 (Sur1) to form Sur1-Trpm4 channels, which play a critical role in cytotoxic edema, accidental necrotic (oncotic) cell death, blood-brain barrier (BBB) breakdown and formation of vasogenic edema. To date, the expression and molecular interactions of Trpm4 within human cerebral infarcts have not been systematically evaluated. In this study, we examined Trpm4 expression in postmortem specimens obtained from 15 patients within the first 31 days after onset of focal cerebral ischemia. Significant upregulation of Trpm4 protein was found in all cases, relative to controls. De novo transcriptional upregulation of Trpm4 protein was confirmed using in situ hybridization for Trpm4 mRNA. Trpm4 co-localized and co-associated with Sur1 within ischemic endothelial cells and neurons which exhibited membrane thickening and irregularities characteristic of necrotic cell death. Sur1 and Trpm4 co-expression in abnormal endothelial cells also was associated with vasogenic edema, as evidenced by upregulated perivascular TNFα, perivascular extravasation of serum immunoglobulin G and associated inflammation. Upregulated Trpm4 protein persisted up to one month post onset of cerebral ischemia. Furthermore, pharmacological channel blockade by glibenclamide, a selective inhibitor of sulfonylurea receptor, was found to mitigate perivascular TNFα labeling in a rat middle cerebral artery occlusion (MCAo) stroke model. We conclude that the Sur1-Trpm4 channel is upregulated and associated with BBB disruption and cerebral edema formation in human cerebral infarcts. These data suggest that pharmacological targeting of this channel may represent a promising therapeutic strategy for clinical management of ischemic stroke.
Keywords: Transient receptor potential melastatin 4 (Trpm4), Sulfonylurea receptor 1 (Sur1), cerebral infarct, neuron, endothelium, astrocyte, neutrophil, cerebral edema, inflammation
Introduction
Stroke accounts for 5.5 million deaths worldwide each year and is a leading cause of long-term disability (1-3). Despite tremendous progress in our understanding of stroke pathophysiology, ongoing efforts to identify novel molecular targets have yet to yield new therapies. Recombinant tissue plasminogen activator (rtPA) remains the only medication specifically approved by national regulatory agencies for use in patients with ischemic stroke but, for a variety of reasons, it is utilized in less than 20% of stroke victims (4-7). Moreover, its use is associated with an increased risk of intracranial hemorrhage (8). Despite the devastating effects of malignant cerebral edema in large territorial infarctions, the only pharmacotherapeutic option for this complication is osmotherapy, and its effects are unproven. There remains a critical need to better understand the molecular pathogenesis of this devastating neurological condition, in order to identify more effective therapeutic strategies.
Transient receptor potential (Trp) melastatin 4 (Trpm4) is a member of a large protein superfamily consisting of 28 mammalian cation channels (9,10). Most members of the Trp family are permeable to divalent cations. The exceptions, Trpm4 and Trpm5, are Ca2+-impermeable channels that transport monovalent cations exclusively and non-selectively (9,10). Trpm4 channels are activated by an increase in cytosolic Ca2+ or a decrease in cytosolic ATP (11). Accumulating data show upregulation of Trpm4 in microvascular endothelial cells, neurons, and glia in experimental rat models of stroke, spinal cord injury, and subarachnoid hemorrhage (12-15). Emerging evidence suggests that Trpm4 interacts with sulfonylurea receptor 1 (Sur1) to form a novel ion channel, the Sur1-Trpm4 channel (15), which functions critically in the pathophysiology of various acute central nervous system (CNS) injuries. Sur1-Trpm4 channels contribute to cytotoxic edema, serve as end-executioners in accidental necrotic death induced by ATP-depletion or reactive oxygen species (16), and participate in blood-brain barrier breakdown and formation of ionic and vasogenic edema (14,17).
The expression of Trpm4 has been investigated in tissue specimens obtained from patients with multiple sclerosis (18) and subarachnoid hemorrhage (14), but it has not yet been studied in human cerebral infarcts. Here, we sought to determine whether upregulation of Trpm4 protein and mRNA is present in infarcted human cerebral cortex, and whether Trpm4 protein co-associates with Sur1 to form Sur1-Trpm4 channels in focal cerebral ischemia in humans.
Materials and Methods
Human Tissues
The tissue collection protocol was approved by the Institutional Review Board of the University of Maryland, Baltimore. Patients dying within 31 days of documented cerebral ischemia between January, 2010 and December, 2012 and who underwent autopsy were identified retrospectively by reviewing the records of the Department of Pathology, University of Maryland School of Medicine. Within these constraints, 15 focal cerebral infarcts originating from 13 patients were identified. For comparison, contralateral cortex was evaluated as control tissue. Brain specimens from six patients with documented absence of ischemic lesions also were examined as controls. For additional validation of our antibodies, sections of normal colon surgically removed from 2 patients during bypass surgery were also evaluated. Standard tissue fixation protocols (7–10 days in formalin) were applied.
Histological validation of the presence or absence of an ischemic lesion in brain specimens was made in all cases by a neuropathologist. Representative paraffin-embedded tissue blocks were selected from each case for detailed evaluation. For normal brains, blocks encompassing representative frontal or parietal cortices were studied. Blocks were sectioned at 6 μm and were stained with hematoxylin and eosin (H&E), or were prepared for immunohistochemistry.
Rat Middle Cerebral Artery Occlusion (MCAo) Stroke Model
Animal experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland. All experiments were performed in accordance with the relevant guidelines and regulations in the United States National Institutes of Health Guide for the Care and Use of laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Male Wistar rats (275–325 g, Harlan, Indianapolis, IN) were anesthetized (ketamine 60 mg/kg and xylazine 7.5 mg/kg intraperitoneally). SpO2 via pulse oximetry and temperature were carefully regulated, and all surgical procedures were performed aseptically. Middle cerebral artery occlusion (MCAo) was achieved by using an intraluminal thread. A midline incision was made to expose the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA). A 4-0-monofilament nylon suture with a rounded tip approximately 0.26 mm in diameter was inserted via the ECA into the ICA and was advanced approximately 18 mm to occlude the MCA, as monitored by doppler flowmetry. After 120 min, the occluder was removed to allow reperfusion. The ECA stump was ligated and the neck incision was closed. Using an automatic homeothermic blanket control unit, the animal's body temperature was maintained at 37°C throughout the procedure and during post-surgical recovery. Following the procedure, 12 rats were randomly assigned to vehicle (n=6) or glibenclamide (n=6) treatment. At 48 hours, the animals were euthanized and the brains were analyzed for tumor necrosis α (TNFα) expression.
Glibenclamide Treatment
Drug formulation of glibenclamide (#G2539; Sigma, St. Louis, MO) in dimethyl sulfoxide and the preparation of miniosmotic pumps were as described (19). Treatment consisted of administering a single loading dose of glibenclamide (10 μg/kg) or an equivalent volume of vehicle IP within 10 minutes of ischemia as well as continuous infusion via miniosmotic pump (Alzet 2001, 1.0 μL/hr; Alzet Corp, Cupertino, CA) beginning at the end of surgery, resulting in delivery of 200 ng/hr or an equivalent volume of vehicle subcutaneously for 1 week. The dose of glibenclamide used here has been shown to not result in hypoglycemia (20).
Antibodies
The custom anti-Trpm4 and anti-Sur1 antibodies used for this study have been previously described (15). The antigenic peptide for Trpm4 was the N-terminal intracellular domain of mouse Trpm4, corresponding to amino acids 1–612 (NP 780339). Anti-Trpm4 antibodies were raised in chicken, and were used at 1:200 dilution. The antigenic peptide for Sur1 was the intra-cellular nucleotide-binding domain 1 of Sur1 (rat Sur1 cDNA amino acids 598 to 965 of NP_037171). Anti-Sur1 antibodies were raised in rabbit and were used at 1:200 dilution. Other primary antibodies included: rabbit anti-cytokeratin 20 (prediluted; Ventana, Tucson, AZ) for colonic epithelial cells; mouse anti-glial fibrillary acidic protein (GFAP) (1:500; CY3 conjugated; C-9205; Sigma, St. Louis, MO) for astrocytes; mouse anti-NeuN (1:100; MAB377; Chemicon, Temecula, CA) for neurons; goat anti-CD31 (PECAM-1) (1:200; sc-1506; Santa Cruz Biotechnology, Santa Cruz, CA) for endothelial cells; goat anti-IgG (1:500; FITC conjugated; NB7477; Novus Biologicals, Littleton, CO) for immunoglobulin G (IgG); goat anti-TNFα (1:100; sc-1350; Santa Cruz Biotechnology, Santa Cruz, CA) for tumor necrosis factor α; rabbit anti-myeloperoxidase (MPO) (1:200, A0398; Dako, Carpinteria, CA) for neutrophils; and mouse anti-rat endothelial cell antigen (Reca-1) (1:100; MA1-81510; Thermo Fisher, Rockford, IL) for rat endothelium.
Immunohistochemistry and Förster Resonance Energy Transfer (FRET)
Deparaffinized sections were rinsed in ethanol and washed with phosphate-buffered saline (PBS; 10 mM, pH 7.4). For antigen retrieval, slides were placed in citrate buffer (10 mM, pH 8.0) and heated in a microwave oven at 900 W for 10 min, then washed in PBS. Slides were incubated with a mixture of 5% goat serum (Sigma) and 0.2% Triton X-100 for 1 hr at room temperature, prior to incubation overnight at 4 °C with anti-Trpm4 and/or anti-Sur1 antibodies and, in some cases, with a cell-specific or anti-IgG primary antibody. The slides were rinsed again in PBS. Single-label immunohistochemistry for Trpm4 was developed using biotin-conjugated secondary antibody. Sections were incubated for 30 min in PBS with 0.3% H2O2 to block endogenous peroxidase activity. Following overnight incubation with primary antibody, sections were incubated with biotinylated secondary antibody (BA-1000; 1:500 goat anti-chicken; Vector Laboratories, Burlingame, CA) for 2 hr. After washing in PBS, sections were incubated in avidin biotin solution (Vector Laboratories) and the color was developed in diaminobenzidine chromogen solution (0.02% diaminobenzidine in 0.175 M sodium acetate) activated with 0.01% hydrogen peroxide. Cresyl violet was used as a counterstain to visualize cell nuclei. The sections were rinsed, mounted, dehydrated, and cover-slipped with DPX mounting medium (Electron Microscopy Services, Fort Washington, PA). Omission of primary antibodies was used as a negative control.
To show localization of Trpm4 and/or Sur1 within identified cell types and/or co-expression with IgG, immunofluorescence microscopy was performed using the standard protocol with anti-Trpm4 or anti-Sur1 antibodies in addition to cell-specific and anti-IgG primary antibodies (as above). Slides were incubated for 1 hr with fluorescent-labeled mspecies appropriate secondary antibodies (1:500; Alexa Fluor 488 and Alexa Fluor 555; Invitrogen, Molecular Probes, Eugene, OR) at room temperature. Omission of primary antibody was used as a negative control. The sections were coverslipped with polar mounting medium containing anti-fade reagent and 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Eugene, OR). Immunolabeled sections were visualized using epifluorescence microscopy (Nikon Eclipse 90i; Nikon Instruments Inc., Melville, NY).
Antibody-based FRET was performed on acute lesions (cases 1-4) using anti-Sur1 and anti-Trpm4 antibodies, as described (14,15), except that FRET imaging and measurements of FRET efficiency were performed using a Zeiss LSM710 confocal microscope.
Analysis of Trpm4, Sur1, IgG and TNFα Protein Expression
Trpm4 immunoreactivity was analyzed for each cell-type specific marker, for each case (Table 1). All sections were immunolabeled as a single batch, and all images were collected using uniform parameters of magnification and exposure, as previously described (21,22). Two areas encompassing ischemic lesions or controls were randomly selected for construction of a montage, with each montage composed of 16 images, each 300 × 400 μm, that were acquired at 20× magnification. Images were independently evaluated by 2 observers blinded to demographic and clinical data. Specific Trpm4 immunoreactivity associated with each cell-type specific marker, for each case, was evaluated. Areas of maximum labeling were scored for each case, using a semiquantitative scale ranging from 0 to 4 [0: none; 1: +, weak punctuate labeling present in rare cells (<10%); 2: ++, weak punctate staining in scattered cells or aggregates (<50%); 3: +++, strong punctate staining present in many cells (>50%); 4: ++++, strong diffuse staining of most cells (>90%)]. The overall concordance between the two observers was >90%. In cases of disagreement, independent re-evaluation was performed by both observers to arrive at the final score. Sur1 immunoreactivity for each cell-type specific marker for each case was previously reported (23).
Table 1. Trpm4 protein expression in neurogliovascular cells (semi-quantitative scores).
| Case # | Capillaries | Venules | Arterioles | Neurons | Astrocytes | Neutrophils | Postinfarct interval |
|---|---|---|---|---|---|---|---|
| Focal Ischemic Infarcts | |||||||
| 1 | +++ | ++ | +++ | ++++ | ++ | +++ | 1 d |
| 2 | ++ | ++ | +++ | +++ | ++ | +++ | 3 d |
| 3 | ++++ | ++ | +++ | +++ | ++ | +++ | 5 d |
| 4 | ++ | ++ | ++ | +++ | ++ | ++++ | 5 d |
| 5 | ++++ | +++ | ++++ | +++ | ++ | ++++ | 7 d |
| 6 | ++++ | +++ | ++++ | +++ | ++ | ++++ | 7 d |
| 7a | +++ | ++ | +++ | +++ | ++ | +++ | 7 d |
| 7b | +++ | ++ | +++ | +++ | ++ | ++++ | 7 d |
| 8 | ++ | +++ | ++ | +++ | ++ | +++ | 15 d |
| 9 | ++++ | ++++ | ++++ | ++ | +++ | ++++ | 15 d |
| 10 | ++ | +++ | ++++ | +++ | ++++ | ++++ | 16 d |
| 11 | ++ | +++ | ++++ | ++ | ++++ | ++++ | 26 d |
| 12 | +++ | ++++ | ++++ | ++ | ++++ | +++ | 30 d |
| 13a | +++ | ++ | +++ | ++ | +++ | +++ | 31 d |
| 13b | ++ | ++ | ++ | ++ | ++++ | ++++ | 31 d |
| Contralateral | |||||||
| 1 | + | 0 | + | + | ++ | 0 | 1 d |
| 2 | 0 | 0 | 0 | + | ++ | + | 3 d |
| 3 | + | 0 | + | + | ++ | 0 | 5 d |
| 4 | 0 | 0 | + | 0 | + | 0 | 5 d |
| 5 | + | 0 | + | + | ++ | + | 7 d |
| 6 | 0 | 0 | 0 | 0 | 0 | + | 7 d |
| 7a | 0 | 0 | + | + | ++ | ++ | 7 d |
| 7b | + | 0 | + | ++ | 0 | + | 7 d |
| 8 | + | 0 | 0 | ++ | ++ | 0 | 15 d |
| 9 | 0 | 0 | 0 | 0 | + | + | 15 d |
| 10 | + | 0 | + | + | ++ | 0 | 16 d |
| 11 | 0 | 0 | + | + | + | + | 26 d |
| 12 | 0 | + | + | 0 | + | ++ | 30 d |
| 13a | 0 | 0 | ++ | + | ++ | + | 31 d |
| 13b | 0 | 0 | + | + | ++ | + | 31 d |
| Control Brains | |||||||
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
| 15 | 0 | 0 | 0 | 0 | 0 | + | N/A |
| 16 | + | 0 | 0 | + | + | 0 | N/A |
| 17 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
| 18 | 0 | 0 | + | 0 | + | + | N/A |
| 19 | + | 0 | + | + | + | 0 | N/A |
Unbiased measurements of specific IgG or TNFα labeling within regions of interest (ROIs) were obtained using NIS-Elements AR software (Nikon Instruments, Melville, NY) from sections of acute lesions (cases 1-4) and controls immunolabeled in a single batch, as previously described (24). All ROI images for a given signal were captured using uniform parameters of magnification, area, exposure and gain. To quantify IgG or TNFα, circular ROIs were defined as a perimeter encompassing vessels, with the diameter twice that of the vessel, within the infarct or control brain. For each ROI, a histogram of pixel intensity was constructed to determine the intensity of background labeling. Pixels within the ROI were defined as having specific labeling if their intensity was >3× that of the background. The area occupied by pixels with specific labeling was used to calculate the percent of the region of interest (% ROI) with specific labeling.
In Situ Hybridization for Trpm4 mRNA
Digoxigenin labeled probes, antisense (5′- CCGAGAGTGGAATTCCCGGATGAGGCGGTAACGCTGC-3′) and sense (5′-GCAGCGTTACCGCCTCATCCGGGAATTCCACTCTCGG-3′), designed to hybridize to nucleotides 3288–3324 located within coding sequence of the human Trpm4 gene (NM_017636), were supplied by IDT (Integrated DNA Technologies, Inc., Coralville, IA). In situ hybridization was performed on 10-μm sections of acute lesions (cases 1-4) and controls using an IsHyb In Situ Hybridization (ISH) Kit (Biochain Institute, Inc., Newark, CA) according to the manufacturer's protocol. Deparaffinization and rehydration were performed as described above. Sections were then incubated in diethyl pyrocarbonate (DEPC)-treated PBS and fixed in 4% paraformaldehyde in PBS for 20 min. After being rinsed twice with DEPC-PBS, the slides were treated with 10 μg/ml proteinase K at 37 °C for 10 min. Slides were then washed in DEPC-PBS, rinsed with DEPC-H2O, and prehybridized with ready-to-use prehybridization solution (BioChain Institute, Newark, CA) for 3 hr at 50 °C. The DIG-labeled probe was diluted in hybridization buffer (BioChain Institute, Newark, CA) and applied at 4 ng/μl. Sections then were incubated at 45 °C for 16 hr. Post-hybridization washing and immunological detection, using anti-DIG-alkaline phosphatase (AP) with NBT/BCIP as substrates were performed as recommended by the manufacturer. AP-conjugated anti-DIG antibodies (1:100 PBS diluted, BioChain Institute, Newark, CA), were incubated with slides for 2 hr. Finally, slides were rinsed in distilled H2O and then were immunolabeled for Trpm4 protein using a fluorescent secondary antibody, as above. The dark purple reaction product represents Trpm4 mRNA; green fluorescence indicates immunohistochemical staining for Trpm4 protein. In situ hybridization for Sur1 has been previously reported [23].
Statistical Analysis
Two-tailed Fisher's exact test was used to determine contingency of absent or weak (0/+/++) versus prominent or diffuse (+++/++++) Trpm4 staining in different cell types present within infarcted and non-infarcted cortices. Scores for Trpm4 expression in different cell types were additionally analyzed as a function of post-mortem and post-infarct intervals by calculating Spearman correlation coefficient. IgG and TNFα labeling were analyzed using Student's t-test. Calculations were performed using OriginPro version 8 (Origin Lab Corp., Northampton, MA).
Results
I. Positive and Negative Controls
The custom anti-Trpm4 antibody used here was validated previously in rat and human cerebral tissues (14,15). The specificity of the antibody batch used for this study was further validated in human colon epithelium, which is known to express high levels of Trpm4 (Fig. 1) (25). Omission of primary antibody and tissues obtained from Trpm4 knockout mice were used as negative controls, and confirmed the specificity of labeling (not shown).
Figure 1.
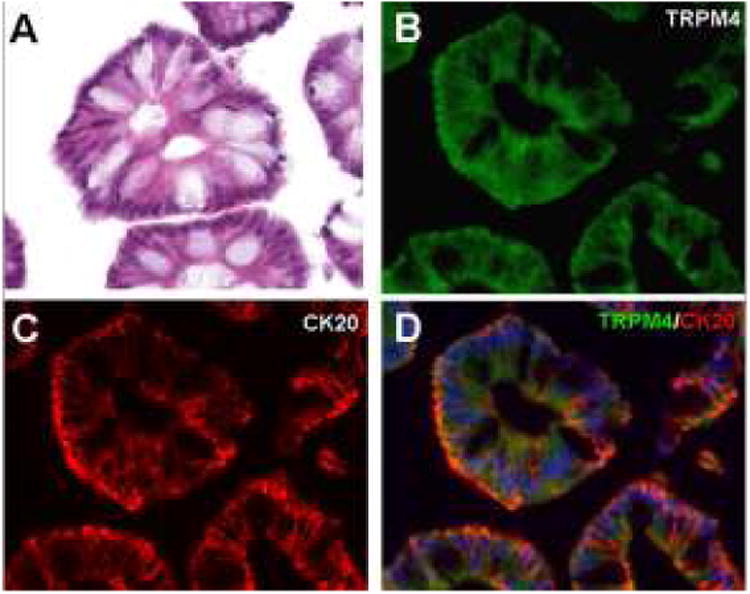
Transient receptor potential melastatin 4 (Trpm4)–positive controls. (A) Hematoxylin-eosin stained section of normal colon shows villus epithelial cells. (B–D) Immunohistochemistry using primary antibodies against Trpm4 protein shows cytoplasmic labeling of colonic epithelial cells (B), and co-localization with cytokeratin 20 (CK20) (C); merged fluorescent image shown in (D). Scale bar, 50 μm; green/FITC, Trpm4; red/CY3, CK20; blue, DAPI.
II. Trpm4 Expression in Infarcted Cerebral Cortex
Ischemic lesions originated from 15 men and 5 women, with age at death ranging from 32 to 84 years (mean, 61 years). Normal specimens originated from 4 men and 2 women who had died rapidly from non-neurological diseases (i.e., acute cardiovascular or respiratory disorders). The post-mortem interval ranged between 12–80 hours. Additional demographic information on these patients have been reported previously (23).
Trpm4 protein expression was analyzed in infarcted and non-infarcted cortices using immunohistochemistry. Representative sections of acute infarcts are shown in Figure 2. Immunoperoxidase preparations revealed prominent Trpm4 immunoreactivity in virtually all neural and vascular cells (Table 1; Fig. 2A), whereas non-infarcted contralateral and control cortices demonstrated only weak immunoreactivity in rare cells and/or background neuropil (Table 1; Fig. 2B).
Figure 2.
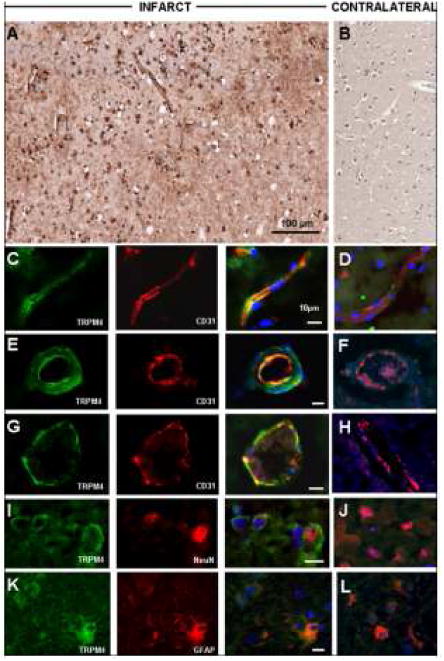
Transient receptor potential melastatin 4 (Trpm4) protein is upregulated in focal cerebral infarcts. (A,B) Low power images illustrating immunohistochemistry with diaminobenzidine chromogen staining, showing upregulation of Trpm4 in neural and vascular cells of infarcted cortex (A), relative to control (B). With fluorescent double labeling, increased Trpm4 protein was observed within CD31-positive capillaries (C), arterioles (E) and venules (G); NeuN-positive neurons (I), and glial fibrillary acidic protein (GFAP)–positive astrocytes (K) in the ischemic tissues (C, E, G, I, K) versus contralateral control tissues (D, F, H, J, L); merged images of double labeling shown in the third and fourth columns. Original magnifications, 20× (A, B) or 40× (C-N); scale bars, 100 μm (A) or 10 μm (C, E, G, I, K, M); green/FITC, Trpm4; red/CY3, PECAM-1 (CD31) or NeuN or GFAP; blue, DAPI; the images shown are from cases 3 and 6.
Immunofluorescent preparations, used for cellular identification, further demonstrated marked upregulation of Trpm4 in neural and vascular cells present in ischemic infarcts (Fig. 2C, E, G, I, K). Endothelial cells of capillaries, arterioles and venules exhibited diffuse membranous and cytoplasmic expression, and co-labeled for the endothelial cell marker PECAM (CD31) (Fig. 2C, E, G). Trpm4 immunolabeling also was prominent in neurons immunoreactive for NeuN (Fig. 2I) and astrocytes immunoreactive for GFAP (Fig. 2K). Neurons, endothelial cells, and astrocytes in non-infarcted contralateral and control cortices exhibited weak or no Trpm4 labeling by immunohistochemistry (Table 1; Fig. 2D, F, H, J, L).
In situ hybridization studies showed de novo transcriptional upregulation of Trpm4 mRNA in acute lesions. There was strong labeling of cortical endothelial cells of capillaries, arterioles, and venules, and upregulation in frequent neurons (Fig. 3, middle column) present within ischemic cortex. In situ hybridization signals for Trpm4 mRNA were present in cells that were strongly immunopositive for Trpm4 protein (Fig. 3, left column), while contralateral and control cortices showed only faint in situ labeling in rare cells (Fig. 3, right column).
Figure 3.
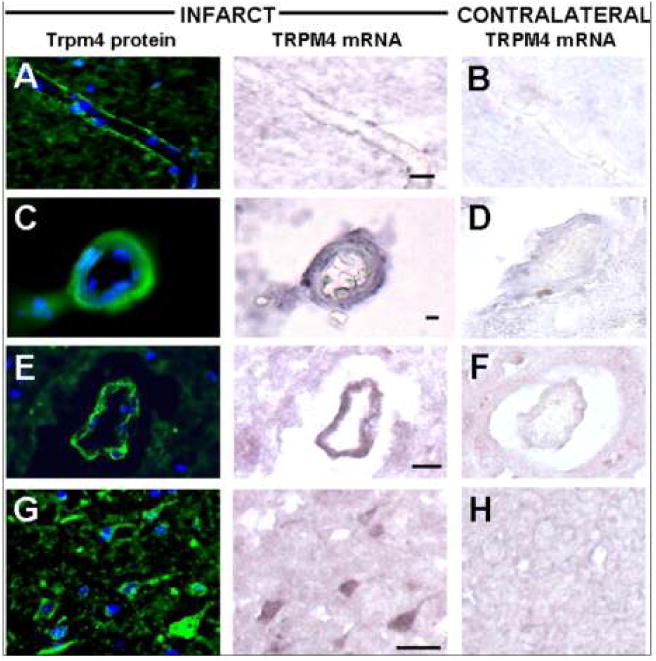
In situ hybridization shows upregulated Trpm4 mRNA in acute ischemic infarcts. Tissues from recently infarcted cortex were hybridized with antisense probe and labeled with immunohistochemical stain directed against Trpm4 protein; dual labeling for Trpm4 mRNA and Trpm4 protein was identified within endothelial cells in capillaries (A), arterioles (C), and venules (E), as well as in scattered neurons (G) in the ischemic tissues (A, C, E, G) versus contralateral cortex (B, D, F, H). Original magnification, 20×; scale bar, 10 μm; the images shown are from cases 3 and 6.
II.A Time Course of Trpm4 Expression in Ischemic Cells
Trpm4 expression was evaluated semi-quantitatively in different cell types at various times after onset of ischemic events. Statistically significant differences were not observed in any cell type as a function of postmortem interval. However, upregulated protein was found in neural and vascular cells as early as one day after clinical onset of ischemia, and remained elevated in specimens obtained from patients who died up to 30 days following clinical onset of stroke. Statistically significant differences in staining intensities were found in all cell types, relative to controls (p<0.05 for astrocytes; p<0.01 for endothelial cells, neurons and neutrophils) (Table 1). Neurons stained prominently in acute lesions, with mild regression of staining noted with post-infarct interval (p<0.01) (Table 1). Endothelial cells and neutrophils exhibited sustained Trpm4 upregulation over time (Table 1). Astrocytes exhibited a unique pattern of Trpm4 immunoreactivity: in non-infarcted cortex, Trpm4 appeared as punctate perinuclear staining, whereas a progressive increase in extent of cytoplasmic and membranous immunopositivity was observed with post-infarct interval (p<0.01) (Table 1; Fig. 4).
Figure 4.
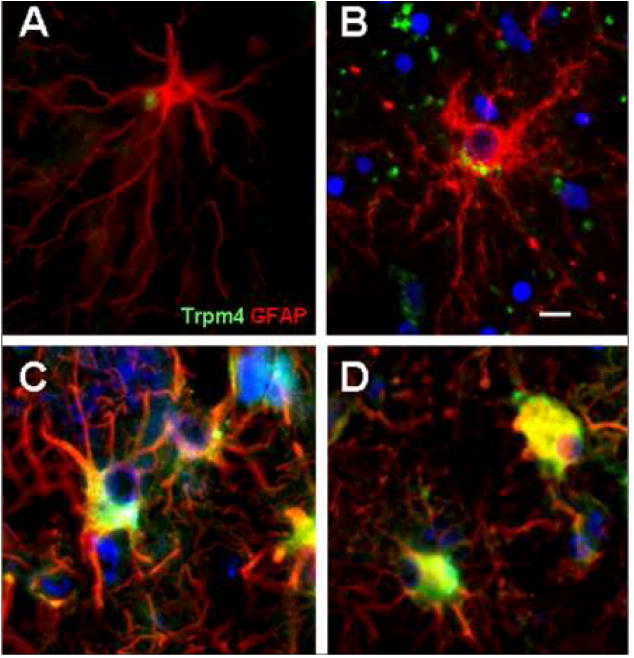
Unique pattern of Trpm4 immunoreactivity in astrocytes. Astrocytes showed perinuclear dot-like immunoreactivity for Trpm4 in normal cortex (A), with prominent perinuclear accentuation in acute infarcts (B) and intensified cytoplasmic and distal process staining in subacute infarcts (C, D). Original magnification, 20×; scale bar, 10 μm; green/FITC, Trpm4; red/CY3, glial fibrillary acidic protein (GFAP); nuclei stained with DAPI; merged images are shown from cases 19, 1, 9, and 13b.
II.B Trpm4 and Sur1 Proteins are Co-Expressed and Co-Associate
Sur1 was previously reported to be upregulated in endothelial cells, neurons, and astrocytes in infarcted cerebral cortices (23). Here, we found that upregulated Trpm4 co-localized with Sur1 in endothelial cells, neurons, and astrocytes in infarcted cerebral cortices (Fig. 5). We used FRET to determine whether the co-localized proteins also co-associated. FRET analysis demonstrated that Sur1 and Trpm4 co-associated in endothelial cells (Fig. 6A-D) and neurons. FRET efficiency, which reflects the proximity of the two fluorophores, was 13% in infarcted cortices, and 0% in controls (Fig. 6E).
Figure 5.
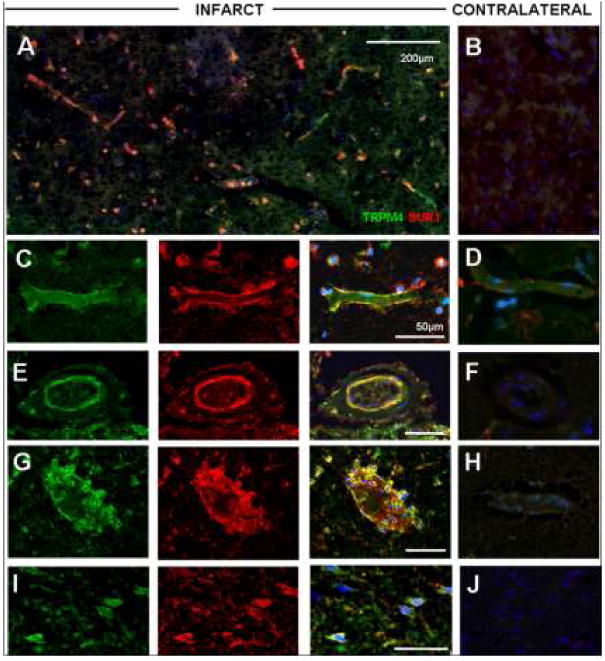
Transient receptor potential melastatin 4 (Trpm4) and sulfonylurea receptor 1 (Sur1) proteins are upregulated and co-localize in acute ischemic infarcts. Fluorescent double labeling studies demonstrated prominent expression in scattered neural and vascular cells of an acute infarct (A), relative to contralateral brain (B). High-power images of infarcted cortex show co-localization of proteins within capillaries (C), arterioles (E), venules and neutrophils (G), as well as neurons (I) present within infracted cortex, with negligible staining in controls (D, F, H, J). Original magnification, 20×, scale bar, 10 μm; green/FITC, Trpm4; red/CY3, Sur1; blue, DAPI; the images shown are from cases 2 and 3.
Figure 6.
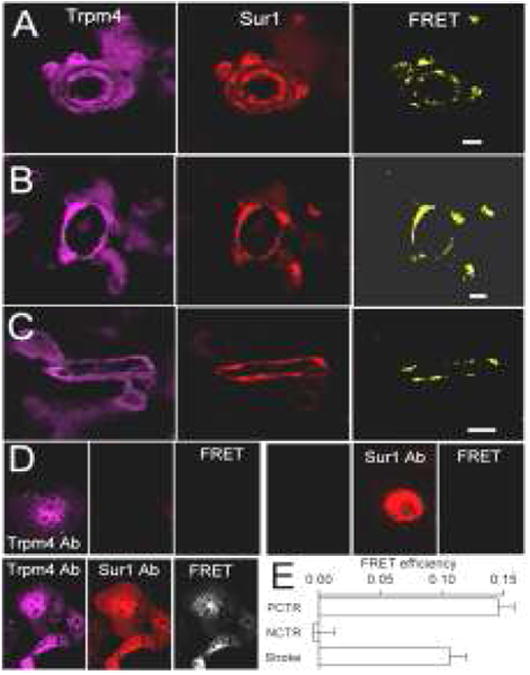
Transient receptor potential melastatin 4 (Trpm4) and sulfonylurea receptor 1 (Sur1) proteins co-associate in acute ischemic infarcts. Immunolabeled sections reveal coexpression of Trpm4 and Sur1, with FRET signals elicited in cortical arterioles (A), venules (B), capillaries (C) and neurons (not shown) present in ischemic infarcts. Validation of antibodies for antibody-based FRET analysis (D): COS-7 cells expressing either Trpm4 or Sur1 (upper panels) or co-expressing both (lower panel) were incubated with either anti-Sur1 or anti-Trpm4 antibodies (upper panel) or both (lower panel), followed by anti-rabbit Cy3-conjugated plus anti-goat Cy5-conjugated secondary antibodies. Fluorescence images of the 2 fluorophores (left and middle columns), as well as FRET images (right column) are shown; data are representative of 6 replicates; note that the primary antibodies against Sur1 and Trpm4 do not cross-react. FRET efficiencies in positive and negative controls (PCTR, NCTR) and in infarcts (Stroke) are shown in (E).
II.C Pathologic Correlates of Sur1-Trpm4 Channel Upregulation in Cerebral Infarcts
Sur1+/Trpm4+ endothelial cells and Sur1+/Trpm4+ neurons present in infarcted cerebral cortex and peri-infarct zones showed prominent membrane irregularities and frank blebbing, consistent with cytotoxic edema (Fig. 7A). Sur1 and Trpm4 co-expression in abnormal-appearing endothelial cells also was associated with vasogenic edema, as evidenced by upregulated perivascular labeling for IgG (Fig. 7B-G), TNFα (Fig. 7I) and MPO (Fig. 7J). Membrane blebbing was not identified in Trpm4-negative neural or vascular cells. Additionally, membrane blebbing was not seen in Trpm4-negative or Trpm4-positive astrocytes present in infarcted or non-infarcted cortices (Fig. 4).
Figure 7.
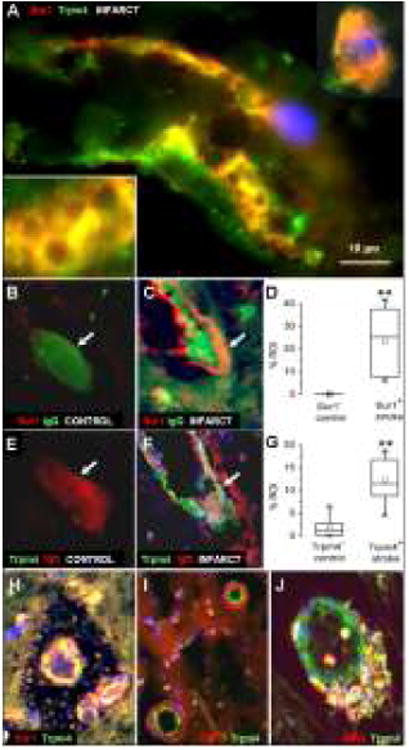
Oncosis and membrane blebbing are present in Sur1+/Trpm4+ cells in acute cerebral infarcts. Note the cytoplasmic vacuolization, membrane thickening and irregularities present in endothelial cells of a small venule and a labeled neuron (A). Intravascular IgG is present within Sur1− microvessels (B), but is extravasated around Sur1+ endothelium in infarcts (C). Similarly, intravascular IgG is present within Trpm4− microvessels (E), but is extravasated around Trpm4+ endothelium in infarcts (F). Quantitative evaluation of IgG labeling in the infarcted and non-infarcted regions of interest (ROI) revealed significant increases in perivascular IgG around Sur1- and Trpm4-expressing versus nonexpressing vessels (D,G); Channel expression in microvessels was also associated with early hemorrhagic transformation (H), perivascular TNFα labeling (I) and inflammation (J). **P<0.01. Original magnification, 100× (A) or 40× (B,C,E,F,H-J); scale bar, 10 μm; green/FITC, Trpm4 or IgG; red/CY3, Sur1 or IgG or TNFα or MPO; DAPI, blue; the images shown are from cases 3 and 6.
III. Inhibition of Sur1 in a Rat MCAo Stroke Model
To assess the consequences of Sur1-Trpm4 channel expression, the effect of pharmacological inhibition of Sur1 was evaluated using glibenclamide in a rat MCAo stroke model. Extravasation of serum proteins is known to promote neuroinflammation. Inhibition of Sur1 was found to mitigate expression of TNFα, an acute phase reactant cytokine (Fig 8), suggesting a critical proinflammatory role of Sur1-Trpm4 channel expression in the context of focal cerebral ischemia.
Figure 8.
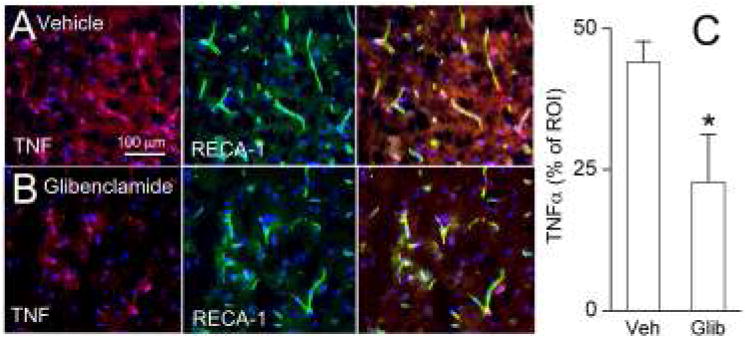
Pharmacologic inhibition of the Sur1-Trpm4 channel in a rat MCAo stroke model mitigates TNFα expression. Penumbral cortex immunolabeled for TNFα (left panel, red) and rat endothelial cell antigen-1 (RECA; middle panel, green) in vehicle (A) or glibenclamide (B) treated rats; merged images are shown in right panel. Bar graph of quantitative ROI analysis is shown (C); *, P<0.05; 6 rats per group.
Discussion
This is the first report to systematically analyze Trpm4 expression in adult human brains following the onset of focal cerebral ischemia. Our findings indicate that Trpm4 mRNA and Trpm4 protein are upregulated in infarcted human cortex. The current study accords with a report of Trpm4 upregulation in a pre-clinical model of stroke (13). In contrast to the findings in the animal model, however, our study showed that Trpm4 upregulation was sustained in infarcted human tissues over the acute and subacute post-ischemic intervals. Analysis of Trpm4 expression in human neurological disease previously was limited to specimens originating from patients with multiple sclerosis (18) or subarachnoid hemorrhage (14). Significant upregulation of Trpm4 was identified in both neuropathologic conditions. In animal models, Trpm4 expression has been associated with inflammation-induced axonal and neuronal injury in demyelinating lesions (18). Also, endothelial upregulation of Trpm4 has been associated with inflammation and BBB permeability in a model of subarachnoid hemorrhage (14), and with capillary fragmentation and secondary hemorrhage in a model of spinal cord injury (12). These published data suggest a critical function of Trpm4 in acute CNS injuries of varying etiology.
In a prior analysis, we showed prominent upregulation of Sur1, a member of the ATP-binding cassette protein superfamily, in neural and vascular cells of these same postmortem infarct specimens (23). The current study complements these prior findings and is the first to report that Trpm4, a voltage-dependent cation channel, co-localizes with Sur1 in neurons, endothelial cells, astrocytes and neutrophils present in human cerebral infarcts. Furthermore, this is the first study to document via antibody-based FRET the co-association of upregulated Trpm4 and Sur1 in human cerebral infarcts. Together, these findings indicate that Sur1-Trpm4 channels (formerly known as Sur1-regulated NCCa-ATP channels) are present in infarcted human cerebral cortex, but not in non-infarcted control specimens.
The Sur1-Trpm4 cation channel is not constitutively present in CNS tissues, but is transcriptionally upregulated de novo under neuropathological conditions. Recent data showed stable heteromultimeric assembly of Sur1 and Trpm4 and demonstrated that Sur1 regulates the calcium-sensitivity of Trpm4, the pore-forming subunit of the Sur1-Trpm4 channel (15). The channel is activated by a rise in intracellular [Ca2+] or by depletion of intracellular ATP. Channel activation induces monovalent cation influx that results in cell membrane depolarization.
As Ca2+ ions are an important second messenger, significant amounts of energy are expended to maintain intracellular concentrations of free Ca2+ at physiological levels (∼10,000-fold lower than extracellular levels). Because a large electrochemical driving force promotes Ca2+ influx into cells, homeostasis requires Ca2+ extrusion, which is achieved through the plasma membrane Ca2+-ATPase (PMCA) pump, the Na+-Ca2+ exchanger, and other energy-dependent mechanisms. Oxygen, glucose and energy restriction during cerebral ischemia rapidly results in increased cytoplasmic [Ca2+] (26). If unchecked, excess Ca2+ can lead to enhanced phospholipase and protease activities, inducing irreversible catabolic processes and producing free radicals that activate multiple cell death subroutines. Thus, there is an important adaptive advantage in upregulating the expression of Sur1-Trpm4 – the membrane depolarization induced by Ca2+-mediated activation of Sur1-Trpm4 channels provides negative feedback that reduces the electrical driving force for inward Ca2+ flux, and thereby acts to protect the cell from excessive Ca2+. However, sustained channel activity, as can be brought about by severe ATP depletion, results in continuous monovalent cation influx, converting an adaptive advantage into a maladaptive phenomenon that leads to cytotoxic edema and necrotic (oncotic) cell death (16).
The potential benefits of Sur1-Trpm4 channel blockade in stroke have been demonstrated in various studies. In a rat MCAo stroke model, Trpm4 gene suppression was associated with preserved vascular integrity, enhanced angiogenesis, reduced infarct volume and improved functional recovery (13). A study using primary cultures of human umbilical vein endothelial cells (HUVEC) showed that suppression of Trpm4, by pharmacological inhibition or siRNA, protected cells against lipopolysaccharide-induced cell death (27). Sulfonylurea therapy via low-dose glibenclamide (a.k.a. glyburide), which binds to Sur1 and potently inhibits the Sur1-Trpm4 channel, has been shown to confer protective effects, including reduced edema, cortical stroke volumes, and overall mortality in various rodent stroke models (28-30). Moreover, sulfonylurea therapy in diabetic stroke patients has been associated with improved neurological function and reduced rates of symptomatic hemorrhagic transformation and death, relative to diabetics managed without sulfonylurea agents (31,32). In a pilot study, stroke patients treated with RP-1127 (glyburide formulated for intravenous infusion) showed improved clinical outcomes, compared to historical controls (31). The current study further supports the hypothesis that Sur1 co-associates with Trpm4 to form functional Sur1-Trpm4 channels in human cerebral ischemia, and suggests that channel expression is associated with post-ischemic BBB disruption and neuroinflammation. As a constitutive function of this channel has not been identified in normal brain, and since both Abcc8 and Trpm4 knockout mice exhibit near-normal phenotypes, pharmacological inhibition of the Sur1-Trpm4 channel may represent a promising novel therapeutic strategy in patients who sustain focal cerebral infarction.
Due to an aging population, the absolute number of stroke events is projected to increase over the next two decades (34), and the management of stroke patients remains a major clinical challenge. Affected patients are at high risk for progressive neurologic deterioration and death due to malignant cerebral edema. While important progress has been made in understanding the pathophysiology of stroke in recent years, specific molecular mechanisms involved in post-ischemic cell death are relatively ill defined. Elucidation of these mechanisms is necessary to optimize outcomes of patients who sustain focal cerebral infarction. The identification of new pharmacotherapies with extended therapeutic time windows remains a critical goal in medicine. The current study is limited to examination of cerebral infarcts originating from stroke patients with poor outcome (i.e,. death). Nonetheless, in light of recent data (14-16,23,33,35,36), these data suggest an important role for the Sur1-Trpm4 channel in pathophysiology of post-ischemic cell death and implicates pharmacological blockade of the Sur1-Trpm4 channel as a possible novel therapeutic strategy to mitigate neuronal loss and malignant cerebral edema in patients who sustain large, territorial cerebral infarction.
Acknowledgments
This work was supported by grants to R.I.M. and J.M.S. from the National Institute of Neurological Disorders and Stroke (NINDS) (K08NS089830; NS061808), and to J.M.S. from the National Heart, Lung and Blood Institute (HL082517). Human tissues were obtained, in part, from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD.
J.M.S. holds a US patent (7,285,574), “A novel non-selective cation channel in neural cells and methods for treating brain swelling”. J.M.S. is a member of the scientific advisory board and holds shares in Remedy Pharmaceuticals. No support, direct or indirect, was provided to J.M.S., or for this project by Remedy Pharmaceuticals.
Footnotes
Disclosure/Conflicts of Interest: All other authors declare no conflict of interest.
References
- 1.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Executive Summary: Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation. 2013;127(1):143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 3.Truelsen T, Begg S, Mathers C. The global burden of cerebrovascular disease. [accesed 12-30-2012];2012 http://www.who.int/healthinfo/statistics/bod_cerebrovasculardiseasestroke.pdf. cited 2013 Jan 10. Available from: URL: http://www.who.int/healthinfo/statistics/bod_cerebrovasculardiseasestroke.pdf.
- 4.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42(7):1952–5. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jauss M, Schutz HJ, Tanislav C, Misselwitz B, Rosenow F. Effect of daytime, weekday and year of admission on outcome in acute ischaemic stroke patients treated with thrombolytic therapy. Eur J Neurol. 2010;17(4):555–61. doi: 10.1111/j.1468-1331.2009.02845.x. [DOI] [PubMed] [Google Scholar]
- 6.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke. 2008;39(3):924–8. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 7.Singer OC, Hamann GF, Misselwitz B, Steinmetz H, Foerch C. Time trends in systemic thrombolysis in a large hospital-based stroke registry. Cerebrovasc Dis. 2012;33(4):316–21. doi: 10.1159/000335816. [DOI] [PubMed] [Google Scholar]
- 8.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 9.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathar I, Jacobs G, Kecskes M, Menigoz A, Philippaert K, Vennekens R. TRPM4. Handb Exp Pharmacol. 2014;222:461–87. doi: 10.1007/978-3-642-54215-2_18. [DOI] [PubMed] [Google Scholar]
- 11.Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handb Exp Pharmacol. 2007;179:269–85. doi: 10.1007/978-3-540-34891-7_16. [DOI] [PubMed] [Google Scholar]
- 12.Gerzanich V, Woo SK, Vennekens R, et al. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat Med. 2009;15(2):185–91. doi: 10.1038/nm.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loh KP, Ng G, Yu CY, et al. TRPM4 inhibition promotes angiogenesis after ischemic stroke. Pflugers Arch. 2014;466(3):563–76. doi: 10.1007/s00424-013-1347-4. [DOI] [PubMed] [Google Scholar]
- 14.Tosun C, Kurland DB, Mehta R, et al. Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke. 2013;44(12):3522–8. doi: 10.1161/STROKEAHA.113.002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM. The sulfonylurea receptor 1 (sur1)-transient receptor potential melastatin 4 (trpm4) channel. J Biol Chem. 2013;288(5):3655–67. doi: 10.1074/jbc.M112.428219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simard JM, Woo SK, Gerzanich V. Transient receptor potential melastatin 4 and cell death. Pflugers Arch. 2012;464(6):573–82. doi: 10.1007/s00424-012-1166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab. 2012;32:1699–717. doi: 10.1038/jcbfm.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schattling B, Steinbach K, Thies E, et al. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2012;18:1805–11. doi: 10.1038/nm.3015. [DOI] [PubMed] [Google Scholar]
- 19.Simard JM, Woo SK, Tsymbalyuk N, Voloshyn O, Yurovsky V, Ivanova S, Lee R, Gerzanich V. Glibenclamide-10-h Treatment Window in a Clinically Relevant Model of Stroke. Transl Stroke Res. 2012;3:286–95. doi: 10.1007/s12975-012-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab. 2012;32:1699–1717. doi: 10.1038/jcbfm.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerzanich V, Ivanov A, Ivanova S, et al. Alternative splicing of cGMP-dependent protein kinase I in angiotensin-hypertension: novel mechanism for nitrate tolerance in vascular smooth muscle. Circ Res. 2003;93:805–12. doi: 10.1161/01.RES.0000097872.69043.A0. [DOI] [PubMed] [Google Scholar]
- 22.Simard JM, Geng Z, Woo SK, et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29:317–30. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta RI, Ivanova S, Tosun C, Castellani RJ, Gerzanich V, Simard JM. Sulfonylurea receptor 1 expression in human cerebral infarcts. J Neuropathol Exp Neurol. 2013;72:871–83. doi: 10.1097/NEN.0b013e3182a32e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel AD, Gerzanich V, Geng Z, Simard JM. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol. 2010;69:1177–90. doi: 10.1097/NEN.0b013e3181fbf6d6. [DOI] [PubMed] [Google Scholar]
- 25.Xu XZ, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci U S A. 2001;98:10692–7. doi: 10.1073/pnas.191360198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristian T, Siesjo BK. Calcium in ischemic cell death. Stroke. 1998;29(3):705–18. doi: 10.1161/01.str.29.3.705. [DOI] [PubMed] [Google Scholar]
- 27.Becerra Al, Echeverría C, Varela D, et al. Transient receptor potential melastatin 4 inhibition prevents lipopolysaccharide-induced endothelial cell death. Cardiovasc Res. 2011;91:677–84. doi: 10.1093/cvr/cvr135. [DOI] [PubMed] [Google Scholar]
- 28.Simard JM, Tsymbalyuk N, Tsymbalyuk O, Ivanova S, Yurovsky V, Gerzanich V. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke. 2010;41(3):531–7. doi: 10.1161/STROKEAHA.109.572644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12(4):433–40. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simard JM, Yurovsky V, Tsymbalyuk N, Melnichenko L, Ivanova S, Gerzanich V. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009;40(2):604–9. doi: 10.1161/STROKEAHA.108.522409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunte H, Schmidt S, Eliasziw M, et al. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke. 2007;38(9):2526–30. doi: 10.1161/STROKEAHA.107.482216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunte H, Busch MA, Trostdorf K, et al. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol. 2012;72(5):799–806. doi: 10.1002/ana.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheth KN, Kimberly WT, Elm JJ, et al. Pilot study of intravenous glyburide in patients with a large ischemic stroke. Stroke. 2014;45(1):281–3. doi: 10.1161/STROKEAHA.113.003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinlay S. Changes in stroke epidemiology, prevention, and treatment. Circulation. 2011 Nov 8;124(19):e494–6. doi: 10.1161/CIRCULATIONAHA.111.069633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simard JM, Kahle KT, Gerzanich V. Molecular mechanisms of microvascular failure in central nervous system injury--synergistic roles of NKCC1 and Sur1/Trpm4. J Neurosurg. 2010;113(3):622–9. doi: 10.3171/2009.11.JNS081052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang E, Liao P. Brain transient receptor potential channels and stroke. J Neurosci Res. 2014 Dec 11; doi: 10.1002/jnr.23529. [DOI] [PubMed] [Google Scholar]


