Abstract
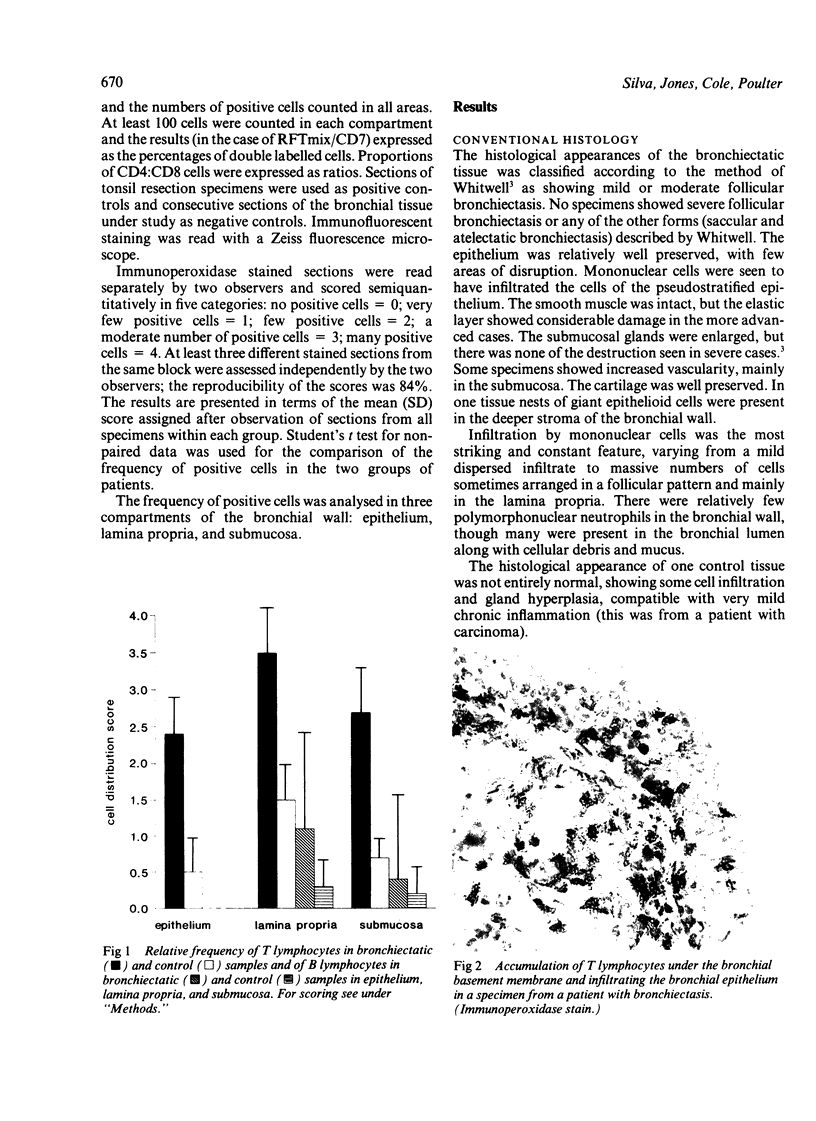
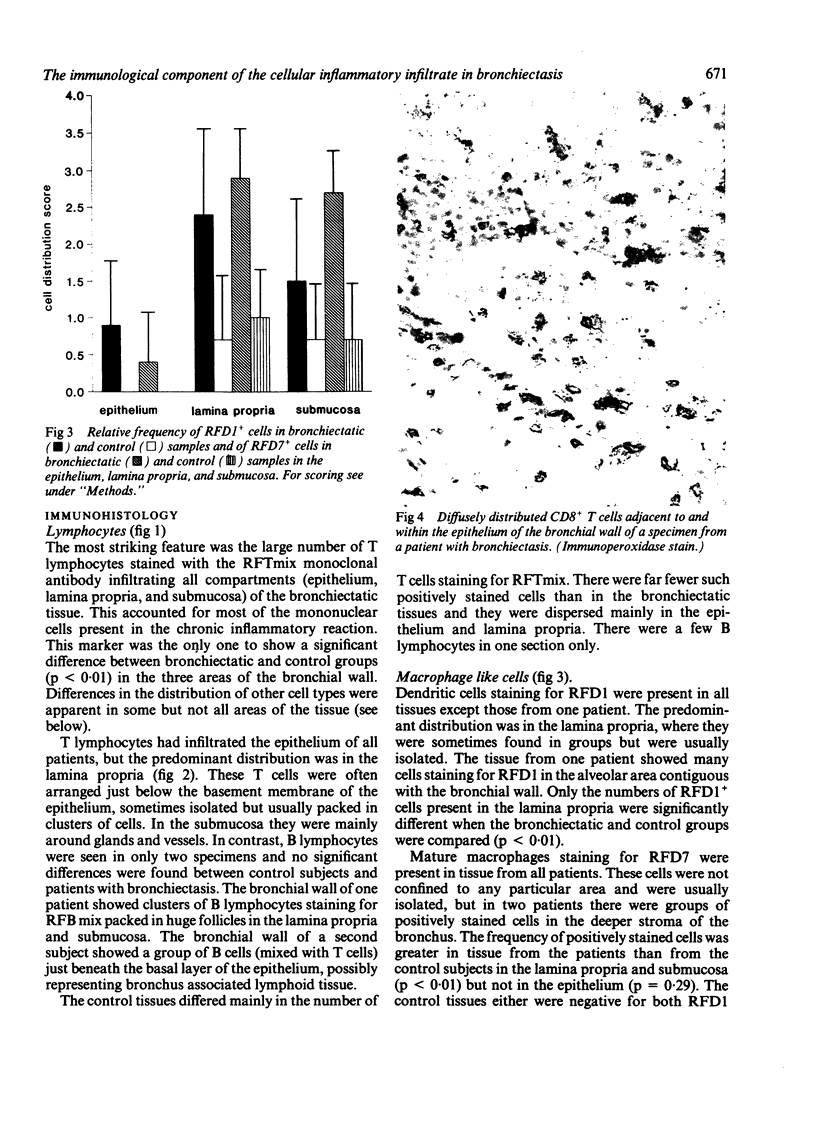
Immunohistological analysis of bronchial biopsy specimens from nine patients with bronchiectasis and four control subjects was performed with a panel of monoclonal antibodies selected to show lymphocyte and macrophage subsets and signs of cellular activation. The cells taking part in the inflammatory response in the bronchial wall of patients with bronchiectasis were almost exclusively mononuclear cells, most of them T lymphocytes. B lymphocytes were observed in biopsy specimens from only two out of nine patients. CD8+ T cells outnumbered CD4+ cells in all patients in a ratio ranging from 2:1 to 10:1. Most T lymphocytes also strongly expressed CD7 antigen and a proportion of them expressed HLA-DR. Most of the lymphocytic infiltration occurred just beneath the basement membrane of the epithelium, though intraepithelial and submucosal infiltration was also seen. Non-lymphoid mononuclear cells expressing the phenotype of dendritic cells and macrophages were found dispersed throughout the infiltrate, most of them expressing HLA-DR. These observations support the hypothesis that cell mediated immunological reactions contribute to the inflammation associated with bronchiectasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienenstock J., Befus D. Gut- and bronchus-associated lymphoid tissue. Am J Anat. 1984 Jul;170(3):437–445. doi: 10.1002/aja.1001700316. [DOI] [PubMed] [Google Scholar]
- Bienenstock J. Mucosal immunological protection mechanisms in the airways. Eur J Respir Dis Suppl. 1986;147:62–71. [PubMed] [Google Scholar]
- Campbell D. A., du Bois R. M., Butcher R. G., Poulter L. W. The density of HLA-DR antigen expression on alveolar macrophages is increased in pulmonary sarcoidosis. Clin Exp Immunol. 1986 Jul;65(1):165–171. [PMC free article] [PubMed] [Google Scholar]
- Collings L. A., Tidman N., Poulter L. W. Quantitation of HLA-DR expression by cells involved in the skin lesions of tuberculoid and lepromatous leprosy. Clin Exp Immunol. 1985 Jul;61(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract. N Engl J Med. 1984 Jan 26;310(4):235–244. doi: 10.1056/NEJM198401263100406. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Prentice H. G. T cell subpopulations, monoclonal antibodies and their therapeutic applications. Clin Haematol. 1982 Oct;11(3):631–660. [PubMed] [Google Scholar]
- Kallenberg C. G., Schilizzi B. M., Beaumont F., Poppema S., De Leij L., The T. H. Expression of class II MHC antigens on alveolar epithelium in fibrosing alveolitis. Clin Exp Immunol. 1987 Jan;67(1):182–190. [PMC free article] [PubMed] [Google Scholar]
- Munro C. S., Campbell D. A., Collings L. A., Poulter L. W. Monoclonal antibodies distinguish macrophages and epithelioid cells in sarcoidosis and leprosy. Clin Exp Immunol. 1987 May;68(2):282–287. [PMC free article] [PubMed] [Google Scholar]
- Munro C. S., Mitchell D. N., Poulter L. W., Cole P. J. Early cellular responses to intradermal injection of Kveim suspension in normal subjects and those with sarcoidosis. J Clin Pathol. 1986 Feb;39(2):176–182. doi: 10.1136/jcp.39.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Collings L. A., Tung K. S., Waters M. F. Parasitism of antigen presenting cells in hyperbacillary leprosy. Clin Exp Immunol. 1984 Mar;55(3):611–617. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Panayi G. S., Hobbs S., Raftery M. J., Janossy G. Activated T lymphocytes of the synovial membrane in rheumatoid arthritis and other arthropathies. Scand J Immunol. 1985 Dec;22(6):683–690. doi: 10.1111/j.1365-3083.1985.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- WHITWELL F. A study of the pathology and pathogenesis of bronchiectasis. Thorax. 1952 Sep;7(3):213–239. doi: 10.1136/thx.7.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]