Abstract
Objectives
Evaluate health plan interventions targeting physician chronic opioid therapy (COT) prescribing.
Methods
In 2006, Group Health’s (GH) integrated group practice (IGP) initiated diverse interventions targeting COT prescriber norms and practices. In 2010, the IGP implemented a COT guideline, including a mandated online course for physicians managing COT. These interventions were not implemented in GH’s network practices. We compared trends in GH-IGP and network practices for 2006–12 in the percent of patients receiving COT and their opioid dose. We compared physician beliefs before versus after the mandated course and pre- to post-course changes in COT dosing for IGP physicians who took the course.
Results
From 2006 to 2012, mean (SE) daily opioid dose among IGP COT patients (intervention setting) declined from 74.1 (1.9) mg. morphine equivalent dose (MED) to 48.3 (1.0) mg. MED. Dose changes among GH network COT patients (control setting) were modest—88.2 (5.0) mg. MED in 2006 to 75.7 (2.3) mg. MED in 2012. Among physicians taking the mandated course in 2011, we observed pre- to post-course changes toward more conservative opioid prescribing beliefs. However, COT dosing trends did not change pre- to post-course.
Discussion
Following initiatives implemented to alter physician prescribing practices and norms, mean opioid dose prescribed to COT patients declined more in intervention than control practices. Physicians reported more conservative beliefs regarding opioid prescribing immediately after completing an online course in 2011, but the course was not associated with additional reductions in mean daily opioid dose prescribed by physicians completing the course.
Keywords: Prescribing practices, physician education, chronic opioid therapy, physician beliefs
INTRODUCTION
A dramatic increase in prescription opioid abuse1,2 and overdose3–6 has followed increased opioid prescribing for chronic non-cancer pain. Risks of opioid overdose and abuse increase with increased dose.7–9 In April 2011, the White House Office of Drug Control Policy, the Drug Enforcement Agency, and the Food and Drug Administration announced a national action plan to reduce prescription opioid abuse and overdose (http://www.whitehouse.gov/ondcp/2011-national-drug-control-strategy).10 Physician education to promote safer opioid prescribing was a key component of this plan.
Given gaps in physician knowledge and performance regarding COT management11, and uncertainties regarding the long-term effectiveness and safety of COT12,13, health plan initiatives and education aimed at influencing opioid prescribing norms and practices could potentially influence opioid prescribing patterns. The potential promise of such initiatives aimed at prescribers is based on two assumptions: (1) prescriber norms (i.e. shared expectations that guide behavior) and beliefs about COT influence prescribing behaviors; and (2) modifying prescriber norms, knowledge, and beliefs will result in changes in prescribing in ways that reduce patient risks and harms. There is evidence that physicians’ beliefs about opioids are better predictors of prescribing than are demographics or factors such as specialized training in chronic pain management.14 According to a national survey of 1,535 physicians, providers’ beliefs regarding opioid effectiveness, Schedule II vs. Schedule III opioids, and tamper-resistant formulations predicted opioid prescribing rates.15 Other surveys have explored relationships between physician beliefs and prescribing behaviors14,16–23, but most did not focus exclusively on COT for patients with chronic non-cancer pain, use objective measures of prescribing, or examine prescriber perceptions regarding the appropriateness of higher-dose opioid regimens. Although several opioid-related studies suggest that educational initiatives can modify physician knowledge and beliefs24–26, it is unknown whether educational initiatives that successfully modify physician knowledge and beliefs alter their opioid-related prescribing behaviors.26 Some have suggested that regulatory measures that influence prescribing norms have a larger impact on opioid prescribing than do educational interventions targeting knowledge and beliefs.27
This study evaluated health plan interventions begun in 2006 that included interventions to influence prescribing norms, beliefs, and behaviors without undue interference with clinical decisions for individual patients. The intent of these efforts was to enhance patient safety. Subsequently, the health plan implemented a COT guideline and multi-faceted risk reduction initiative in 2010 targeting COT patient management. A primary goal of the initiative was to reduce risks of opioid overdose, misuse, abuse, and diversion -- in part, by reducing COT doses. One part of the multi-faceted initiative was a 90-minute mandatory online course for physicians managing COT, implemented in 2011, regarding the COT guideline and management of complex chronic pain. To evaluate these interventions, we first compared trends in the percent of adult patients receiving COT and the opioid doses they received in the segment of the health plan that implemented COT risk reduction interventions relative to the segment of the health plan that did not implement these interventions. We then examined: 1) changes in physician beliefs regarding COT from before to after completing the 2011 course about the COT guideline; and 2) trends in opioid dosing among COT patients of course participants before versus after the course.
MATERIALS AND METHODS
Background and setting
This study was conducted at Group Health (GH), a large health plan in Washington State with Integrated Group Practice (IGP) and network segments. Providers in the IGP exclusively contract with GH to deliver care to about two-thirds of GH members, through its own facilities. The network segment consists of independent providers who contract with GH to deliver care to the remaining one-third of members, primarily in areas with lower population density or market share. The study procedures were approved by GH’s Institutional Review Board.
Health plan initiatives to influence COT prescribing
Beginning in the last half of 2006 in the IGP, more conservative opioid prescribing was encouraged by Rehabilitation Medicine specialists with expertise in chronic pain management who had recently assumed overall responsibility for COT prescribing standards. These changes were also supported by medical staff leaders supervising primary care physicians (PCPs). GH-IGP PCPs are family practitioners and general internists responsible for a panel of patients who have selected that physician as their main source of primary healthcare. From 2006 to 2010, the Medical Director of Rehabilitation Medicine (RB) delivered occasional voluntary educational presentations regarding management of chronic pain and opioid prescribing. Attendance at these presentations varied, but typically amounted to about one-fourth of IGP PCPs. A clinical policy was established making PCPs responsible for overall opioid management of their COT patients. PCPs and clinic medical directors were given lists of their COT patients that flagged those receiving high opioid doses (120+ mg. Morphine Equivalent Dose (MED)). Physicians with unusually large numbers of COT patients receiving high opioid doses received feedback and supervisory guidance from clinic medical directors.
In 2010–11, the IGP implemented a multi-faceted COT risk reduction initiative in accord with the Washington State COT guideline that had recently been enacted into state law.28 As previously detailed,29 the initiative components included, for all COT patients: a standard guideline establishing minimum standards for risk-stratified COT monitoring (including urine drug testing), designation of the PCP as responsible for a patient’s COT management; standardized care plans documented in the electronic health record; periodic monitoring visits; and prescription refill process modifications to prevent urgent refill requests. Implementation was supported by practice tools (patient education materials, care plan template, an online calculator for estimating MED); performance measures; medical staff leader advocacy; clinician access to expert consultants in each primary care clinic; and financial incentives for completion of care plans for COT patients. Patient education materials were made available to clinicians in pdf-format through GH’s clinical information systems, but the extent of use of these educational materials is unknown. In addition, completion of a 90-minute continuing medical education (CME) course about chronic pain management and opioids was mandated by medical staff leadership. After taking the course, clinicians in each clinic met for one hour to discuss issues related to implementation of GH’s COT guideline and their reactions to the online course.
CME course and online survey
The 90-minute online course was available to GH IGP providers starting in April 2011. Completion of the course was monitored, but participants did not receive incentives for course completion other than CME credits. The course was originally developed by the Department of Veterans Affairs (VA), where it has been taken by over 6,000 prescribers (Anthony Mariano, Ph.D., unpublished data, February, 2014). The VA course was substantially modified to include content specific to GH COT guidelines and to the GH model of clinician-patient communication (the Four Habits of Effective Communication).30 The Four Habits of Effective Communication are: 1) Invest in the beginning; 2) Elicit the patient’s perspective; 3) Demonstrate empathy; and, 4) Invest in the end. The GH online course was organized in three segments: (1) Perspectives on chronic pain management and the role of COT; (2) Guidance on COT; and (3) Patient scenarios for difficult situations that arise in implementing COT. The Perspectives section presented the chronic pain cycle and the application of the Four Habits of Effective Communication30 to management of chronic pain. The Four Habits of Effective Communication define approaches to organizing patient-centered clinical encounters appropriate for primary care settings. The online course applied this general framework for clinician-patient communication to issues in chronic pain and COT management. Guidance on COT was organized in a five-step approach: (a) identifying COT patients; (b) assessing patients and stratifying risks; (c) educating patients; (d) setting goals and developing a care plan; and (e) following-up and modifying treatment as necessary. The Scenarios modeled 11 difficult situations that arise in implementing COT, including patient requests for increased dosages, with approaches to their management in primary care settings. A major goal of the course was to provide up-to-date information regarding the potential benefits and risks of COT, especially those related to high doses. Immediately before and immediately after taking the course, providers completed a 16-item, non-anonymous, online survey concerning their views regarding COT and chronic pain management.
Comparators for opioid prescribing 2006–2012
We evaluated health plan interventions by comparing opioid prescribing trends for GH-IGP patients versus GH network patients. GH network physicians were not exposed to the health plan interventions, the voluntary education, the multi-faceted risk reduction initiative, or the mandated online CME, whereas GH-IGP physicians were exposed to these interventions to varying degrees. We estimated trends in mean daily COT dose and the percent of adult patients on COT each quarter from 2006 to 2012. Patients of IGP and network physicians were included in a particular quarter if they were 18+ years of age and were enrolled in the health plan for the entire quarter. Because our focus was on non-cancer pain, we excluded patients for a particular quarter who received an opioid prescription from an oncologist in that quarter, or had two or more visits with cancer (excluding non-melanoma skin cancer) diagnoses, or admission to hospice during the relevant calendar year.
Comparators for CME course and pre- and post-course surveys
Change in survey responses
Physicians who completed both the pre- and post-course surveys were used to assess changes in beliefs from before to after the course.
Pre- to post-course dosing trends among course participants 2009–2012
We examined longitudinal trends in mean daily opioid doses among COT patients of GH-IGP physicians who completed the 2011 mandated online course. Trends were examined for each calendar quarter from January 2009 through December 2012 to assess whether course completion was associated with pre- to post-course changes in opioid dosing. We restricted these analyses to physicians who maintained a panel of patients for every calendar quarter in the analysis. We did not examine trends prior to 2009 for these analyses to reduce attrition of the number of physicians included in the analyses. All course participants were managing COT patients during this time period.
Measures
Physician beliefs
The survey questions (see Appendix) were developed for course purposes to assess changes in physicians’ beliefs from before to after the course. The pre- and post-survey questions were identical and all had 1 – 5 response scales, with anchors of “strongly disagree” (1) to “strongly agree” (5) or “not at all confident” (1) to “extremely confident” (5).
To assess changes in physician beliefs regarding COT, we created two scales. The “Confidence” Scale consisted of five questions (Appendix items 1–5) assessing “confidence in treating chronic pain and prescribing opioids for patients with chronic pain” (higher scores indicate greater confidence). The “Liberal Prescribing Beliefs” scale consisted of five questions (items 12–16) that assessed physicians’ beliefs about the relative benefits versus risks of COT for patients with different risk profiles (higher scores indicate more liberal attitudes toward COT). Both scales had high internal consistency (Cronbach’s alpha = 0.89 for the Confidence Scale and 0.81 for the Liberal Prescribing Beliefs Scale). For each scale, values were set to missing if physicians answered fewer than four of the five items.
We examined three other items potentially relevant to physician COT prescribing decisions. These questions asked physicians how strongly they agreed or disagreed that: (1) “Providers are obligated to provide immediate pain relief for patients with chronic pain,” (2) “Opioid dose should be increased until patients report satisfactory pain relief,” and (3) “Pain needs to be significantly reduced before chronic pain patients can address other life problems.”
Opioid dose
We estimated COT patients’ mean daily doses from GH’s automated pharmacy files.31 We estimated mean daily doses32 by dividing the total MED received by the patient in the relevant 90-day time period by 90. We defined high opioid dose as a mean daily dose of 120+ mg. MED.28
Physician variables
Physician age, gender, panel size, tenure at GH, and practice specialty were obtained from GH electronic data.
Patient variables
Patient age, gender, GH enrollment history, and PCP were obtained from GH electronic databases.
Analyses
Trend analyses
In order to assess the effects of the overall health plan initiatives on COT prescribing, we evaluated the dosing trends between GH network and GH-IGP patients on COT by estimating dosing levels for patients over time. Specifically, we examined quarterly trends between 2006 and 2012 for adult enrollees receiving COT (defined as 70+ days’ supply of opioids in a calendar quarter, 90 days duration) and mean daily opioid dose among COT patients. We standardized probabilities and means to account for different age and gender distributions within groups over time as well as between network and IGP patients. We standardized each quarter to have the same age and gender distributions of the population of COT patients in the final quarter of 2012, both GH-IGP and network patients combined. We used 10 groups (gender and age-categorized as: 18–34, 35–44, 45–64, 65–74, and 75+ years).
Dose increase and reduction analyses
To understand differences in COT dosing practices between GH-IGP and network practices, we conducted a series of secondary, exploratory analyses. From October 2005 to July 2010, we examined quarterly rates of COT users whose daily doses were dramatically different compared to 1 year prior. In particular, for each quarter we calculated the number of patients per 1,000 COT patients who had moderate or large increases in daily dose one year later, and moderate or large decreases in opioid dose one year later. Moderate increases in dose consisted of increases from an initial daily dose of 40 – < 120 mg. MED to a dose of ≥120 mg. MED one year later. Large increases in daily dose consisted of increases from an initial dose of <40 mg. MED to ≥120 mg. MED one year later. Moderate decreases in dose consisted of reductions from an initial dose of ≥120 mg. MED to a dose of 40 to <120 mg. MED one year later. Large decreases in dose consisted of reductions from an initial dose of ≥ 120 mg. MED to a dose of <40 mg. MED one year later. These secondary analyses were not age-gender standardized.
Evaluation of CME course participation
We compared the characteristics of GH-IGP physicians who did and did not complete the pre-course survey using chi-square and t-tests with significance defined as a p-value of < 0.05. We also compared characteristics of physicians who completed both the pre- and post-course surveys (a proxy for course completion) to those physicians who just completed the pre-course survey. For descriptive analyses, we classified physicians into three categories based on their scores on each of the two scales: 1) High: ≥3.5 (indicating, on average, endorsement of “very” or “extremely” confident responses to Confidence Scale items or “agree” or “strongly agree” responses to Liberal Prescribing Beliefs items); 2) Moderate: 2.5 – < 3.5 (indicating, on average, endorsement of “average” confidence or “neutral” responses to Liberal Prescribing Beliefs items); and 3) Low: < 2.5 (indicating, on average, endorsement of “none” or “minimal” confidence or “strongly disagree” or “disagree” responses to Liberal Prescribing Beliefs Scale items). We also calculated the percent of physicians who agreed, disagreed, or were neutral on each of the three individual items. We used paired t-tests to evaluate whether responses to the two scales and the three individual items changed significantly from the pre- to post-course surveys.
To examine pre- to post-course longitudinal quarterly trends in dosing among course participants, we calculated mean daily opioid dose for each calendar quarter during 2009–12 for all of their COT patients. To adjust for demographic changes in the patient population over time, mean daily doses were age- and gender-standardized. All analyses were performed using SAS Software, Version 9.3 (SAS Institute Inc. 2011).33
RESULTS
Trends in COT prevalence in GH-IGP and network patients
For analyses examining the percent of adult enrollees receiving COT and opioid dosing trends between January 2006 and December 2012 among COT patients of all IGP and network physicians, the number of IGP PCPs in each quarter ranged from 230 to 291 and the number of GH network PCPs in each quarter was between 400 and 600.
The percent of GH adult enrollees receiving COT increased gradually from 2006 through 2012 among both IGP and network practices. The age- and gender-standardized percent of GH-IGP enrollees receiving COT increased from 1.9% (1.81, 1.92) at the beginning of 2006 to 2.8% (2.70, 2.83) at the end of 2012. The percent of GH network enrollees receiving COT also increased during this time period, from 1.3% (1.26, 1.42) at the beginning of 2006 to 2.3% (2.23, 2.40) at the end of 2012. The trends in the percent of adult enrollees receiving COT were linear over this time period, with no apparent divergence in the steady rate of increase between the IGP and the network practices over the study period (data not shown).
Trends in opioid dosing in GH-IGP and GH network COT patients
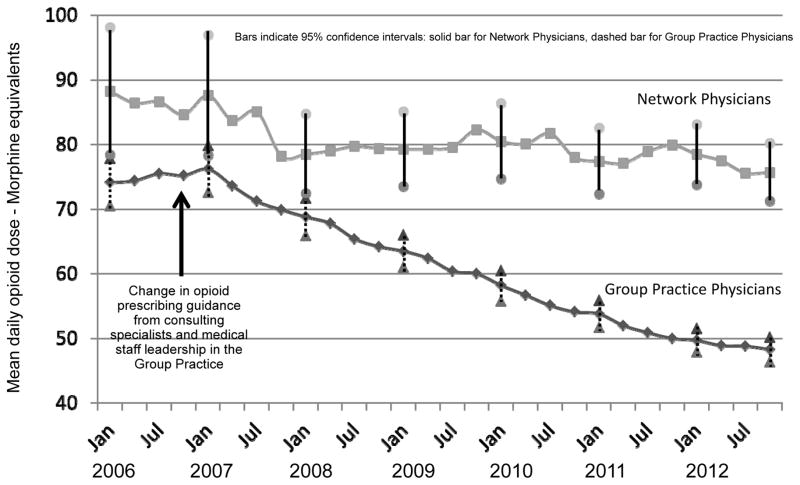
In contrast, large differences in trends in mean daily opioid dose were observed, with notable differences beginning to emerge in 2008. Figure 1 shows quarterly trends in mean daily doses for COT patients of all GH-IGP and network physicians between 2006 and 2012. As shown in the figure, there was an initial drop in mean daily dose in both the IGP (from 76.2 [72.6, 79.8] to 68.8 [65.9, 71.7]) and in the network practices (from 87.6 [78.3, 96.9] to 78.5 [72.4, 84.7]) from the beginning of 2007 to the beginning of 2008. But, by 2008, the trends in mean daily dose in the network practices had leveled off, whereas mean daily dose continued to decline in the IGP practices. Daily doses for COT patients of GH-IGP physicians fell almost 35% from a mean of 74.1 mg. (70.5, 77.8) MED in January 2006 to a mean of 48.3 mg. (46.4, 50.2) MED in December 2012. In contrast, mean daily doses among COT patients of network physicians fell only 14%, from 88.2 mg. (78.4, 98.1) MED to 75.7 mg. (71.2, 80.2) MED over this time period.
Figure 1.
Trends in Mean Daily Opioid Dose among Chronic Opioid Therapy Patients: Group Health Integrated Group Practice vs. Network Physicians
Dose increase and reduction exploratory analyses
Large dose increases
The percent of COT patients who were escalated from low opioid daily doses (< 40 mg. MED) in consecutive baseline quarters to high opioid doses (≥120 mg. MED) in the corresponding follow-up quarters one year later was consistently lower among GH-IGP patients than among GH network patients. For the four consecutive baseline quarters in 2006, rates of large dose increases ranged from 8 to 11 per 1,000 COT patients in the IGP. In contrast, they ranged from 12 to 19 per 1,000 among network COT patients. For four consecutive baseline quarters in 2009 (the last complete calendar year with baseline data), the rates of large dose increases in the corresponding quarters one year later ranged from 3 to 6 per 1,000 COT patients in the IGP. In contrast, these rates ranged from 5 to 8 per 1,000 among network COT patients.
Moderate dose increases
The differences in moderate dose increases over the study period (i.e., from 40–<120 mg. MED in consecutive baseline quarters to ≥120 mg. MED in the corresponding quarter one year later) were more pronounced. In 2006, the quarterly rates of moderate dose increases ranged from 108 to 127 per 1,000 COT patients in the IGP. In contrast, they ranged from 136 to 147 per 1,000 among network COT patients. In 2009, the quarterly rates of moderate dose increases ranged from 54 to 71 per 1,000 COT patients in the IGP. Among network COT patients, these rates of moderate dose increases ranged from 90 to 103 per 1,000 across the four consecutive baseline quarters in 2009. It is noteworthy that rates of moderate dose increases declined from 2006 to 2009 in both the IGP and the network, but across the entire time period, these rates were consistently lower in any given quarter in the IGP than in the network practices in the same quarter.
Moderate dose reductions
Consistent differences were also observed between the IGP and network practices in moderate dose reductions (i.e. from ≥120 mg. MED in the relevant baseline quarter to 40–<120 mg. MED in the corresponding quarter one year later). For the four baseline quarters in 2006, the rates of moderate dose reductions ranged from 119 to 145 per 1,000 COT patients in the IGP. In contrast, they ranged from 94 to 129 per 1,000 among network COT patients. In 2009, the quarterly rates of moderate dose reductions ranged from 158 to 182 per 1,000 COT patients in the IGP. In contrast, they ranged from 99 to 146 per 1,000 among network COT patients across the four baseline quarters in 2009. The increase in moderate dose reductions rates tended to be larger in the IGP than in the network practices.
Large dose reductions
No consistent differences were observed in rates of large dose reductions between the IGP and the network COT patients. In both the IGP and network practices, the rates of large dose reductions were generally between 50 and 85 per 1,000 COT patients with no discernable trends toward increased or reduced rates over the study period.
Participation in CME course and pre- and post-surveys
Two hundred twenty-four IGP physicians completed the pre-survey questions prior to taking the online course. This was 87% of the GH-IGP physicians with primary care panels in June 2011. These physicians did not differ significantly from non-participants (n=33) in age, gender, tenure at Group Health, panel size, percent of panel receiving COT, or mean daily doses among COT patients. Among physicians who completed the pre-course survey, 88% (n=198) also completed the course and the post-course survey. Post-course survey completers did not differ significantly from the 26 pre-survey completers who did not complete the post-survey on any measure examined (physician age and gender, “pre” physician beliefs measured by the five survey variables, or mean daily doses among COT patients in the 90 days before the survey). Because course participants included in the dosing trends analyses were required to continuously maintain panels of patients between 2009 and 2012, the sample was limited to 140 of the 198 physicians who completed the “post” survey..
Table 1 shows the characteristics of the 224 physicians who completed the pre-survey and the COT patients (N=4,721) on their panels at that time. The mean daily dose of their COT patients was about 40 mg. MED ([Interquartile Range 28.0–53.2]). A median of 5.6% of their COT patients received a high dose (120+ mg. MED/day), but this proportion ranged from 0 to 42% across physicians.
Table 1.
Characteristics of Primary Care Physicians (N=224) who completed the pre-survey before the on-line CME course and their Chronic Opioid Therapy (COT)* patients
| Physician characteristics | Mean (SD), %, or Median [IQR] |
|---|---|
| Age, years | 48.8 (10.6) |
| 29–44 | 37.5% |
| 45–54 | 25.0% |
| 55–68 | 37.5% |
| Female | 49.6% |
| Tenure at Group Health, years | 13.7 (10.4) |
| 0–5 | 33.9% |
| 6–20 | 34.0% |
| 21–40 | 32.1% |
| Panel± size | 972.4 (301.8) |
| <800 | 31.3% |
| 800–1100 | 31.7% |
| >1100 | 37% |
| Family practice specialty | 95.5% |
| Date of survey | |
| April–June 2011 | 53.1% |
| July–Sept 2011 | 33.9% |
| Oct–Dec 2011 | 13.0% |
|
| |
| COT Patient characteristics∞ | |
|
| |
| Age, years | 58.0 [54.1–61.6] |
| Female | 63.3% [50.0–77.3] |
|
| |
| Physician COT prescribing characteristics£ | |
|
| |
| Daily opioid MED, mg.β | 39.4 [28.0–53.2] |
| Receiving 120+ mg. MED/day | 5.6 [0, 12.5] |
Chronic Opioid Therapy is defined as the receipt of 70+ days’ supply of oral opioid medications in the 90 days before the physician took the survey.
“Panel” refers to the group of patients who have selected a given physician as their main source of primary healthcare.
Patient characteristics expressed as the median [Interquartile Range] of the panel-level means calculated across the 224 physicians.
Physician prescribing characteristics expressed as the median [Interquartile Range] of the panel-level means calculated across the 224 physician panels.
MED = Morphine Equivalent Dose.
Pre-Survey Responses
Table 2 presents information on the physician “pre” survey scales and individual items. Before the course, most physicians (68.3%) indicated a moderate level of confidence in treating patients with chronic pain, including confidence in using opioids long-term (see Appendix for data on individual scale items). The median Confidence Scale score was 3.0 (corresponding to “average” confidence); 26.8% of physicians expressed high levels of confidence. Relatively few physicians expressed consistently liberal attitudes toward COT prescribing; only 5.9% had high scores on the Liberal Prescribing Beliefs Scale. About half (52.7%) expressed neutral beliefs concerning the relative risks versus benefits of COT. Only 8% of the physicians agreed that opioid doses should be increased until the patient reports adequate pain relief, but one-fifth were neutral on this item.
Table 2.
Primary care physician chronic pain therapy beliefs prior to the on-line Continuing Medical Education course (N=224).
| Physician Belief | Mean (SD), Median [(IQR], or n (%) |
|---|---|
| Confidence Scale* | |
| Mean (SD) | 3.2 (0.5) |
| Median [IQR] | 3.0 [3.0, 3.6] |
| High scores (3.5+), n (%) | 60 (26.8%) |
| Moderate scores (2.5 – < 3.5), n (%) | 153 (68.3%) |
| Low scores (< 2.5), n (%) | 11 (4.9%) |
| Liberal Prescribing Beliefs Scale± | |
| Mean (SD) | 2.6 (0.6) |
| Median [IQR] | 2.6 [2.2, 3.0] |
| High scores (3.5+), n (%) | 13 (5.9%) |
| Moderate scores (2.5 – < 3.5), n (%) | 117 (52.7%) |
| Low scores (< 2.5), n (%) | 92 (41.4%) |
| Providers are obligated to provide immediate pain relief for patients with chronic pain. | |
| Agree or Strongly Agree, n (%) | 10 (4.5%) |
| Neutral, n (%) | 32 (14.2%) |
| Disagree or Strongly Disagree, n (%) | 182 (81.3%) |
| Opioid dose should be increased until patients report satisfactory pain relief (dose escalation). | |
| Agree or Strongly Agree, n (%) | 19 (8.5%) |
| Neutral, n (%) | 43 (19.2%) |
| Disagree or Strongly Disagree, n (%) | 162 (72.3%) |
| Pain needs to be significantly reduced before chronic pain patients can address other life problems. | |
| Agree or Strongly Agree, n (%) | 23 (10.3%) |
| Neutral, n (%) | 42 (18.7%) |
| Disagree or Strongly Disagree, n (%) | 159 (71.0%) |
The Confidence Scale ranges from 1 to 5, with “1” signifying “Not at all Confident” and “5” signifying “Extremely Confident.”
The Liberal Prescribing Beliefs Scale ranges from 1 to 5, with “1” signifying “Strongly Disagree” and “5” signifying “Strongly Agree.”
Change in survey responses
Table 3 shows the pre- to post-survey changes in physician beliefs. Changes in mean scores on the scales and the individual items were statistically significant (p-values < 0.0001), with physicians expressing more confidence and more conservative beliefs after the course. The percent of physicians expressing high levels of confidence in treating patients with chronic pain, including using opioids long-term, more than doubled pre- to post-course. After the course, over half expressed conservative views toward COT on the Liberal Prescribing Beliefs Scale and almost no physicians (1.5%) agreed that opioid doses should be increased until the patient reports satisfactory pain relief (down from 8.1% prior to the course).
Table 3.
Change in physician chronic pain therapy beliefs from before to after taking the CME course (N=198)*
| Physician Belief | Pre-Survey Mean (SD) or n (%) |
Post-Survey Mean (SD) or n (%) |
|---|---|---|
| Confidence Scale± | ||
| Mean (SD) | 3.25 (0.55) | 3.63 (0.53) |
| High scores (3.5+), n (%) | 56 (28.3%) | 121 (61.1%) |
| Moderate scores (2.5 – < 3.5), n (%) | 132 (66.6%) | 75 (37.9%) |
| Low scores (< 2.5), n (%) | 10 (5.1%) | 2 (1.0%) |
| Liberal Prescribing Beliefs Scale∞ | ||
| Mean (SD) | 2.59 (0.63) | 2.39 (0.64) |
| High scores (3.5+), n (%) | 12 (6.1%) | 9 (4.6%) |
| Moderate scores (2.5 – < 3.5), n (%) | 104 (53.1%) | 80 (40.8%) |
| Low scores (< 2.5), n (%) | 80 (40.8%) | 107 (54.6%) |
| Providers are obligated to provide immediate pain relief for patients with chronic pain. | ||
| Mean (SD) | 2.03 (0.74) | 1.64 (0.67) |
| Agree or Strongly Agree, n (%) | 8 (4.0%) | 4 (2.0%) |
| Neutral, n (%) | 29 (14.7%) | 6 (3.0%) |
| Disagree or Strongly Disagree, n (%) | 161 (81.3%) | 188 (94.6%) |
| Opioid dose should be increased until patients report satisfactory pain relief (dose escalation). | ||
| Mean (SD) | 2.1 (0.8) | 1.6 (.6) |
| Agree or Strongly Agree, n (%) | 16 (8.1%) | 3 (1.5%) |
| Neutral, n (%) | 37 (18.7%) | 5 (2.5%) |
| Disagree or Strongly Disagree, n (%) | 145 (73.2%) | 190 (96.0%) |
| Pain needs to be significantly reduced before chronic pain patients can address other life problems. | ||
| Mean (SD) | 2.2 (0.8) | 1.7 (0.6) |
| Agree or Strongly Agree, n (%) | 20 (10.1%) | 3 (1.5%) |
| Neutral, n (%) | 37 (18.7%) | 10 (5.1%) |
| Disagree or Strongly Disagree, n (%) | 141 (71.2%) | 185 (93.4%) |
All p-values (obtained from paired t-tests) comparing pre and post means are < 0.0001
The Confidence Scale ranges from 1 to 5, with “1” signifying “Not at all Confident” and “5” signifying “Extremely Confident.”
The Liberal Prescribing Beliefs Scale ranges from 1 to 5, with “1” signifying “Strongly Disagree” and “5” signifying “Strongly Agree.”
Pre- to post-course dosing trends among course participants
Figure 2 shows COT dosing trends from 2009 through 2012 for GH-IGP physicians who completed the CME course. Doses followed a downward trajectory before the course, with mean daily doses in January 2009 of 63.0 mg. MED [95% CI (59.6, 66.3)], falling to 53.4 mg. MED [95% CI 50.7, 56.0] in March 2011. Mean doses were relatively flat after the course was implemented, with no obvious indication that course participation influenced physician dosing behaviors in terms of before-after changes.
Figure 2.
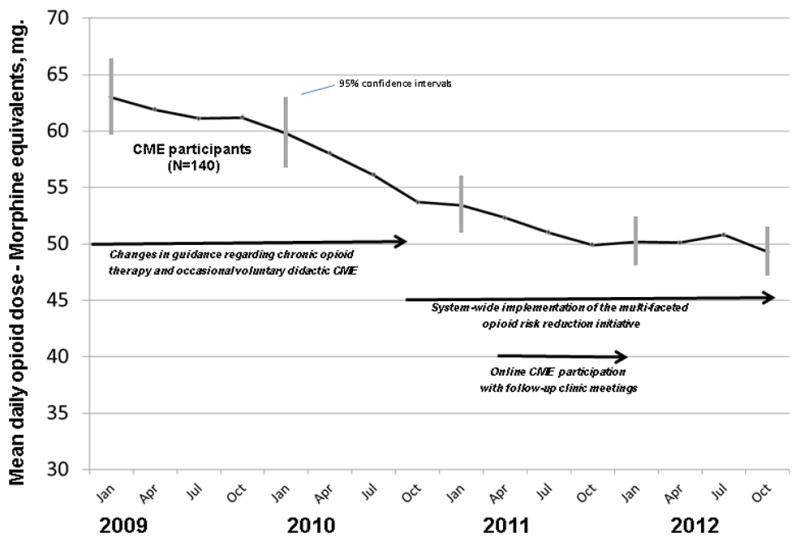
Trends in Mean Daily Opioid Dose among Chronic Opioid Therapy Patients of Online Course Participants
DISCUSSION
Interventions implemented at Group Health, intended to foster more conservative opioid prescribing norms and practices among physicians prescribing to COT patients, were followed by large reductions in opioid dose over a 5 to 6 year period. Substantial and sustained opioid dose reductions were observed in the GH-IGP segment. These dose reductions began around the time that Washington State announced its guideline in 2007 that encouraged lower opioid doses, but the dose reduction trends were sustained from 2008 through 2011. In contrast, the network practices that did not implement the interventions showed an initial reduction in mean daily dose in 2007, but the dose reduction quickly leveled off and further reductions were not observed over the following four years. These results suggest that the Washington State Guideline may have contributed to a modest initial reduction in opioid dose levels, but that additional interventions were necessary to sustain opioid dose reductions over time. The health plan interventions evaluated here fixed responsibility for COT management in primary care, sought to change physician opioid prescribing norms, provided feedback about COT patients receiving high opioid doses, offered supervisory guidance to physicians with unusually large numbers of COT patients on high opioid doses, and delivered voluntary physician education encouraging more conservative opioid prescribing standards. These changes were followed by large reductions in opioid doses among COT patients over an extended time period. Reductions in average daily opioid dose in the COT patient population appeared to be explained by lower rates of dose escalation and higher rates of modest dose reduction in the intervention (IGP) practices compared to the control (network) practices. There were no differences in rates of large reductions in opioid dose (including termination of opioids among patients on high dose regimens) between the IGP and network practices. At this time, we are not able to determine whether these changes were associated with more or less favorable patient clinical outcomes.
Physicians who completed a mandated online course and the pre- and post-course-surveys showed significant before-after changes in their beliefs and confidence levels regarding COT prescribing. They reported greater confidence in treating patients with chronic pain, including treatment with opioids, and less liberal views towards COT prescribing. Almost no physicians endorsed opioid dose titration to pain-relieving effect in the post-survey, compared to 8.1% in the pre-survey. However, changes in confidence and beliefs were not accompanied by pre- to post-course changes in these physicians’ COT dosing.
Our finding that changes in prescriber beliefs were not accompanied by changes in opioid prescribing from before to after the mandated course suggests that brief, one-time physician education courses are not potent strategies for changing prescribing behaviors, an interpretation consistent with findings of prior research. For example, a 2-day course on opioid prescribing offered to Canadian physicians did not affect prescribing rates, whereas rates were dramatically reduced among physicians who were notified by a medical regulator of a complaint or investigation.27 The state of Utah targeted opioid prescribing through an approach including provider detailing, continuous quality improvement dialogs among members of a care team, prescribing guidelines, and practice tools such as patient education materials, treatment plans, and a statewide database of all controlled prescriptions. The physician education intervention, along with concurrent efforts to educate the public, patients, and insurers, was associated with a 14% reduction in opioid-overdose deaths.34
Consistent with recommendations in the literature about physician education programs, 35–37 GH’s opioid risk reduction initiative featured multiple exposures and multiple instructional techniques, one of which was the mandated online CME course. The multi-faceted initiative implemented starting in 2010 was preceded by years of voluntary, yet consistent, efforts by the GH-IGP to encourage more conservative opioid prescribing, primarily by targeting prescribing norms. The multi-faceted approach to changing practice norms through the influence of relevant specialists and medical staff leaders was followed by dramatic dose reductions for IGP COT patients. These changes appeared to begin leveling off about the time the mandated online CME course was administered. Thus, our finding that the mandated online CME course did not lead to meaningful changes in prescribing might reflect not only its short-term, didactic nature, but also that many physicians had already adjusted their prescribed dosages downward prior to the course, reducing potential for further dose reductions.
Our findings should be viewed within the broader environment of changes affecting opioid prescribing in Washington State and nationally over this time period. In 2007, Washington State agencies implemented the initial phase of an opioid dosing guideline on safe prescribing for chronic noncancer pain. In the Washington State workers’ compensation population, incident opioid users were significantly less likely to go on to chronic opioid use and significantly less likely to achieve high (≥ 120 mg. MED/day) doses after the guideline was implemented (April 2007–December 2010) as compared to before (April 2004–March 2007).38 Furthermore, the mean daily long-acting opioid dose, the percentage of claimants receiving work disability benefits with doses ≥120 mg. MED/day, and the number of unintentional opioid poisoning deaths all decreased after 2007.39 In January 2012, a new law took effect in Washington that required physicians who prescribe opioids to complete CME including education on long acting opioids and a consultation with a pain specialist for patients with doses greater than 120 mg. MED/day. The GH guideline was based, in part, on the Washington State guideline, and several persons working on implementation of the 2010 Group Health risk reduction program (RB, MVK) also helped develop the Washington State Guideline. While the prevailing changes in Washington State likely facilitated the changes in prescribing norms and practices in the GH-IGP, it is significant that comparable reductions in opioid dose were not observed in the GH network practices, also located in Washington State. The comparison of prescribing trends in two sets of practices in the same geographic area controls for effects of concurrent influences on opioid prescribing at the state and national level--both IGP and network physicians were exposed to Washington State and national initiatives that encouraged changes toward more conservative opioid prescribing.
At the same time mean opioid doses among GH COT patients were decreasing, rates of COT prescribing among GH-IGP and GH network patients were increasing. A prior study reported that increased levels of physician confidence in prescribing opioids was associated with greater numbers of patients receiving opioids.23 The CME course evaluated in this study increased physician confidence levels in opioid prescribing—it’s possible that the earlier interventions also increased physician confidence, leading to the observed increases in COT prescribing rates. However, rates of COT prescribing at GH were on the rise long before the dose reduction efforts,40 a phenomenon observed nationwide beginning in the 1990s.41 Also, rates were increasing in network practices that were not exposed to the interventions. The increasing prevalence of COT use in both the GH-IGP and network practices may reflect a long-term secular trend toward increased opioid use that had not yet reached steady-state during the time period examined in this evaluation due to the long average duration of opioid use among COT patients.
This study has several limitations. The post-survey beliefs were measured immediately after the course, when the information was most salient. It is possible that the course did not produce enduring changes in beliefs. The CME course was administered to physicians belonging to an integrated group practice in the midst of a multi-year effort to improve the safety of opioid prescribing. Thus, it is not clear to what degree physician beliefs expressed before and after the CME course generalize to other settings. It is unknown what effect the CME course may have had on opioid prescribing if delivered in a different setting to physicians who had not been exposed to an extended, multi-faceted initiative. Study strengths include the high course participation rate, the specific focus on COT for chronic non-cancer pain, and the use of electronic health records to measure prescribing behaviors.
Based on the Group Health Integrated Group Practice experience, relative to a set of comparable practices in the same geographic area, it appears that a multi-faceted health plan intervention that sought to influence opioid prescribing norms, that provided feedback and supervisory guidance for physicians with unusual patterns of high dose prescribing, and that included voluntary physician education was able to achieve large reductions in opioid dose over a five- to six-year time period. However, a mandated online CME course did not lead to additional reductions in opioid doses beyond those previously achieved, although physician beliefs changed in the direction of greater caution regarding opioid prescribing in general and higher dose regimens in particular after completing the course. Our results are consistent with prior research42–43 suggesting that a single-session didactic CME course is unlikely to produce robust changes in targeted prescribing practices. It is possible that health plan interventions preceding the online course reduced the potential for further dose reductions, but our results do not provide empirical support for mandated prescriber education. Rather, large changes in opioid prescribing appear to have been achieved through sustained, multi-faceted interventions targeting physician opioid prescribing norms.
Acknowledgments
This research was supported by grants from the National Institute on Aging (AG034181, Von Korff) and the Patient-Centered Outcomes Research Institute (R-IHS-1306-02198, Von Korff). The Group Health Foundation provided funding for modifications made to the online CME program that was originally developed by the Department of Veterans Affairs.
Appendix
Survey questions administered on-line before and after the CME course. Responses are from the “pre” survey (N=224).
The response scale for items 1–5 is 1 = “Not at all confident”, 2 = “Minimal confidence”, 3 = “Average confidence”, 4 = “Very confident”, and 5 = “Extremely confident.”
| % very or extremely confident | % with average confidence | % not at all or minimally confident | |
|---|---|---|---|
| Rate your level of confidence … | |||
| 1. in your ability to evaluate and treat chronic pain. | 27.2 | 68.8 | 4.0 |
| 2. in prescribing opioid medications for chronic pain. | 26.3 | 70.1 | 3.6 |
| 3. addressing psychosocial issues with chronic pain patients taking opioid medications. | 28.1 | 61.2 | 10.7 |
| 4. to carry out a planned visit to consider whether long-term opioid use is appropriate. | 32.1 | 59.9 | 8.0 |
| 5. in counseling patients about long-term opioid use when you think it is not an appropriate option. | 31.3 | 56.6 | 12.1 |
The response scale for items 6–16 is 1 = “Strongly disagree”, 2 = “Disagree”, 3 = “Neutral”, 4 = “Agree”, and 5 = “Strongly agree.”
| % agree or strongly agree | % neutral | % disagree or strongly disagree | |
|---|---|---|---|
| 6. Providers are obligated to provide immediate pain relief for patients with chronic pain. | 4.5 | 14.2 | 81.3 |
| 7. Opioid dose should be increased until patients report satisfactory pain relief. | 8.5 | 19.2 | 72.3 |
| 8. Pain needs to be significantly reduced before chronic pain patients can address other life problems. | 10.3 | 18.7 | 71.0 |
| 9. In managing chronic pain, objective findings are more important than patient reports of chronic pain severity. | 15.6 | 31.7 | 52.7 |
| 10. Pain is the primary problem of most chronic pain patients. | 9.8 | 15.9 | 74.6 |
| 11. For chronic pain patients, self-management is more important than medical treatment. | 76.6 | 14.8 | 8.6 |
| The benefits of COT for pain and function typically outweigh medical, psychosocial and addiction risks for… | |||
| 12. Typical chronic pain patients treated with low opioid daily dose. | 59.0 | 28.8 | 12.2 |
| 13. Typical chronic pain patients treated with medium opioid daily dose. | 32.4 | 41.9 | 25.7 |
| 14. Typical chronic pain patients treated with high opioid daily dose. | 7.2 | 29.7 | 63.1 |
| 15. Chronic pain patients with a history of substance abuse. | 1.8 | 11.2 | 87.0 |
| 16. Chronic pain patients who are clinically depressed. | 5.4 | 27.8 | 66.8 |
Footnotes
Conflicts of Interest and Source of Funding: Ms. Saunders owns stock in Merck. Dr. Shortreed has received funding from research grants awarded to Group Health Research Institute (GHRI) by Bristol-Myers Squibb. Dr. Von Korff is the Principal Investigator of grants to GHRI from Pfizer Inc. that concern opioids. These grants also support Ms. Saunders. Dr. Von Korff was the Principal Investigator of a grant to GHRI from Johnson & Johnson concerning prediction of clinical pain outcomes. This grant also supported Ms. Saunders and Drs. Turner and LeResche. Dr. Von Korff is Vice President of Physicians for Responsible Opioid Prescribing (PROP), an organization that has advocated for more cautious and selective prescribing of opioids for chronic pain. He has received no compensation or reimbursement for travel (etc.) expenses for his work with PROP.
The remaining authors report no conflicts. This research was supported by grants from the National Institute on Aging (AG034181, Von Korff) and from the Patient-Centered Outcomes Research Institute (R-IHS-1306-02198, Von Korff).
References
- 1.Compton W, Volkow N. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug Alcohol Depend. 2006;81(2):103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2012 National Survey on Drug Use and Health: Summary of national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. [Google Scholar]
- 3.Warner M, Chen L, Makuc D. NCHS data brief, no. 22. National Center for Health Statistics; 2009. [Accessed February 19, 2014]. Increase in fatal poisonings involving opioid analgesics in the United States, 1999–2006. Available at: http://www.cdc.gov/nchs/data/databriefs/db22.htm. [PubMed] [Google Scholar]
- 4.Coben J, Davis S, Furbee P, et al. Hospitalizations for poisoning by prescription opioids, sedatives, and tranquilizers. Am J Prev Med. 2010;38(5):517–524. doi: 10.1016/j.amepre.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363 (21):1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. CDC Grand Rounds: Prescription drug overdoses--a U.S. epidemic. MMWR. 2012;61:10–13. [PubMed] [Google Scholar]
- 7.Dunn K, Saunders K, Rutter C, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes T, Mamdani M, Dhalla I, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. JAMA Intern Med. 2011;171(7):686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- 9.Bohnert A, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 10.Office of National Drug Control Policy. [Accessed February 19, 2014];Epidemic: Responding to America’s Prescription Drug Abuse Crisis. 2011 Apr 11; [updated 2011 Apr 11; cited 2011 Jun 10]. Available at: http://www.whitehousedrugpolicy.gov/publications/pdf/rx_abuse_plan.pdf.
- 11.Krebs E, Ramsey D, Miloshoff J, et al. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011;12(5):740–746. doi: 10.1111/j.1526-4637.2011.01099.x. [DOI] [PubMed] [Google Scholar]
- 12.Chou R, Fanciullo G, Fine P, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papaleontiou M, Henderson C, Turner B, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1353–1369. doi: 10.1111/j.1532-5415.2010.02920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchinson K, Moreland AME, de C Williams AC, et al. Exploring beliefs and practice of opioid prescribing for persistent non-cancer pain by general practitioners. Eur J Pain. 2007;11(1):93–98. doi: 10.1016/j.ejpain.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Wilson H, Dansie E, Kim M, et al. Clinicians’ Attitudes and Beliefs about Opioids Survey (CAOS): Instrument development and results of a national physician survey. J Pain. 2013;14(6):613–627. doi: 10.1016/j.jpain.2013.01.769. [DOI] [PubMed] [Google Scholar]
- 16.Turk DC, Brody MC, Okifuji EA. Physicians’ attitudes and practices regarding the long-term prescribing of opioids for non-cancer pain. Pain. 1994;59:201–208. doi: 10.1016/0304-3959(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 17.Potter M, Schafer S, Gonzalez-Mendez E, et al. Opioids for chronic nonmalignant pain. J Fam Pract. 2001;50(2):145–151. [PubMed] [Google Scholar]
- 18.Bhamb B, Brown D, Hariharan J, et al. Survey of select practice behaviors by primary care physicians on the use of opioids for chronic pain. Curr Med Res Opin. 2006;22(9):1859–1865. doi: 10.1185/030079906X132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upshur C, Luckmann RS, Savageau JA. Primary care provider concerns about management of chronic pain in community clinic populations. J Gen Intern Med. 2006;21(6):652–655. doi: 10.1111/j.1525-1497.2006.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JD, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23(9):799–803. doi: 10.1097/AJP.0b013e3181565cf1. [DOI] [PubMed] [Google Scholar]
- 21.Dobscha SK, Corson K, Flores JA, et al. Veterans Affairs primary care clinicians’ attitudes toward chronic pain and correlates of opioid prescribing rates. Pain Med. 2008;9 (5):564–571. doi: 10.1111/j.1526-4637.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 22.McCracken LM, Velleman SC, Eccleston C. Patterns of prescription and concern about opioid analgesics for chronic non-malignant pain in general practice. Prim Health Care Res Dev. 2008;9(2):146–156. [Google Scholar]
- 23.Wenghofer EF, Wilson L, Kahan M, et al. Survey of Ontario primary care physicians’ experiences with opioid prescribing. Can Fam Physician. 2011;57(3):324–332. [PMC free article] [PubMed] [Google Scholar]
- 24.Elhwaris H, Reznich C. An educational strategy for treating chronic, noncancer pain with opioids: A pilot test. J Pain. 2010;11:1368–1375. doi: 10.1016/j.jpain.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan M, Gaster B, Russo J, et al. Randomized trial of web-based training about opioid therapy for chronic pain. Clin J Pain. 2010;26:512–517. doi: 10.1097/AJP.0b013e3181dc7adc. [DOI] [PubMed] [Google Scholar]
- 26.McCracken L, Boichat C, Eccleston C. Training for general practitioners in opioid prescribing for chronic pain based on practice guidelines: A randomized pilot and feasibility trial. J Pain. 2012;13(1):32–40. doi: 10.1016/j.jpain.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Kahan M, Gomes T, Juurlink D, et al. Effect of a course-based intervention and effect of medical regulation on physicians’ opioid prescribing. Can Fam Physician. 2013;59:231–239. [PMC free article] [PubMed] [Google Scholar]
- 28.Washington State Agency Medical Directors’ Group. [Accessed February 19, 2014];Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy. 2010 update. Available at: http://www.agencymeddirectors.wa.gov/Files/OpioidGdline.pdf.
- 29.Trescott CE, Beck RM, Seelig MD, et al. Group Health’s initiative to avert opioid misuse and overdose among patients with chronic non-cancer pain. Health Aff. 2011;30(8):1420–1424. doi: 10.1377/hlthaff.2011.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein T, Frankel R, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns. 2005;58(1):4–12. doi: 10.1016/j.pec.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Saunders K, Davis R, Stergachis A Group Health Cooperative. In: Pharmacoepidemiology. Strom B, editor. West Sussex, England: John Wiley and Sons; 2005. pp. 223–239. [Google Scholar]
- 32.Von Korff M, Saunders K, Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SAS Institute Inc. SAS/STAT® 9.3 User’s Guide. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 34.Cochella S, Bateman K. Provider detailing: An intervention to decrease prescription opioid deaths in Utah. Pain Med. 2011;12(Suppl 2):S73–S76. doi: 10.1111/j.1526-4637.2011.01125.x. [DOI] [PubMed] [Google Scholar]
- 35.Grol R, Grimshaw J. From best evidence to best practice: Effective implementation of change in patients’ care. Lancet. 2003;362(9391):1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 36.Davis D, Galbraith R. Continuing medical education effect on practice performance: Effectiveness of continuing medical education: American College of Chest Physicians evidence-based guidelines. Chest. 2009;135 (3_suppl):428–488. doi: 10.1378/chest.08-2517. [DOI] [PubMed] [Google Scholar]
- 37.Marinopoulos SS, Dorman T, Ratanawongsa N, et al. Effectiveness of continuing medical education. (Johns Hopkins Evidence-based Practice Center Evidence Report/Technology Assessment No. 149. Agency for Healthcare Research and Quality, Rockville MD) Agency for Healthcare Research and Quality. 2007 Jan;(149):1–69. [Google Scholar]
- 38.Garg R, Fulton-Kehoe D, Turner J, et al. Changes in opioid prescribing for Washington workers’ compensation claimants after implementation of an opioid dosing guideline for chronic noncancer pain: 2004 to 2010. J Pain. 2013;14(12):1620–1628. doi: 10.1016/j.jpain.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Franklin GM, Mai J, Turner J, et al. Bending the prescription opioid dosing and mortality curves: Impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55:325–331. doi: 10.1002/ajim.21998. [DOI] [PubMed] [Google Scholar]
- 40.Boudreau D, Von Korff M, Rutter C. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18(12):1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caudill-Slosberg M, Schwartz L, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Davis D, Thompson O’Brien MA, Freemantle N, et al. Impact of formal continuing medical education. Do conferences, workshops, rounds and other traditional continuing education activities change physician behavior or health care outcomes? JAMA. 1999;282(9):867–874. doi: 10.1001/jama.282.9.867. [DOI] [PubMed] [Google Scholar]
- 43.Mansouri M, Lockyer J. A meta-analysis of continuing medical education effectiveness. J Contin Educ Health Prof. 2007;27(1):6–15. doi: 10.1002/chp.88. [DOI] [PubMed] [Google Scholar]