Abstract
The penta-ethyl ester prodrug of diethylenetriaminepentaacetic acid (DTPA), which exists as an oily liquid, was incorporated into a solid dispersion for oral administration by the solvent evaporation method using blends of polyvinylpyrrolidone (PVP), Eudragit® RL PO and α-tocopherol. D-optimal mixture design was used to optimize the formulation. Formulations that had a high concentration of both Eudragit® RL PO and α-tocopherol exhibited low water absorption and enhanced stability of the DTPA prodrug. Physicochemical properties of the optimal formulation were evaluated using Fourier transform infrared (FTIR) spectroscopy and differential scanning calorimetry (DSC). In vitro release of the prodrug was evaluated using the USP Type II apparatus dissolution method. DSC studies indicated that the matrix had an amorphous structure, while FTIR spectrometry showed that DTPA penta-ethyl ester and excipients did not react with each other during formation of the solid dispersion.. Dissolution testing showed that the optimized solid dispersion exhibited a prolonged release profile, which could potentially result in a sustained delivery of DTPA penta-ethyl to enhance bioavailability. In conclusion, DTPA penta-ethyl ester was successfully incorporated into a solid matrix with high drug loading and improved stability compared to prodrug alone.
Keywords: Solid dispersion, DTPA, decorporation, oral drug delivery, statistical optimization
Introduction
Diethylenetriaminepentaacetic acid (DTPA) is a chelating agent that can be used for decorporation of plutonium (Pu), americium (Am) and curium (Cm) after accidental or deliberate internal contamination in humans.[1] After such a contamination event, DTPA is usually administered to the patient by intravenous (i.v.) injection.[2, 3] However, injection of DTPA requires a well-trained medical professional, which poses a logistical challenge for treating patients in a mass casualty scenario. To facilitate drug distribution and allow for self-dosing, a number of strategies, including DTPA-entrapped dry powder for pulmonary delivery, have been pursued.[4, 5] An oral dosage form that could be distributed readily and to large numbers of people would be ideal in the case of a wide-spread contamination emergency. DTPA, however, is hydrophilic, highly ionizable and, thus, is poorly absorbed from the gastrointestinal tract with a bioavailability of <1% in humans.[6] To overcome this drawback, we prepared a lipophilic prodrug of DTPA by esterifying its five carboxylic acid functional groups and measured the octanol-water partition coefficient of DTPA penta-ethyl ester at various pH values which indicated that this prodrug would be a good candidate for intestinal delivery.[7] We demonstrated that DTPA penta-ethyl ester was absorbed after oral administration to rats and enzymatically converted to partially-de-esterified metabolites capable of chelating transuranic elements.[8] We further demonstrated the radionuclide decorporation efficacy of DTPA penta-ethyl ester when administered orally in soybean oil or acetate buffer to rats that had been contaminated with 241Am, an alpha particle-emitting transuranic radionuclide.[9] Because DTPA penta-ethyl ester exists as an oily liquid, formulating it in a solid dosage form would be very desirable in terms of ease of packaging, handling and administering. The solid dispersion approach has been used previously[10, 11]; it allows for a more uniform product than does mechanical mixing of liquids with solids. In addition, solid dispersions of poorly water soluble active pharmaceutical ingredients can increase solubility, dissolution rate and bioavailability.[12]
In this study, the DTPA penta-ethyl ester-containing matrix was prepared by solid dispersion using water-soluble polyvinylpyrrolidone (PVP), water insoluble Eudragit® RL PO polymers and α-tocopherol. PVP is commonly used as a disintegrant in pharmaceutical tablets[13, 14] and has been used for the preparation of binary solid dispersion systems for various drugs.[15, 16] Eudragit® RL PO is composed of poly(ethylacrylate-methylmethacrylate-trimethylammonioethyl methacrylate chloride) copolymers, and is inert to digestive tract contents, pH independent, and capable of swelling due to the presence of quaternary ammonium groups, and, thus, is utilized for formulating extended-release dosage forms.[17, 18] It was expected that utilizing Eudragit® RL PO would enhance the absorption of DTPA penta-ethyl ester after oral administration. The antioxidant α-tocopherol was used to maintain the stability of DTPA penta-ethyl ester.[19] The DTPA penta-ethyl ester has five ester groups, which can be hydrolyzed and oxidized in high relative humidity and temperature environments;[20] thus, our design maximized drug stability and drug loading, and minimized water absorption. In this investigation, the D-optimal mixture design[21, 22] was used to select the optimum formulation. Interaction of DTPA penta-ethyl ester in the solid dispersion were investigated by Fourier transform infrared (FTIR) spectroscopy and differential scanning calorimetry (DSC). In vitro release of DTPA penta-ethyl ester from the optimized formulation in 0.1 N HCl (pH 1.2) and phosphate buffer (pH 6.8) were investigated.
Materials and methods
Materials
PVP (MW: 360,000 g mol−1) was supplied by Fluka (Switzerland), Eudragit® RL PO was obtained from Rohm Pharma GmbH (Darmstadt, Germany), α-tocopherol was from Sigma-Aldrich (St Louis, MO), absolute ethanol, isopropanol and acetonitrile were purchased from Acros Organics (New Jersey, USA), and DTPA penta-ethyl ester was prepared as described previously.[7]
Preparation of solid dispersions of DTPA penta-ethyl ester and optimization
Preparation of solid dispersions of DTPA penta-ethyl ester
Solid dispersions of DTPA penta-ethyl ester entrapped in the excipients (PVP, Eudragit® RL PO and α-tocopherol) were prepared by the solvent evaporation method.[12] The amount of DTPA penta-ethyl ester was fixed at 375 mg (75%) and excipients at 125 mg (25%). DTPA penta-ethyl ester with different ratios (Table 1) of the three excipients were dissolved in 0.3 mL absolute ethanol and stirred for 1 h. A 0.15 mL aliquot of the DTPA penta-ethyl ester excipient mixture was subsequently transferred to a round bottom 96-well plate. The resulting solid dispersion was dried at 40°C for 24 h.
Table 1.
Observed responses in D-optimal mixture design for DTPA penta-ethyl ester matrixes.
| Independent Variables | Dependent Variables | |||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | |
| Batch | PVP (%) |
Eudragit® RL PO (%) |
α-Tocopherol (%) |
DTPA penta-ethyl ester loading (%) |
Water absorption (%) |
DTPA penta-ethyl ester remaininga (%) |
| 1 | 25 | 0 | 0 | 66.0 | 8.3 | 45.3 |
| 2 | 13.5625 | 11.0625 | 0.375 | 64.2 | 5.6 | 62.0 |
| 3 | 13.5625 | 10.3125 | 1.125 | 65.2 | 5.6 | 69.0 |
| 4 | 10 | 13.5 | 1.5 | 64.7 | 4.3 | 82.0 |
| 5 | 23.5 | 0 | 1.5 | 65.5 | 7.5 | 71.5 |
| 6 | 10 | 15 | 0 | 64.1 | 5.1 | 57.4 |
| 7 | 24.25 | 0 | 0.75 | 66.0 | 7.3 | 66.4 |
| 8 | 20.6875 | 3.5625 | 0.75 | 65.5 | 7.7 | 69.0 |
| 9 | 17.5 | 7.5 | 0 | 64.5 | 6.2 | 57.2 |
| 10 | 25 | 0 | 0 | 66.2 | 7.4 | 42.2 |
| 11 | 17.5 | 7.5 | 0 | 65.0 | 6.8 | 53.9 |
| 12 | 10 | 15 | 0 | 64.6 | 5.0 | 61.4 |
| 13 | 23.5 | 0 | 1.5 | 65.4 | 7.8 | 72.3 |
| 14 | 10 | 13.5 | 1.5 | 64.4 | 4.5 | 76.0 |
Stability after storage at 40 ± 2°C and 75 ± 5% relative humidity for one month.
D-optimal mixture design
In order to optimize the formulation, the Design of Expert software program (version 8, Stat-Ease Inc., Minneapolis, USA) was used. The goal was to maximize the percent drug loading and stability while minimizing percent water absorption. A 14-run, 5-replicate, D-optimal mixture design was employed to evaluate the effect of PVP (X1), Eudragit® RL PO (X2), and α-tocopherol (X3) concentrations on drug loading (Y1), water absorption (Y2) and stability (Y3).
Characterization of solid dispersions of DTPA penta-ethyl ester
Evaluation of drug loading
In our previous study,[7] we demonstrated that a single-dose treatment of 40 mg/kg DTPA penta-ethyl ester improved decorporation of 241Am in rats, compared to a no-treatment control group. Thus, we designed a matrix that could contain a high weight/weight percentage of prodrug to accommodate large doses. We were concerned that loading of the prodrug could be affected by the ratio of PVP to Eudragit® RL PO. Thus, the amount of DTPA penta-ethyl ester in various samples was quantified using an HPLC system (Shimadzu fitted with a Corona charged aerosol detector (CAD) (Dionex/ESA, USA). The DTPA penta-ethyl ester matrix was weighed accurately and then dissolved in acetonitrile/ethanol (1:1). For each formulation, four matrices were analyzed by the HPLC-CAD method, and drug-free excipient mixtures served as controls. The drug loading was calculated as follows:
| [Eq. 1] |
The column was an Alltima™ C18 (250 mm × 2.1 mm; 5 µm) (Grace Davison Discovery Sciences) and maintained at 40 °C. The flow rate was 0.20 mL min−1; the mobile phase was water with 0.1% trifluoroacetic acid (A), acetonitrile (B), and isopropanol (C). The mobile phase mixture (A–B–C) followed a linear gradient from 94:4:2 to 10:60:30 over 6.5 min, followed by an isocratic phase of 10:60:30 for 4.5 min.[7] The HPLC was validated as described previously.[23]
Evaluation of weight variation
Twenty DTPA penta-ethyl ester matrices of each formulation were selected randomly and the average weight was determined. Individual matrices were then weighed and these were compared to the average weight.
Evaluation of water absorption
Water absorption capacity of the DTPA penta-ethyl ester matrix was determined by weighing DTPA penta-ethyl ester matrices before and after storage at room temperature and exposure to a relative humidity of approximately 80% (a saturated solution of sodium chloride in the glass chamber). After storage and exposure, the weight was monitored until no further change was observed. The water absorption capacity of the DTPA penta-ethyl ester matrix was calculated using the Equation 2; an average of ten readings is reported.
| [Eq. 2] |
where Wa is weight after storage of DTPA penta-ethyl ester matrix and Wb is weight before storage of DTPA penta-ethyl ester matrix.
Fourier transform infrared (FTIR) spectroscopy
FTIR spectra of DTPA penta-ethyl ester, PVP, α-tocopherol, Eudragit® RL PO and DTPA penta-ethyl ester matrix were obtained using a Shimadzu Prestige-21 FTIR spectrometer and IRsolution FTIR control software. Thirty two scans were obtained at a resolution of 4 cm−1 from 4,000 to 400 cm−1.
Differential scanning calorimetry (DSC)
The thermal behavior of the DTPA penta-ethyl ester, PVP, α-tocopherol,Eudragit® RL PO and DTPA penta-ethyl ester matrix was determined using DSC (Perkin Elmer DSC 6, USA). Samples of approximately 10 mg were placed into a crimped aluminum pan and scanned from 40 to 180°C at a heating rate of 5°C min−1. Samples were maintained under a nitrogen atmosphere, which was fed at a flow rate of 40 mL min−1.
Dissolution studies
The dissolution profiles of neat DTPA penta-ethyl ester were obtained using a USP dissolution Type II apparatus (Hanson Research Elite 8, Chatsworth, CA) with a paddle rotation speed of 50 rpm in 900 mL 0.1 N HCl at 37°C for 2 h. An HPMC capsule (Size: 0) was filled with 200 mg of DTPA penta-ethyl ester and subsequently placed in the dissolution medium. For dissolution studies with the optimized formulation of DTPA penta-ethyl ester-matrix, 300 mg of matrices containing 195 mg DTPA penta-ethyl ester were placed into 900 mL of 0.1 N HCl (pH 1.2) and incubated at 37°C for 2 h. The DTPA penta-ethyl ester matrices were then transferred to 900 mL of phosphate buffer, pH 6.8, and incubated at 37°C for an additional 2 h. At specific time intervals, an aliquot (0.5 mL) was withdrawn and passed through a 0.2 µm PVDF filter. The DTPA penta-ethyl ester content was then determined by HPLC-CAD. Dissolution studies were performed in triplicate and calculated mean values of cumulative drug release were used for plotting the release curves.
Stability assessment
DTPA penta-ethyl ester matrix samples were stored in closed glass screw-cap vials at 40 ± 2 °C and 75 ± 5% relative humidity for one month for the accelerated stability tests. Drug remaining in the matrices was analyzed using a previously developed HPLC-CAD method.
Statistical analysis
Data are presented as the mean ± SEM. Each experiment was repeated at least three times. An unpaired t test was used to establish the significance of differences among groups. Differences were considered statistically significant at P < 0.05.
Results and discussion
Solid dispersion of DTPA penta-ethyl ester
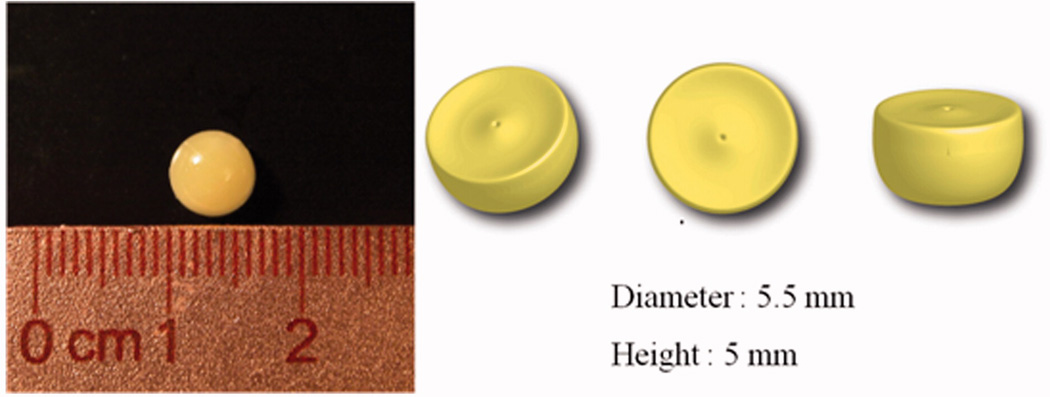
The DTPA penta-ethyl ester matrix is shown in Figure 1. Its diameter and height were 5.5 mm and 5 mm, respectively, and its weight approximately 70 ± 4 mg. There were no significant differences in weight among the DTPA penta-ethyl ester matrixes formulated (P > 0.05).
Figure 1.
Appearance of the DTPA penta-ethyl ester matrix.
Data analysis
Observed responses in D-optimal mixture design for the DTPA penta-ethyl ester matrix
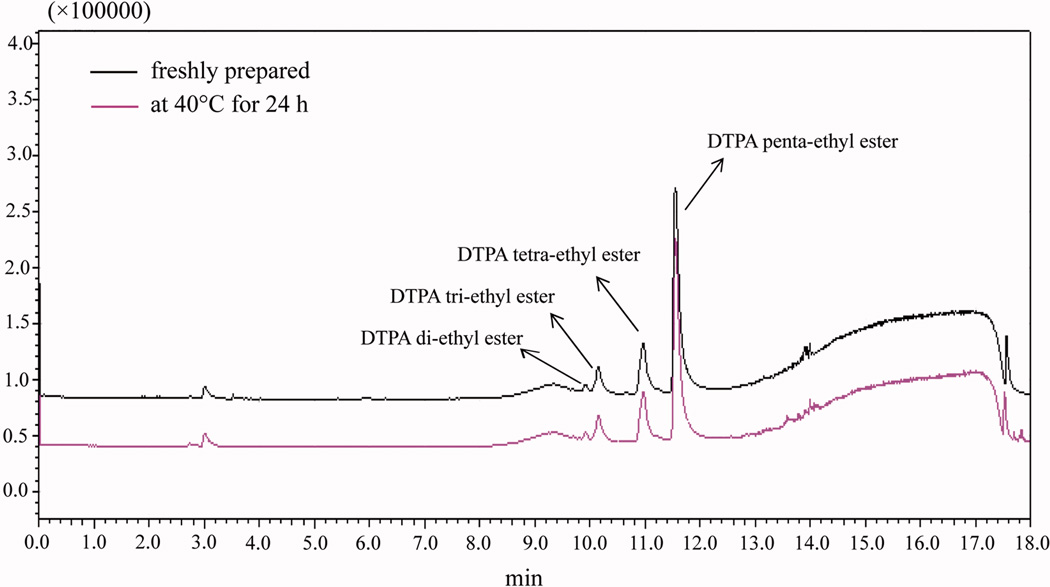
The values for percent drug loading, water absorption and stability of the prodrug are presented in Table 1. A drug loading of 75% was chosen because the resulting weight of DTPA penta-ethyl ester in the matrix would correspond to the dose required in vivo, 40 mg kg−1 (75 µmol kg−1), to improve decorporation of 241Am after oral administration to rats.[7] For drug loading, there were no significant differences among the prepared DTPA penta-ethyl ester matrixes (P > 0.05). Table 2 shows the area of DTPA penta-ethyl ester peak decreased while drying at 40°C for 24 h. The result demonstrated that approximately 4% of the DTPA penta-ethyl ester underwent degradation after 24 h of storage at 40°C. Water absorption and stability were dependent upon the concentration of the three excipients, PVP, Eudragit® RL PO and α-tocopherol. The minimum water absorption (4.3%) corresponds to the highest Eudragit® RL PO concentration (13.5%) and lowest PVP concentration (10%). Increasing the fraction of PVP, a hydrophilic polymer which readily absorbs up to 40% of its own weight under 80% relative atmospheric humidity,[24] resulted in an increase in the percent water absorbed. There was no phase separation observed in any of the formulations, and the stability results showed that the percent of intact DTPA penta-ethyl ester in the matrix after one month of storage under accelerated storage conditions ranged from 42.2–82.0%. The greatest stability was observed when using high concentrations of α-tocopherol (1.5%) and Eudragit® RL PO (13.5%). Stability studies with neat DTPA penta-ethyl ester showed that only 34% of the drug remained after storage at 40 ± 2°C (75 ± 5% relative humidity) for 1 month (data not shown), which is lower than that in all matrixes. Thus, it appears that the stability of DTPA penta-ethyl ester can be increased after formulating it in a solid dispersion. Due to the presence of five esters in its structure, the prodrug is unstable in high-temperature, high-humidity environments, as ester prodrugs are susceptible to degradation by hydrolytic cleavage.[25] Our pre-formulation studies showed that hydrolysis is the major mechanism by which the prodrug degrades. An examination of the HPLC chromatograms (Figure 2) of degraded samples revealed the presence of DTPA tetra-ethyl ester, DTPA tri-ethyl ester, and DTPA di-ethyl ester.
Table 2.
The area of DTPA penta-ethyl ester with freshly prepared and storage for 24 h at 40°C
| Retention time (min) | Area | |
|---|---|---|
| Freshly prepared | 11.547 | 1428280 |
| 24 hr (40°C) | 11.553 | 1372973 |
Figure 2.
Chromatograms of freshly prepared DTPA penta-ethyl ester that was stored at 40°C for 24 h.
Data fitting
Models for fitting the data were considered significant when the p value was <0.05.[26] From the p values presented in Table 3, it can be concluded that the best model for Y1, Y2 and Y3 is linear. R2 values for Y1, Y2 and Y3 were 0.791, 0.935 and 0.885, respectively, indicating good correlation between dependent and independent variables. Furthermore, the three linear models had large adjusted and predicted R2 values as well as small standard deviations (SD). These predicted residual sum of squares (PRESS), shown in Table 3, further exemplifying their suitability to fit the data. The following equations show the relationship between components that influence drug loading, water absorption and stability.
| [Eq. 3] |
| [Eq. 4] |
| [Eq. 5] |
Table 3.
Model comparison: Summary of statistics for Y1, Y2 and Y3 responses.
| Models | p-value | R2 | Adjusted R2 | Predicted R2 | SD | PRESSa | Remarks |
|---|---|---|---|---|---|---|---|
| Response (Y1) | |||||||
| Linear | 0.0002 | 0.7909 | 0.7529 | 0.6706 | 0.35 | 2.12 | Suggested |
| Quadratic | 0.1059 | 0.8987 | 0.8354 | 0.7415 | 0.29 | 1.66 | |
| Special Cubic | 0.1389 | 0.9276 | 0.8655 | 0.6333 | 0.26 | 2.35 | |
| Response (Y2) | |||||||
| Linear | < 0.0001 | 0.9351 | 0.9233 | 0.8983 | 0.37 | 2.41 | Suggested |
| Quadratic | 0.4495 | 0.9525 | 0.9229 | 0.8511 | 0.37 | 3.53 | |
| Special Cubic | 0.2531 | 0.9611 | 0.9278 | 0.8104 | 0.36 | 4.49 | |
| Response (Y3) | |||||||
| Linear | < 0.0001 | 0.8847 | 0.8638 | 0.8170 | 4.18 | 304.83 | Suggested |
| Quadratic | 0.2332 | 0.9305 | 0.8870 | 0.7988 | 3.80 | 335.12 | |
| Special Cubic | 0.0798 | 0.9565 | 0.9193 | 0.8529 | 3.22 | 245.0 |
PRESS is predicted residual sum of square
A positive value indicates a synergistic effect and a negative value represents an antagonistic effect. The coefficients of X1 for Y2 responses show that a synergistic effect was observed for water absorption. Thus, as expected, an increase in PVP resulted in an increase in water absorption; therefore, a lower ratio of PVP is preferred here. The coefficients of the X3 for Y3 responses demonstrate that α-tocopherol is the most important component for stability of the prodrug in the matrix.
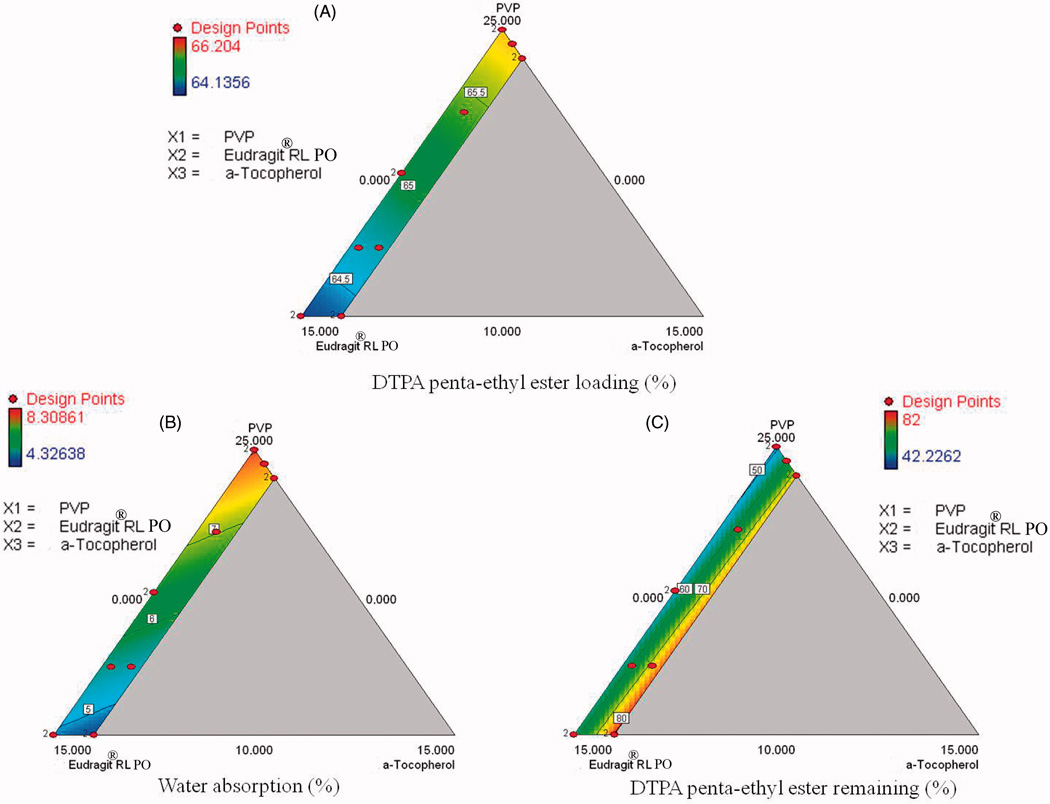
Contour plot analysis
Two dimensional contour plots are presented in Figure 3 and show the effects of three components (PVP, Eudragit® RL PO and α-tocopherol) on dependent variables. The contour plot shows that drug loading is not significantly affected by the ratio of formulation ingredients (Figure 3A). The water absorption of the formulations was dependent on the concentration of PVP and Eudragit® RL PO (Figure 3B). Increasing the fraction of Eudragit® RL PO decreased water absorption as expected due to its hydrophobic properties. Contour plots for stability showed that increasing concentrations of α-tocopherol and Eudragit® RL in the formulation resulted in an increase in the stability of DTPA penta-ethyl ester in the matrix (Figure 3C).
Figure 3.
Contour plots showing the effect of PVP, Eudragit® RL PO and α-tocopherol on drug loading (A), water absorption (B) and 1 month stability of DTPA penta-ethyl ester at 40°C/80% relative humidity (C).
Optimization of the DTPA penta-ethyl ester-matrix formulation
The optimum formulation of DTPA penta-ethyl ester-matrix (batch 4) was selected based on maximum percent drug loading, stability and minimum water absorption. The optimized formulation was achieved with 10% PVP, 13.5% Eudragit® RL PO and 1.5% α-tocopherol; it had drug loading of 64.7% with water absorption and stability of 4.3% and 82.0%, respectively.
FTIR spectroscopy
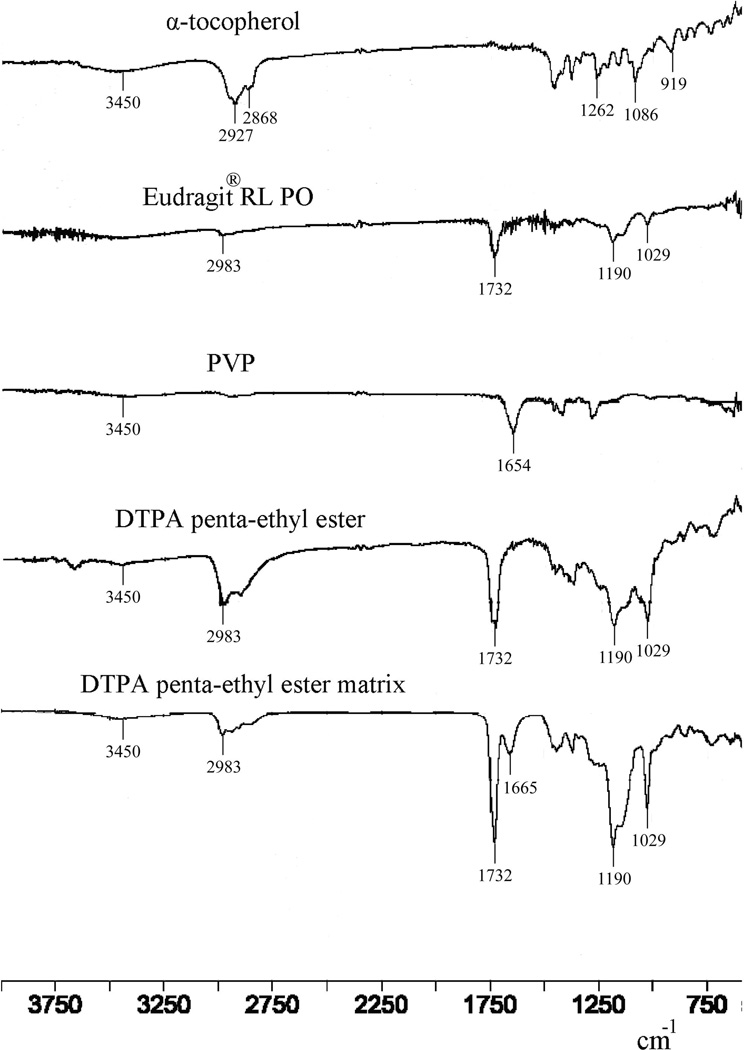
Interactions between drug and carrier can lead to identifiable changes in the FTIR spectrum of a solid dispersion. Thus, FTIR spectra of DTPA penta-ethyl ester, PVP, Eudragit® RL PO, α-tocopherol and the DTPA penta-ethyl ester matrix were obtained (Figure 4). The spectra of DTPA penta-ethyl ester and Eudragit® RL PO have absorptions at 2983, 1732, 1190 and 1029 cm−1, which correspond to sp3 CH2 and CH3, C=O, C-O and C-N stretching vibrations, respectively. In the PVP spectrum, the absorption at 1654 cm−1 corresponds to the stretching vibration of the C=O on the pyrrolyl ring.[13, 27] The α-tocopherol spectrum has absorptions at 3450 cm−1 (O-H stretching), 2927 and 2868 cm−1 (asymmetric and symmetric sp3 CH2 and CH3 stretching), 1,262 cm−1 (CH2 bending), 1,086 cm−1 (CH in-plane bending on the phenyl ring) and 919 cm−1 (-CH=CH-(trans) stretching).[28] In the FTIR spectrum of the DTPA penta-ethyl ester matrix, absorptions associated with DTPA penta-ethyl ester and excipients are observed that do not differ significantly in position from their individual spectra. These results suggest that the DTPA penta-ethyl ester and excipients do not exhibit solid-solid interactions with each other during formulation of the solid dispersion.
Figure 4.
FTIR spectra of the optimized DTPA penta-ethyl ester matrix, neat DTPA penta-ethyl ester, PVP, Eudragit® RL PO and α-tocopherol.
Thermal analysis
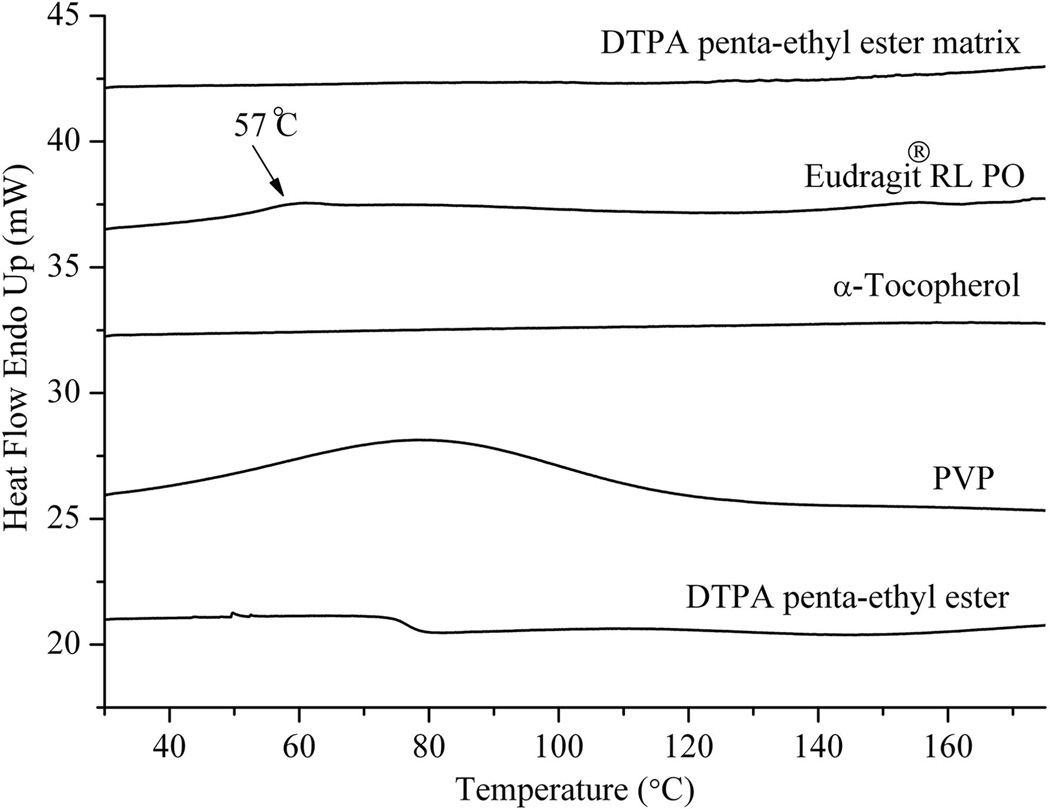
DSC curves obtained for neat DTPA penta-ethyl ester, PVP, α-tocopherol, Eudragit® RL PO and the DTPA penta-ethyl ester matrix (batch 4) are shown in Figure 5. No thermal phase transitions were observed for neat DTPA penta-ethyl ester or α-tocopherol, when the temperature was raised from 40 to 180°C. Eudragit® RL PO had a glass transition temperature at 57°C, and during the scanning of PVP, a broad endothermic peak ranging from 60 to 120°C was observed, probably due to the presence of water. As expected, no peaks were present in the thermogram of the solid dispersion of the optimized formulation indicating that the matrix had an amorphous structure.
Figure 5.
DSC traces of neat DTPA penta-ethyl ester, PVP, α-tocopherol, EudragitRL PO and DTPA penta-ethyl ester matrix at a scanning rate of 5°C min-1.
Dissolution
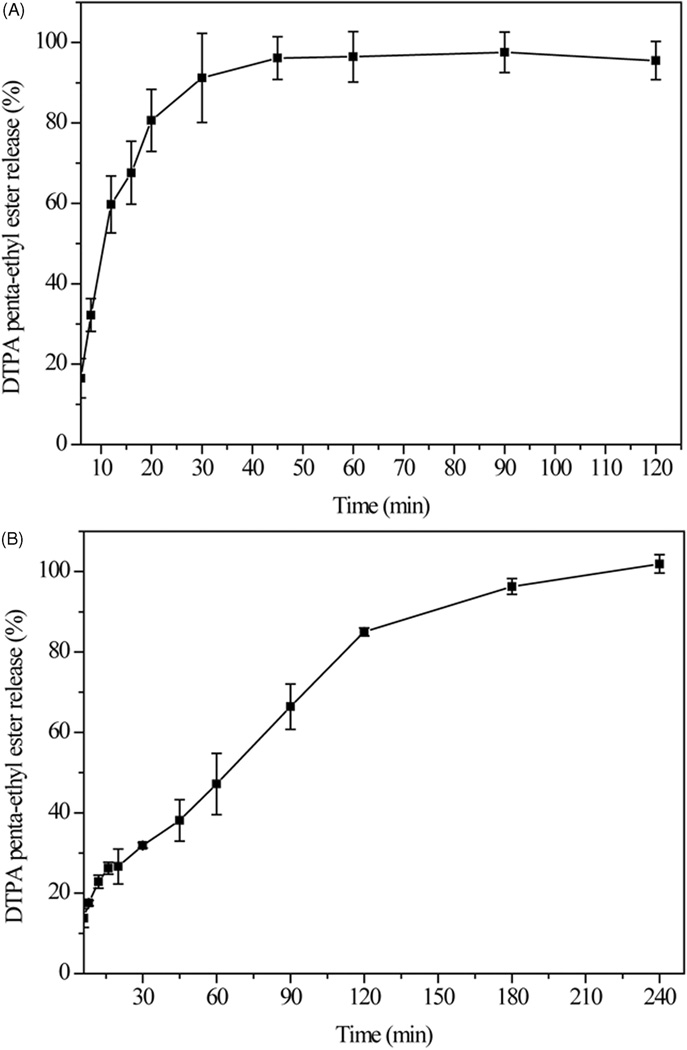
The dissolution profiles of neat DTPA penta-ethyl ester in 0.1 N HCl (pH 1.2) and the optimized DTPA penta-ethyl ester matrix formulation (batch 4) in 0.1 N HCl and phosphate buffer(pH 6.8) are shown in Figure 6. The dissolution rate of DTPA penta-ethyl ester was faster than that of the DTPA penta-ethyl ester matrix in pH 1.2 medium in the first two hours. Approximately 96% of the drug was released after 45 min when using neat DTPA penta-ethyl ester. In contrast, a slower release of drug was observed when using the optimized formulation; approximately 85% of drug was released in the acidic dissolution medium after two hours, and 100% two hours after the medium was changed to a pH 6.8 solution. The solid dispersion polymer of DTPA penta-ethyl ester in the matrices strongly affected drug dissolution rate, and thehe slower release of DTPA penta-ethyl ester from the matrix was likely due to the addition of Eudragit® RL PO polymer in the formulation. Eudragit RL is a water-insoluble polymer, and it shows the pH-independent sustained drug release attributed to the quaternary ammonium groups.[17, 18] The optimized solid dispersion appears to allow for sustained delivery of DTPA penta-ethyl ester to the intestines. This is advantageous for radionuclide decorporation therapy where sustained blood levels of chelating agents are desirable for removing circulating radionuclides from the bloodstream.
Figure 6.
Amount released of the neat DTPA penta-ethyl ester in 0.1 M HCl (2 h) (A) and from matrix in 0.1 M HCl (2 h) and then PBS, pH 6.8 (2 h) (B).
Conclusions
DTPA penta-ethyl ester was successfully incorporated into a solid matrix with high drug loading and improved stability compared to neat prodrug. The results of the D-optimal mixture design indicated that having a high concentration of both Eudragit® RL PO and α-tocopherol favored low water absorption and enhanced stability of DTPA penta-ethyl ester in the matrix; no significant differences between formulations in percent loading of the prodrug were ever observed, however. DSC studies indicated that the matrix has an amorphous structure, and FTIR spectrometry showed that there are no interactions between drug molecule and excipients in the solid dispersion. Future efforts are being devoted to preparing a polymer-coated solid dispersion to further protect the active ingredient from hydrolytic and oxidative degradation.
Acknowledgments
The authors would like to thank Dr. Weiling He for her contributions on analytical method development.
This work was funded by the National Institute of Health, U.S. Department of Health and Human Services under contracts HHSN266200500045C and HHSN272201000030C. The authors alone are responsible for the content and writing of the paper.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Bair WJ, Thompson RC. Plutonium: biomedical research. Science. 1974;183:715–722. doi: 10.1126/science.183.4126.715. [DOI] [PubMed] [Google Scholar]
- 2.Breustedt B, Blanchardon E, Berard P, Fritsch P, Giussani A, Lopez MA. Biokinetic modelling of DTPA decorporation therapy: the CONRAD approach. Radiat Prot Dosimetry. 2009;134:38–48. doi: 10.1093/rpd/ncp058. [DOI] [PubMed] [Google Scholar]
- 3.Fritsch P, Serandour AL, Gremy O, Phan G, Tsapis N, Fattal E. Structure of a single model to describe plutonium and americium decorporation by DTPA treatments. Health Phys. 2010;99:553–559. doi: 10.1097/HP.0b013e3181c1cccd. [DOI] [PubMed] [Google Scholar]
- 4.Gervelas C, Serandour AL, Geiger S, Grillon G, Fritsch P, Taulelle C. Direct lung delivery of a dry powder formulation of DTPA with improved aerosolization properties: effect on lung and systemic decorporation of plutonium. J Control Release. 2007;118:78–86. doi: 10.1016/j.jconrel.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Serandour AL, Tsapis N, Gervelas C, Grillon G, Frechou M, Deverre JR. Decorporation of plutonium by pulmonary administration of Ca-DTPA dry powder: a study in rat after lung contamination with different plutonium forms. Radiat Prot Dosimetry. 2007;127:472–476. doi: 10.1093/rpd/ncm300. [DOI] [PubMed] [Google Scholar]
- 6.Cassatt DR, Kaminski JM, Hatchett RJ, DiCarlo AL, Benjamin JM, Maidment BW. Medical countermeasures against nuclear threats: radionuclide decorporation agents. Radiat Res. 2008;170:540–548. doi: 10.1667/rr1485.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sueda K, Sadgrove MP, Fitzsimmons JM, Jay M. Physicochemical characterization of a prodrug of a radionuclide decorporation agent for oral delivery. J Pharm Sci. 2012;101:2844–2853. doi: 10.1002/jps.23218. [DOI] [PubMed] [Google Scholar]
- 8.Leed M, Pacyniak E, Sadgrove M, Kagel J, Armour R, Zamboni W. Biodistribution and pharmacokinetic analysis of a multi-ester prodrug following administration to rats. AAPS Journal. 2011;13:W4417. [Google Scholar]
- 9.Sadgrove MP, Leed MGD, Shapariya S, Madhura DB, Jay M. Evaluation of a DTPA prodrug as an orally bioavailable radionuclide decorporation agent. drug development research. Special issue on radiation drugs: A Hot Topic. 2012;73:243–251. [Google Scholar]
- 10.Chiou WL, Smith LD. Solid dispersion approach to the formulation of organic liquid drugs using polyethylene glycol 6000 as a carrier. J Pharm Sci. 1971;60:125–127. doi: 10.1002/jps.2600600127. [DOI] [PubMed] [Google Scholar]
- 11.Barker SA, Yap SP, Yuen KH, McCoy CP, Murphy JR, Craig DQ. An investigation into the structure and bioavailability of alpha-tocopherol dispersions in Gelucire 44/14. J Control Release. 2003;91:477–488. doi: 10.1016/s0168-3659(03)00261-x. [DOI] [PubMed] [Google Scholar]
- 12.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60. doi: 10.1016/s0939-6411(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 13.Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272:1–10. doi: 10.1016/j.ijpharm.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Modi A, Tayade P. Enhancement of dissolution profile by solid dispersion (kneading) technique. AAPS PharmSciTech. 2006;7:68. doi: 10.1208/pt070368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tros de Ilarduya MC, Martin C, Goni MM, Martinez-Oharriz MC. Solubilization and interaction of sulindac with polyvinylpyrrolidone K30 in the solid state and in aqueous solution. Drug Dev Ind Pharm. 1998;24:295–300. doi: 10.3109/03639049809085623. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita K, Nakate T, Okimoto K, Ohike A, Tokunaga Y, Ibuki R. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int J Pharm. 2003;267:79–91. doi: 10.1016/j.ijpharm.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Kuksal A, Tiwary AK, Jain NK, Jain S. Formulation and in vitro, in vivo evaluation of extended- release matrix tablet of zidovudine: influence of combination of hydrophilic and hydrophobic matrix formers. AAPS PharmSciTech. 2006;7:E1. doi: 10.1208/pt070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahoo J, Murthy PN, Biswal S, Manik Formulation of sustained-release dosage form of verapamil hydrochloride by solid dispersion technique using Eudragit RLPO or Kollidon SR. AAPS PharmSciTech. 2009;10:27–33. doi: 10.1208/s12249-008-9175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzsimmons J, Sueda K, He W, Lu X, McNamara PJ, Mumper RJ. DTPA prodrug as an orally bioavailable actinide decorporation agent, presented at the 10th international conference on health effects of incorporated radionuclides. Health Phys. 2010;99:285. doi: 10.1097/HP.0b013e3181e735a3. [DOI] [PubMed] [Google Scholar]
- 20.Stemmler K, Glod G, von Gunten U. Oxidation of metal-diethylenetriamine-pentaacetate (DTPA)-complexes during drinking water ozonation. Water Res. 2001;35:1877–1886. doi: 10.1016/s0043-1354(00)00457-7. [DOI] [PubMed] [Google Scholar]
- 21.Holm R, Jensen IH, Sonnergaard J. Optimization of self-microemulsifying drug delivery systems (SMEDDS) using a D-optimal design and the desirability function. Drug Dev Ind Pharm. 2006;32:1025–1032. doi: 10.1080/03639040600559024. [DOI] [PubMed] [Google Scholar]
- 22.Nahata T, Saini TR. D-optimal designing and optimization of long acting microsphere-based injectable formulation of aripiprazole. Drug Dev Ind Pharm. 2008;34:668–675. doi: 10.1080/03639040701836545. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Sadgrove MP, Sueda K, Yang YT, Pacyniak EK, Kagel JR, Braun BA, Zamboni WC, Mumper RJ, Jay M. Nonaqueous gel for the transdermal delivery of a DTPA penta-ethyl ester prodrug. The AAPS J. 2013:523–532. doi: 10.1208/s12248-013-9459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haaf F, Sanner A, Straub F. Polymers of N-Vinylpyrrolidone: synthesis, characterization and uses. Polym J. 1985:143–152. [Google Scholar]
- 25.Langner M, Maibach H. Many common drugs in dermatology are light, temperature, or moisture-sensitive. Skin Therapy Lett. 2009:3–5. [PubMed] [Google Scholar]
- 26.Anderson-Cook CM, Goldfarb HB, Borror CM, Montgomery DC, Canter KG, Twist JN. Mixture and mixture–process variable experiments for pharmaceutical applications. Pharmaceut Statist. 2004;3:247–260. [Google Scholar]
- 27.Zhang X, Sun N, Wu B, Lu Y, Guan T, Wu W. Physical characterization of lansoprazole/PVP solid dispersion prepared by fluid-bed coating technique. Powder Technol. 2008:480–485. [Google Scholar]
- 28.Silva SD, Rosa NF, Ferreira AE, Boas LV, Bronze MR. Rapid determination of α-tocopherol in vegetable oils by fourier transform infrared spectroscopy. Food Anal Methods. 2009:120–127. [Google Scholar]