Abstract
Endothelial dysfunction with impaired bioavailability of nitric oxide (NO) is the hallmark in the development of cardiovascular disease. Endothelial dysfunction leads to atherosclerosis, characterized by chronic inflammation of the arterial wall and stepwise narrowing of the vessel lumen. Atherosclerosis causes deprivation of adequate tissue blood flow with compromised oxygen supply. To overcome this undersupply, remodeling of the vascular network is necessary to reconstitute and sustain tissue viability. This physiological response is often not sufficient and therapeutic angiogenesis remains an unmet medical need in critical limb ischemia or coronary artery disease. Feasible approaches to promote blood vessel formation are sparse. Administration of pro-angiogenic factors, gene therapy, or targeting of microRNAs has not yet entered the daily practice. Nitric oxide is an important mediator of angiogenesis that becomes limited under ischemic conditions and the maintenance of NO availability might constitute an attractive therapeutic target. Until recently it was unknown how the organism provides NO under ischemia. In recent years it could be demonstrated that NO can be formed independently of its enzymatic synthesis in the endothelium by reduction of inorganic nitrite under hypoxic conditions. Circulating nitrite derives from oxidation of NO or reduction of inorganic nitrate by commensal bacteria in the oral cavity. Intriguingly, nitrate is a common constituent of our everyday diet and particularly high concentrations are found in leafy green vegetables such as spinach, lettuce, or beetroot. Evidence suggests that dietary nitrate supplementation increases the regenerative capacity of ischemic tissue and that this effect may offer an attractive nutrition-based strategy to improve ischemia-induced revascularization. We here summarize and discuss the regenerative capacity of dietary nitrate on the vascular system.
Keywords: Dietary, Nitrate, Vasculature, Regeneration, Hind limb
Core tip: Nitrate is a common constituent of our everyday diet and particularly high concentrations are found in leafy green vegetables such as spinach, lettuce, or beetroot. Evidence suggests that dietary nitrate supplementation increases the regenerative capacity of ischemic tissue and that this effect may offer an attractive nutrition-based strategy to improve ischemia-induced revascularization. We here summarize and discuss the regenerative capacity of dietary nitrate on the vascular system.
BREAKDOWN OF NITRIC OXIDE AVAILABILITY IS THE HALLMARK OF CARDIOVASCULAR DISEASE
Nitric oxide (NO) is biosynthesized endogenously from the amino acid L-Arginine (L-Arg) and oxygen by various NO synthases (NOS) which are termed either according to their distribution within the body or allowing for the order where they first purified and cloned. NOS produce NO by catalyzing a five-electron oxidation of guanidino nitrogen of L-Arg that requires binding of five cofactors. These cofactors are: flavin adenine dinucleotide, flavin mononucleotide, heme iron, tetrahydrobiopterin, and calcium-calmodulin[1]. If any of these co-factors becomes limited, NO production from NOS is restricted and NOS produce superoxide (O2-) instead. This mechanism has been termed “NOS uncoupling”[2]. Consequently, a physiological oxygen concentration as well as sufficient substrate supply is necessary for a proper NOS function. NO is involved in a wide variety of regulatory mechanisms of the cardiovascular system, including vascular tone (as a major mediator of endothelium dependent vasodilatation), vascular structure (inhibition of smooth muscle cell proliferation), and cell-cell interactions in blood vessels (inhibition of platelet adhesion and aggregation and inhibition of monocyte adhesion). Risk factors like hypercholesterolemia, hypertension, diabetes mellitus or cigarette smoking lead to the inability of the endothelium to produce NO[3-5]. A decrease of endothelial NO formation due to insufficient oxygen and cofactor supply or inactivation by reactive oxygen species is the hallmark of endothelial dysfunction. Importantly, this is the key element and a facilitative factor in the development of atherosclerosis. Lack of NO in turn promotes aggregation and invasion of inflammatory cells in the vessel wall and aggravates sclerosis of arteries. Thus, a vicious cycle takes place that results in progressive deprivation of blood supply with hypoxia of tissues and organs. Growth of new vessels result as an adaptive mechanism in response to tissue hypoxia or ischemic injury, called angiogenesis. The physiological repair response, however, is often not sufficient and therapeutic angiogenesis remains an unmet medical need.
ROLE OF NO IN ANGIOGENESIS
Angiogenesis is strongly stimulated in response to tissue hypoxia or ischemic injury and requires several key processes, including dissolution of matrix, endothelial cell proliferation and migration, and organization into tubes followed by lumen formation. One of the most potent angiogenic growth factors is represented by the vascular endothelial growth factor (VEGF) that induces proliferation, migration, survival and permeability of endothelial cells[6,7]. VEGF upregulates the expression of endothelial NO synthase (eNOS) and stimulates the release of endothelium-derived NO what is believed to play a critical role in the angiogenic action of this factor[8]. In line with these findings, it could be demonstrated that eNOS gene delivery promotes angiogenesis in animal models of ischemia[9]. On the contrary, the angiogenic response following hind limb ischemia in mice is impaired in eNOS- deficient mice and this cannot be reversed by VEGF substitution[10]. From these findings, NO appears to be a downstream mediator of VEGF-induced endothelial cell proliferation and migration and is suggested to even regulate VEGF expression[11]. However, it should be noted that a major limitation of these investigations is the use of NO donors. The drawback of this approach is that the influence of the released NO might be masked by the NO-independent actions of donating compounds or their derivatives. Likewise, animal models using eNOS overexpression to determine whether the effect of NO on VEGF synthesis could be achieved in ischemic limb neglect that eNOS is dysfunctional in ischemic tissues[12].
NO GENERATION WITHOUT NOS: THE NITRATE-NITRITE-NO PATHWAY
Since the classical NO-pathway is not functional during ischemia, NOS independent mechanisms must exist to maintain NO homeostasis under hypoxic conditions. The reduction of nitrite, the oxidation product of NO, by several “nitrite-reductases” under hypoxia was identified to be such an alternative pathway (Figure 1 and Table 1)[13-17]. These nitrite reductases operate along the physiological and pathological oxygen gradient and allow a graded nitrite reduction to NO according to the circulating and metabolic need. The reduction of nitrite to NO reflects a major mechanism by which the NO homeostasis is maintained independent of NOS. New insights evidence that nitrate and nitrite metabolism occurs in blood and tissues to recycle NO and other bioactive nitrogen oxides[18,19]. Commensal bacteria in the crypts of the tongue own a nitrate reductase enzyme that is utilized for energy metabolism in the absence of oxygen[20,21]. It was known that nitrate is taken up by the salivary glands and concentrated in the saliva. However, the reason for this active process could not be explained until the finding that nitrate serves as substrate for the nitrate reductase enzyme of bacteria in the mouth. These bacteria reduce both plasma extracted nitrate as well as dietary nitrate to form nitrite resulting in salivary nitrite levels that are 1000-fold higher than those found in human plasma[22]. When nitrite-rich saliva meets the acidic gastric juice after swallowing, nitrite is protonated to form nitrous acid (HNO2), which then decomposes to NO. This acidic disproportionation takes part in the human defense against pathogens entering via the alimentary tract. Furthermore it could provide protection against ulcers from drugs or stress[23-25]. Beside the intragastric formation of NO it has been demonstrated that ingested nitrite reaches the systemic circulation, thus making it systemically available[22]. Nitrite in turn can be reduced in vivo via numerous pathways to form bioactive NO. These include the reduction via deoxygenated myoglobin within the heart muscle, deoxygenated hemoglobin, intracellular xanthin oxidoreductase, enzymes of the mitochondrial respiratory chain, cytochrome P-450 and even via the NOS[13,15,17,26-29]. Thus, several mechanisms exist by which NO is generated in the body, including the NOS enzymes or the non-enzymatically acidic reduction of nitrite. Nitrite mediates hypoxic vasodilation, enhances blood flow and matches oxygen supply to increased metabolic demands under hypoxic conditions[30]. Moreover, application of exogenous nitrite bears the potential to reduce myocardial damage after myocardial ischemia and reperfusion injury[26,31]. In addition, dietary approaches using nitrate to elevate circulating nitrite levels are emerging as a potential treatment regimen for high blood pressure[32]. Considering the upcoming evidence that nitrite and nitrate mediate cytoprotective effects in human physiology and especially under pathophysiological conditions, it is not unlikely that dietary nitrate and nitrite may positively affect human health and disease. Recognizing that NO is the most important molecule in regulating blood pressure and maintaining vascular homeostasis, food sources rich in NO compounds may provide beneficial effects primarily to the heart and vessels. Although there are clear reports on certain foods and diets that have shown a benefit in terms of preventing cancer and cardiovascular disease, the specific nature of the active constituents responsible for the cardioprotective effects of certain foods is still unknown. Viable candidates are fibers, minerals or antioxidants. High intake of fruits and vegetables is indeed associated with reduced risk for coronary artery disease and apoplectic stroke and the strongest protection against coronary heart disease was seen with high intake of green leafy vegetables[33]. Dietary intakes of nitrate-rich vegetables lowers blood pressure in subjects with borderline hypertension to the same extend as mono-therapy with a standard antihypertensive drug[34,35]. Likewise, blood pressure lowering effects could be demonstrated for dietary nitrate and ingestion of beetroot juice respectively[32,36]. We could recently demonstrate that dietary supplementation with inorganic nitrate improves prognostic relevant outcome measures that have been shown to predict cardiovascular events, namely endothelial dysfunction, vascular stiffness and systolic blood pressure in the elderly with moderately increased cardiovascular risk[37]. Improvements in blood pressure following the nitrate rich diet were associated with reductions of pro-inflammatory cytokines, which points to the potential anti-inflammatory actions of the nitrate-nitrite-NO pathway[38,39]. The hypothesis that dietary nitrate might provide cardiovascular benefit is further encouraged by animal models of myocardial infarction, where dietary supplementation of these anions provided beneficial effects on I/R injury[40]. Interestingly, a diet rich in vegetables, such as the Mediterranean and the traditional Japanese diets, contains more nitrate than the recommended acceptable daily intake by the World Health Organization[41]. Even a portion of spinach consumed in one serving of salad can exceed the acceptable daily intake for nitrate[22]. Taken together, the current evidence supports the conclusion of the European Food Safety Authority that benefits of vegetable and fruit consumption outweigh any perceived risk of developing cancer from the consumption of nitrate and nitrite in these foods. The outlined above data from observational epidemiologic and human clinical studies support the hypothesis that nitrates and nitrites of plant origin play essential physiologic roles in supporting cardiovascular health.
Figure 1.
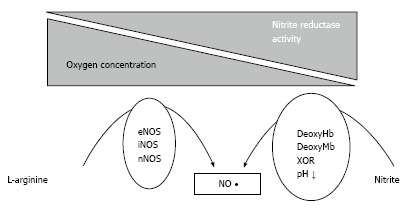
Non-enzymatic nitric oxide formation. One-electron reduction of nitrite (NO2-) to NO by ferrous heme proteins like hemoglobin in the blood or myoglobin in the heart can occur under conditions of low oxygen (O2); the nitrite-reductase activity of these proteins contributes to NOS independent NO formation. eNOS: Endothelial NO synthase; iNOS: Inducible NO synthase; nNOS: Neuronal NO synthase.
Table 1.
Nitric oxide generation pathways
| Nitrate-nitrite-NO pathway | |
| NO synthases | |
| Endothelial NO synthase | |
| Neuronal NO synthase | |
| Inducible NO synthase | |
| Nitrate reductases | |
| Xanthin oxidoreductase | |
| Mitochondrial respiratory chain enzymes | |
| Cytrochrome P-450 | |
| Acidic reduction | |
| Myoglobin | |
| Neuroglobin | |
| Hemoglobin | |
NO: Nitric oxide.
REGENERATIVE CAPACITY OF DIETARY NITRATE ON VASCULATURE
Increased oxygen radicals (ROS) and subsequent reduced NO bioavailability impair vascular growth and remodeling, which highlights important targets for therapeutic angiogenesis in ischaemic myocardial infarction, peripheral vascular disease, as well as stroke. Significant advances have been made with respect to quantitative analytical methods to detect specific ROS and NO species. This must be employed in future studies to enhance the understanding of the relationship between ROS levels and stimulation of vascular remodeling in conjunction with NO bioavailability. Moreover, studies indicate impaired eNOS function in diabetes and atherosclerosis as a result of increased vascular generation of ROS and reduced NO bioavailability[42,43]. Despite endeavors to promote blood vessel formation by administration of pro-angiogenic factors, gene therapy or targeting of microRNAs, clinically applicable strategies have not been developed yet or are still in a preclinical phase[44-47]. In this context, dietary nitrate becomes an attractive candidate in the field. A nitrate rich diet can be achieved via consumption of leefy green vegetables, as spinach or lettuce, or by consumption of beet-root. However, for comparative reasons, most studies have used nitrate in the pure chemical form of sodium nitrate or potassium nitrate. Mechanistically, nitrate and nitrite can be viewed as stable storage pools for NO-like bioactivity. To further determine the mechanisms of a healthy diet through dietary nitrate on the vasculature we investigated the age-related changes that occur at a molecular level and determined the age-related vascular transcriptome altered by dietary nitrate[48,49]. Intriguingly, a chronic nitrate supplementation was shown to act as a modifier of gene expression, highlighting the plethora of putative mechanisms, which dietary nitrate influences[49]. Our results highlight the potential of a dietary approach counteracting the compromised cardiovascular system. Further investigations in ischemic tissues applying the hind limb model in mice highlighted the potential of dietary nitrate in angiogenesis[50]. A cytoprotective role of nitrite in the setting of myocardial, liver, kidney, and brain ischemia-reperfusion (I/R) injury has previously been demonstrated and continuous pharmacological intervention with nitrite injections increases vascular density in hind limb models[51-55]. We could show that dietary nitrate supplementation strongly augments perfusion recovery in chronic hind limb ischemia in vivo via a significant increase in capillary density. This improvement was associated with an increase in circulating nitrite concentrations, an elevated mobilization of CD34+/Flk-1+ cells and migration of bone marrow-derived CD31+/CD45- cells into ischemic tissue[50]. The mobilization of circulating angiogenic cells following dietary nitrate supplementation was recently supported by a phase I clinical study[56]. A further effect of the dietary nitrate supplementation to drinking water was an attenuated apoptosis in myoblasts in chronic hind limb ischemia. Intriguingly, disruption of the nitrate-NO pathway by chronic eradication of the oral bacteria completely abolished beneficial effects of dietary nitrate supplementation and likewise effectively suppressed circulating nitrite levels, which were observed after intake of nitrate-rich food or dietary nitrate supplementation in drinking water. In line with these findings, the results of this study further point to a distinct contribution of dietary nitrate supplementation on tissue viability. Dietary nitrate ameliorated the remarkable capacity of adult skeletal muscles to regenerate myofibers after damage. This rapid repair process is mainly carried out by satellite cells (SCs) with contribution of NO[57,58]. Quiescent SCs become active and proliferate upon injury and display the regenerative capacity of the muscle. Committed daughter cells, the myoblasts, continue to proliferate followed by definite differentiation as initialized by a coordinated cellular signaling[59]. The precise pathways that are influenced by the nitrate-nitrite-NO pathway are under intensive investigations and not fully understood yet.
In summary dietary nitrate supplementation increases the regenerative capacity of ischemic tissue and may offer an attractive nutrition-based strategy to improve ischemia-induced revascularization.
Footnotes
P- Reviewer: Ilgenli TF, Lin GM, Simkhovich BZ S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Supported by The DFG (Ra969/7-2) to Rassaf T.
Conflict-of-interest statement: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 27, 2015
First decision: July 27, 2015
Article in press: September 16, 2015
References
- 1.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galle J, Mülsch A, Busse R, Bassenge E. Effects of native and oxidized low density lipoproteins on formation and inactivation of endothelium-derived relaxing factor. Arterioscler Thromb. 1991;11:198–203. doi: 10.1161/01.atv.11.1.198. [DOI] [PubMed] [Google Scholar]
- 4.Busse R, Fleming I. Nitric oxide, nitric oxide synthase, and hypertensive vascular disease. Curr Hypertens Rep. 1999;1:88–95. doi: 10.1007/s11906-999-0078-6. [DOI] [PubMed] [Google Scholar]
- 5.Mäkimattila S, Virkamäki A, Groop PH, Cockcroft J, Utriainen T, Fagerudd J, Yki-Järvinen H. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–1282. doi: 10.1161/01.cir.94.6.1276. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 7.Holmes DI, Zachary IC. Vascular endothelial growth factor regulates stanniocalcin-1 expression via neuropilin-1-dependent regulation of KDR and synergism with fibroblast growth factor-2. Cell Signal. 2008;20:569–579. doi: 10.1016/j.cellsig.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RS, Lin KF, Agata J, Chao L, Chao J. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2002;22:1279–1285. doi: 10.1161/01.atv.0000026613.18742.67. [DOI] [PubMed] [Google Scholar]
- 10.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 12.Namba T, Koike H, Murakami K, Aoki M, Makino H, Hashiya N, Ogihara T, Kaneda Y, Kohno M, Morishita R. Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation. 2003;108:2250–2257. doi: 10.1161/01.CIR.0000093190.53478.78. [DOI] [PubMed] [Google Scholar]
- 13.Rassaf T, Flögel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100:1749–1754. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 16.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 17.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 19.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 21.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Dykhuizen RS, Frazer R, Duncan C, Smith CC, Golden M, Benjamin N, Leifert C. Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense. Antimicrob Agents Chemother. 1996;40:1422–1425. doi: 10.1128/aac.40.6.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyoshi M, Kasahara E, Park AM, Hiramoto K, Minamiyama Y, Takemura S, Sato EF, Inoue M. Dietary nitrate inhibits stress-induced gastric mucosal injury in the rat. Free Radic Res. 2003;37:85–90. doi: 10.1080/1071576021000086632. [DOI] [PubMed] [Google Scholar]
- 25.Jansson EA, Petersson J, Reinders C, Sobko T, Björne H, Phillipson M, Weitzberg E, Holm L, Lundberg JO. Protection from nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers by dietary nitrate. Free Radic Biol Med. 2007;42:510–518. doi: 10.1016/j.freeradbiomed.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Kozlov AV, Dietrich B, Nohl H. Various intracellular compartments cooperate in the release of nitric oxide from glycerol trinitrate in liver. Br J Pharmacol. 2003;139:989–997. doi: 10.1038/sj.bjp.0705323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci. 2007;64:96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, et al. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luedike P, Hendgen-Cotta UB, Sobierajski J, Totzeck M, Reeh M, Dewor M, Lue H, Krisp C, Wolters D, Kelm M, et al. Cardioprotection through S-nitros(yl)ation of macrophage migration inhibitory factor. Circulation. 2012;125:1880–1889. doi: 10.1161/CIRCULATIONAHA.111.069104. [DOI] [PubMed] [Google Scholar]
- 32.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 33.Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Hennekens CH, Spiegelman D, Willett WC. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 34.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 35.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 36.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J Am Coll Cardiol. 2014;63:1584–1585. doi: 10.1016/j.jacc.2013.08.691. [DOI] [PubMed] [Google Scholar]
- 38.Rammos C, Hendgen-Cotta UB, Pohl J, Totzeck M, Luedike P, Schulze VT, Kelm M, Rassaf T. Modulation of circulating macrophage migration inhibitory factor in the elderly. Biomed Res Int. 2014;2014:582586. doi: 10.1155/2014/582586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rammos C, Hendgen-Cotta UB, Sobierajski J, Adamczyk S, Hetzel GR, Kleophas W, Dellanna F, Kelm M, Rassaf T. Macrophage migration inhibitory factor is associated with vascular dysfunction in patients with end-stage renal disease. Int J Cardiol. 2013;168:5249–5256. doi: 10.1016/j.ijcard.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katan MB. Nitrate in foods: harmful or healthy? Am J Clin Nutr. 2009;90:11–12. doi: 10.3945/ajcn.2009.28014. [DOI] [PubMed] [Google Scholar]
- 42.Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047–1060. doi: 10.1161/01.hyp.31.5.1047. [DOI] [PubMed] [Google Scholar]
- 43.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doebele C, Bonauer A, Fischer A, Scholz A, Reiss Y, Urbich C, Hofmann WK, Zeiher AM, Dimmeler S. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010;115:4944–4950. doi: 10.1182/blood-2010-01-264812. [DOI] [PubMed] [Google Scholar]
- 45.Tirziu D, Simons M. Angiogenesis in the human heart: gene and cell therapy. Angiogenesis. 2005;8:241–251. doi: 10.1007/s10456-005-9011-z. [DOI] [PubMed] [Google Scholar]
- 46.Webster KA. Therapeutic angiogenesis: a complex problem requiring a sophisticated approach. Cardiovasc Toxicol. 2003;3:283–298. doi: 10.1385/ct:3:3:283. [DOI] [PubMed] [Google Scholar]
- 47.Mughal NA, Russell DA, Ponnambalam S, Homer-Vanniasinkam S. Gene therapy in the treatment of peripheral arterial disease. Br J Surg. 2012;99:6–15. doi: 10.1002/bjs.7743. [DOI] [PubMed] [Google Scholar]
- 48.Rammos C, Hendgen-Cotta UB, Deenen R, Pohl J, Stock P, Hinzmann C, Kelm M, Rassaf T. Age-related vascular gene expression profiling in mice. Mech Ageing Dev. 2014;135:15–23. doi: 10.1016/j.mad.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Rammos C, Totzeck M, Deenen R, Köhrer K, Kelm M, Rassaf T, Hendgen-Cotta UB. Dietary nitrate is a modifier of vascular gene expression in old male mice. Oxid Med Cell Longev. 2015;2015:658264. doi: 10.1155/2015/658264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hendgen-Cotta UB, Luedike P, Totzeck M, Kropp M, Schicho A, Stock P, Rammos C, Niessen M, Heiss C, Lundberg JO, et al. Dietary nitrate supplementation improves revascularization in chronic ischemia. Circulation. 2012;126:1983–1992. doi: 10.1161/CIRCULATIONAHA.112.112912. [DOI] [PubMed] [Google Scholar]
- 51.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci USA. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. JAMA. 2005;293:1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- 54.Lu P, Liu F, Yao Z, Wang CY, Chen DD, Tian Y, Zhang JH, Wu YH. Nitrite-derived nitric oxide by xanthine oxidoreductase protects the liver against ischemia-reperfusion injury. Hepatobiliary Pancreat Dis Int. 2005;4:350–355. [PubMed] [Google Scholar]
- 55.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH, Langston W, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci USA. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heiss C, Meyer C, Totzeck M, Hendgen-Cotta UB, Heinen Y, Luedike P, Keymel S, Ayoub N, Lundberg JO, Weitzberg E, et al. Dietary inorganic nitrate mobilizes circulating angiogenic cells. Free Radic Biol Med. 2012;52:1767–1772. doi: 10.1016/j.freeradbiomed.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 57.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Wozniak AC, Kong J, Bock E, Pilipowicz O, Anderson JE. Signaling satellite-cell activation in skeletal muscle: markers, models, stretch, and potential alternate pathways. Muscle Nerve. 2005;31:283–300. doi: 10.1002/mus.20263. [DOI] [PubMed] [Google Scholar]
- 59.Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]


