Abstract
Maternal smoking during pregnancy is associated with enduring psychopathology, such as increased likelihood of substance use, in offspring. Various animal models demonstrate that continuous nicotine exposure produces teratogenic effects in offspring, as well. In the present experiment, a novel intravenous (IV) exposure model was utilized to determine if gestational nicotine (GN) treatment produced alterations in methamphetamine-induced sensitization and the expression of brain derived neurotrophic factor (BDNF) in the mesocorticolimbic dopamine system of adolescent offspring. Dams were injected with IV saline or nicotine (0.05 mg/kg/injection) 3x/day on gestational days 8–21. Habituation was measured on postnatal day (PND) 25–27 and baseline activity on PND 28. On PND 29–35, offspring were injected with saline or methamphetamine (0.3 mg/kg) and locomotor activity was measured after the first and seventh injections. On PND 36, brains were removed, flash frozen, and BDNF protein levels in the nucleus accumbens (NAcc), dorsal striatum (Str), frontal cortex (FC), and hippocampus (Hipp) were analyzed. GN did not affect habituation or the induction of methamphetamine-induced sensitization. Interestingly, GN, but not adolescent methamphetamine treatment, elevated levels of BDNF in the NAcc and Str; however, the GN-induced increase in BDNF in the FC was attenuated by adolescent methamphetamine treatment. Both GN and adolescent methamphetamine treatment increased BDNF in the Hipp. These findings indicate that GN exposure will result in increased levels of BDNF protein throughout the mesocorticolimbic dopamine system during adolescent development, and suggests that methamphetamine abuse will modulate the expression of BDNF in motivational circuitries of adolescent offspring exposed to GN.
Keywords: Gestational nicotine, adolescence, methamphetamine, locomotor activity, BDNF
Introduction
Maternal tobacco smoking during pregnancy is associated with enduring psychopathology in offspring. Neurobehavioral disorders, such as conduct disorder (Cornelius et al., 2007; Fergusson et al., 1998; Stene-Larsen et al., 2009), attention deficit hyperactivity disorder (Button et al., 2007; Thapar et al., 2003), and substance use disorder (SUD; Buka et al., 2003; Kandel et al., 1994; Weissman et al., 1999), are observed in gestational tobacco smoke-exposed offspring at a higher incidence than non-exposed individuals. Maternal tobacco smoking thus increases the vulnerability to neurodevelopmental disorders in offspring that are manifest during adolescent development. The neural substrates affected by maternal smoke exposure, which also contribute to these enduring maladaptive behaviors, however, are not well understood.
The influence of prenatal nicotine exposure on offspring development, apart from other constituents in tobacco smoke, has been investigated with rodent models of gestational nicotine (GN) exposure. This research clearly shows that nicotine delivered during gestation has teratogenic effects on neurodevelopment (Dwyer et al., 2008; Slotkin, 1998). Contemporary models administer nicotine either continuously via a subcutaneous osmotic minipump (Dwyer et al., 2008; Slotkin, 1998); orally through drinking water (Pauly et al., 2004; Zhu et al., 1996), or intravenously (Lacy et al., 2011; LeSage et al., 2006). Studies utilizing the continuous route have demonstrated that nicotine exposure during the gestational period alters cell replication, cell survival, and synaptogenesis in utero, relative to saline-treated animals (GS; Navarro et al., 1989; Slikker et al., 2005; Slotkin, 2004). Moreover, GN produced neurodevelopmental alterations in the mesocorticolimbic dopamine (DA) system, which, in part, mediates motivated behavior (Edwards and Koob, 2010; Everitt et al., 2008; Kalivas, 2009; Robinson and Berridge, 2003; Wise and Bozarth, 1987). Thus, continuous GN exposure altered DA neurons in fetal (Navarro et al., 1989; Ribary and Lichtensteiger, 1989) and preweanling rats (Muneoka et al., 1997) and resulted in decreased DA concentrations and D2 receptors in the striatum of weanlings (Richardson and Tizabi, 1994). Adolescent offspring exhibited increased c-fos expression in the infralimbic cortex and nucleus accumbens (NAcc) core (Park et al., 2006) and decreased nicotine-evoked DA release in the NAcc shell, relative to GS controls (Kane et al., 2004). These findings indicate that continuous GN exposure produces long-lasting changes in the activity of neurons that comprise the motivational system, and demonstrate that nicotine alone produces long-lasting neurobiological changes that may contribute to the psychopathology observed in offspring exposed to prenatal tobacco smoke.
That GN produces increased neuronal activity in brain areas that are known to mediate reward and motivation suggests other neurobiological alterations, such as increases in activity-dependent neurotrophic factors, may also change as a result of prenatal nicotine exposure (Kane et al., 2004; Park et al., 2006). Neurotrophic factors aid in the proliferation, differentiation and survival of neurons (Thoenen, 1995), and are known to play a role in motivated behavior (Thomas et al., 2008). For example, brain derived neurotrophic factor (BDNF) is important for synaptic plasticity and the survival of mesocorticolimbic DA neurons (Hyman et al., 1991), and microinfusion of BDNF into the ventral tegmental area altered cocaine-induced changes in locomotor behavior (Horger et al., 1999). A novel hypothesis is that GN exposure alters expression of BDNF protein in the mesocorticolimbic dopamine system of developing offspring.
An enduring influence of abused drugs such as amphetamine, cocaine, and nicotine, on BDNF protein and BDNF mRNA expression has been demonstrated. For example, postnatal amphetamine exposure has been shown to decrease protein levels of BDNF in the occipital cortex and hypothalamus (Angelucci et al., 2007; Banerjee et al., 2009), and in contrast, cocaine or nicotine treatment has been shown to increase BDNF in the cortex, striatum, and NAcc, (Correll et al., 2009; Graham et al., 2007; Maggio et al., 1998). Second, Wei et al. (2011) characterized numerous potential cell survival and cell death pathways associated with continuous GN exposure in adolescent offspring. Gestational nicotine was shown to result in greater mRNA expression of survival related growth factors, including BDNF, in the NAcc of adolescent offspring. In addition, increased expression of BDNF mRNA was observed in the striatum, whereas the prefrontal cortex showed no change in growth factors. These results are consistent with the general finding that nicotine increases BDNF expression and protein in the striatum (Correll et al., 2009; Maggio et al., 1998), and suggests that GN induces changes in BDNF within the mesocorticolimbic system that are present well after birth.
Alterations in the behavior of GN exposed juvenile, adolescent, and adult offspring have been investigated. GN exposure has been reported to produce hypo- and hyper-active spontaneous locomotor behavior (LeSage et al., 2006; Pauly et al., 2004; Paz et al., 2007; Peters and Tang, 1982; Peters et al., 1979; Richardson and Tizabi, 1994; Romero and Chen, 2004; Tizabi et al., 2000; Vaglenova et al., 2004), as well as altered nicotine and cocaine self-administration in adolescent and adult offspring (Franke et al., 2008; Levin et al., 2006). Behavioral sensitization refers to increased locomotor responding following repeated injection of a psychostimulant drug (Post, 1980), and continuous GN enhanced cocaine-induced behavioral sensitization in adolescent rats (Franke et al., 2007). These findings demonstrate that animal models of GN exposure result in behavioral changes in offspring that are related to the brain’s reward systems (see Dwyer et al., 2008).
The present research utilized a novel, low, intermittent IV exposure model in which pregnant dams receive nicotine, 0.05 mg/kg/injection, 3 times per day from PND 8–21. The IV route of administration is of interest because it closely models the pharmacokinetics of nicotine via the route of inhalation (Benowitz et al., 2009; Booze et al., 1999; Mactutus, 1989; Russell and Feyerabend, 1978). The elimination half-life for IV 0.05 mg/kg/infusion nicotine is ~ 50 minutes (Booze et al., 1999) and thus this exposure method represents a unique model in that the dam and fetuses experience the bolus delivery of nicotine to the brain followed by a rather precipitous clearance (LeSage et al., 2006; Mactutus, 1989; Russell and Feyerabend, 1978).
The current experiments investigated whether the IV GN exposure model (see Lacy et al., 2011) produced alterations in the induction of methamphetamine-mediated behavioral sensitization and induced changes in protein levels of BDNF throughout the mesocorticolimbic system during the adolescent phase of brain development (~ postnatal day [PND] 28–42; Spear, 2000). Specifically, the present experiment determined (1) if IV GN exposure altered spontaneous locomotor behavior and/or behavioral sensitization induced by methamphetamine in adolescent rat offspring; and (2) whether IV GN exposure, adolescent methamphetamine treatment, or the combination altered the expression of BDNF protein levels in the NAcc, dorsal striatum (Str), frontal cortex (FC), and hippocampus (Hipp) of adolescent offspring.
Materials and Methods
IV gestational nicotine exposure
Animals
A total of 42 adult male and female Sprague-Dawley rats were acquired from Harlan Laboratories, Inc., (Indianapolis, IN) for breeding. Thirty-two nulliparous females were implanted with IV access ports at Harlan Laboratories. The females, together with 10 male breeders, were housed in a vivarium located at the University of South Carolina. Rodent food (Teklad Rodent Diet [W] 8604) was provided ad lib. The colony located in the department of psychology was maintained at 21 ± 2° C, 50% ± 10% relative humidity and a 12L:12D cycle with lights on at 0700 h. The protocol for this research methodology was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina.
Breeding
Following a 7-day habituation period, females were housed three per cage, and in the evenings one male rat was placed in a cage with females for breeding. Males remained with the females from approximately 1700 to 0900. Daily vaginal lavage was conducted and samples were analyzed with a microscope to identify pregnant females. If a sample was sperm-positive, the corresponding female was single-caged and that day represented gestational day (GD) 0. If the result was sperm-negative the animal remained group housed and potential mating occurred the following evening(s). This cycle continued until females were pregnant. The weights of the pregnant dams were recorded on GD 1, 7, 14, and 21.
IV Catheter Surgery
The catheterized females used in the present experiment are commercially available from Harlan Industries. Catheterization was performed at Harlan Industries according the methods of Mactutus et al. (1994). Briefly, animals were anesthetized with a mixture of ketamine hydrochloride (100 mg/kg/ml) and xylazine (3.3 mg/kg/ml). Following anesthesia a sterile Intracath IV catheter (Becton, Dickinson and Co., Franklin Lakes, NJ) with a Luer-lock injection cap (Medex, Inc., Carlsbad, CA) was implanted dorsally in a subcutaneous pouch. The distal end of the catheter was inserted into the left jugular vein and advanced toward the heart. On the day following surgery, catheters were flushed with 0.2 ml of heparinized saline.
Drugs
Nicotine hydrogen tartrate and methamphetamine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). Nicotine was weighed as base and was dissolved in physiological saline (0.9%; Hospira, Inc. Lake Forest, IL). The pH of the nicotine solution was neutralized to ~ 7.0 with NaOH. Control dams were administered saline at the volumetric equivalent of nicotine. Heparin (APP Pharmaceuticals, Schaumburg, IL; 1000 U) was added to saline and the 2.5% heparinized saline solution was used to flush the IV catheters once per day. Methamphetamine was weighed as salt and was dissolved in physiological saline.
Prenatal Nicotine Treatment
GN (0.05 mg/kg/injection) or GS (0.9% sterile saline) was injected 3×/day to pregnant dams via internalized IV catheters on gestational day (GD) 8–21. IV injections occurred via the Luer-lock injection cap that was located subcutaneously, on the rat’s dorsal surface. Thus, IV access was achieved by guiding a syringe through the skin and into the injection port. Previous research shows that repeated IV injections of 0.05 mg/kg/injection of nicotine produced behavioral sensitization (Booze et al., 1999; Harrod et al., 2004), and altered DA transporter and DA receptor expression in adult males and females, demonstrating that IV injection of this dose alters motivational circuitry in rodents. Moreover, male and female rats self-administer nicotine within a dose range that includes the 0.05 mg/kg/injection dose (Chaudhri et al., 2005). All prenatal injections were performed during the light portion of the photoperiod, and they were administered daily beginning at 1000, 1300, and 1600 h. The 3-hour intervals that separated IV injections were chosen based on the pharmacokinetic analysis for this dose of nicotine. Booze et al. (1999) showed that male and female rats exhibited an elimination half life of approximately 50 minutes following acute, IV injection of the 0.05 mg/kg/injection dose of nicotine. It was of interest to use an inter-injection-interval that allowed for substantial (2–3 half-lives), but not “complete” (6 half-lives) elimination of nicotine. Thus, across injections, particularly with advancing pregnancy and an associated increased volume of distribution, one would anticipate some accumulation of nicotine levels as occurs with cigarette smoking. Immediately following the first two injections the catheters were flushed with 0.2 ml of saline, and the third injection was followed by 0.2 ml of heparinized saline in order to maintain catheter patency. Dams had ad libitum access to food and water during the experiment.
Surrogate fostering, litter composition, and postnatal testing
The day of birth was considered PND 0. On PND 1 litters were culled to 10, and were composed of 5 males and 5 females whenever possible. All pups were surrogate fostered to timed-pregnant, drug naïve dams on PND 1 to prevent the possibility of poor maternal care. Pups derived from IV saline treated dams were also surrogate fostered to timed-pregnant, drug naïve dams. The righting reflex, negative geotaxis, and eye opening measures were assessed on PND 3–5, 8–10, and 13–17, respectively, to determine if prenatal IV nicotine exposure altered the expression of these developmental milestones, relative to IV saline treated controls. The righting reflex was measured by placing offspring on to their backs, and the time from the release to the completion of the righting reflex was recorded. This procedure occurred in blocks of 3 consecutive trials on PND 3–5, and the maximum latency for each animal to right itself was 25 seconds per trial. Negative geotaxis was measured by placing animals on a wire mesh grid that was positioned at a 25°angle, with their heads aimed towards the downward slope of the apparatus. The latency for animals to right themselves (i.e., turn 180°) was recorded for every trial. A maximum latency of 30 sec was allowed for these trials. Negative geotaxis was tested in three trial blocks across three consecutive days on PND 8–10. Eye opening, which was conducted on PND 13–17, entailed checking every animal’s left and right eyes for the degree of openness. The degree of openness was rated on a scale of 0–3: Zero = completely closed; 1 = any part of the eye visible; 2 = partially open; 3 = fully open. All animals’ weights were recorded on PND 1, 7, 14, and 21. Rats were weaned on PND 21.
Locomotor activity
Animals
Adolescent male (n =34) and female (n =36) offspring were used. Animals were weaned on PND 21 into fresh plastic cages and were housed four, same sex rats/cage. Rats were pair-housed, same sex, on PND 28 for the remainder of the experiment.
Experimental design and procedure
Locomotor activity
Offspring, prenatally exposed to GS or GN, were randomly assigned to the saline (S) or methamphetamine (M) groups, and this yielded the GS-S (females = 8; males = 7), GS-M (females = 8; males = 8), GN-S (females = 9; males = 10), and GN-M (females = 10; males = 10) treatment conditions. Each treatment group was randomly assigned one male and one female per litter (Holson and Pearce, 1992).
Apparatus
The activity monitors were 16 square (40 X 40 cm) chambers (Kinder Inc., Poway, CA) that detected free movement of animals by infrared photocell interruptions. This equipment used an infrared photocell grid (32 emitter/detector pairs) to measure horizontal and vertical locomotor activity. The chambers were converted into round (~ 40 cm diameter) compartments by adding clear Plexiglas inserts. The photocells were tuned by the manufacturer for the extra perspex width. All activity monitors were located in a single, isolated room. One-to-two males and females from each treatment group were represented in each 60-min activity test.
Locomotor activity was repeatedly measured from all animals during the habituation, baseline, and treatment phases of the experiment. Animals were habituated to locomotor activity chambers for three 60-min sessions, one/per day on PND 25–27. No injections were administered during habituation trials. On PND 28 all rats were administered a subcutaneous (sc) saline injection, and five minutes later, were placed into the activity chambers for 60-min to measure baseline activity. Day 28 is referred to as “saline baseline”. On PND 29, rats were injected with saline or methamphetamine (0.3 mg/kg; sc) and were placed into locomotor activity chambers five minutes later. On PND 30–34, rats received injections in the home cage, and on PND 35, animals were administered saline or methamphetamine identically to that of PND 29. Thus the animals’ locomotor response to saline or methamphetamine was assessed on PND 28, 29, and 35 for 60 minutes. This method was used to limit methamphetamine-induced context conditioning (Anagnostaras and Robinson, 1996; Bevins and Palmatier, 2003; Itzhak et al., 1998), because it was of interest to assess the behavioral sensitization produced by methamphetamine alone, in the absence of conditioned hyperactivity elicited from the context. Previous research indeed shows that mice prenatally treated with nicotine exhibited a greater magnitude of conditioned responding in a conditioned place preference procedure (i.e., conditional response to contextual cues) and in a standard fear conditioning procedure (i.e., response to a punctate auditory cue; Paz et al., 2007). Total horizontal activity, which represents all movements detected by the photocells in the horizontal plane, was the dependent measure.
BDNF levels in the NAcc, Str, FC, and Hipp
Twenty-four hours after the last saline or methamphetamine injection, animals were rapidly decapitated. The brains were immediately frozen with isopentane and were then stored in a −80°C freezer. BDNF was assessed on 6–8 animals per drug treatment group. The FC, NAcc, Str, and Hipp were dissected from whole brains and stored at −80°C. In brief, 250 μl of a RIPA cell lysis buffer (150mM NaCl, 50mM Tris-Hcl, 1.0% NP-40, 0.5% Sodium deoxycholate and 0.1% SDS) plus protease and phosphatase inhibitors (P5726, P8340, P0044, Sigma-Aldrich, St. Louis, MO) was added to each tissue sample. Brain regions were homogenized on ice with 10 passes of a Teflon pestle homogenizer. Homogenates were centrifuged at 14,000g for 20 minutes at 4°C and the resulting supernatants were removed and stored at −80°C until use. All samples were analyzed according to manufacturer’s instructions using a BDNF sandwich ELISA kit purchased from Promega (Kit G7610, Madison, WI). For the BDNF assay, anti-BDNF monoclonal antibody (mAb) was added to a carbonate coating buffer (pH 9.7, per specifications included with the Promega protocol for BDNF) and 100 μl of the coating buffer was added to each well of a 96-well polystyrene ELISA plate (MaxiSorb, Nalge Nunc International, Rochester, NY) and incubated overnight at 4°C. All wells were washed using a TBST wash buffer, incubated at room temperature for one hour, and nonspecific binding was blocked through adding block and sample 1× buffer to each well and incubated at room temperature for 1h. The BDNF standard curve was prepared using the BDNF standard supplied from the manufacturer (1μg/ml). The standard was diluted in Block & Sample 1× buffer to achieve a concentration range of 0 to 500 pg/ml pg/ml. Tissue samples were further diluted 1:2 prior to being assayed. The standards and samples were incubated with shaking at room temperature for 2h. Anti-Human BDNF pAB was then added to each well plate, incubated at room temperature (2h), which was followed by incubation (1h) with Anti-IgY horseradish peroxidase (HRP) conjugate. Visualization was achieved by adding TMB one solution to each well followed by an incubation period of 10 min at room temperature. The reaction was stopped by adding 1N hydrochloric acid to each well and plates were read within 30 minutes of stopping the reaction. Optical density was measured using a Bio-Rad (Hercules, CA) 96-well plate reader.
Data Analysis
The data were analyzed using mixed factorial analysis of variance (ANOVA) techniques (SPSS 2009, Version 17.0). An alpha level of 0.05 was used to determine statistical significance for all analyses.
Litter parameters
A one-way ANOVA was conducted for the total number of pups born to GN and GS dams. The between-subjects factors for the litter parameter analyses were sex and gestational treatment (saline or nicotine) and the within-subjects factors were PND and GD. A 2 × 2 (sex × gestational treatment) factorial ANOVA was used to analyze the ratio of males to females born to GN and GS dams. A 2 × 2 × 4 mixed factorial ANOVA (gestational treatment × sex × PND) was conducted for the pup weight gain, righting reflex, negative geotaxis and eye opening data. A 2 × 4 (gestational treatment x gestational day) ANOVA determined if there were differences between GS and GN dams on the measure of maternal weight gain.
Locomotor activity
Separate mixed factorial ANOVAs were conducted on the habituation (PND 25, 26, 27) and locomotor test days (PND 28, 29, 35). A 2 × 2 × 3 (gestational treatment × sex × habituation day) ANOVA was conducted on the habituation data; and a 2 × 2 × 2 × 3 (gestational treatment × sex × adolescent treatment x test day) ANOVA was used for the locomotor test days. The between-subjects factors for these analyses were gestational treatment, adolescent treatment (saline or methamphetamine), and sex. The within-subjects factor was testing days. Within-subjects comparisons were conducted between days one and seven to determine if repeated methamphetamine injection produced behavioral sensitization.
BDNF assay
Separate 2 × 2 × 2 mixed factorial ANOVAs were conducted on the protein levels (pg/mg tissue) derived from ELISA assays for the NAcc, Str, FC, and Hipp. The between-subjects factors for each analysis were gestational treatment, adolescent treatment, and sex. Post hoc comparisons were conducted using the Newman-Keuls test.
Results
Litter Parameters: Pup and Dam Weight Gain, Righting Reflex, Negative Geotaxis, Eye Opening
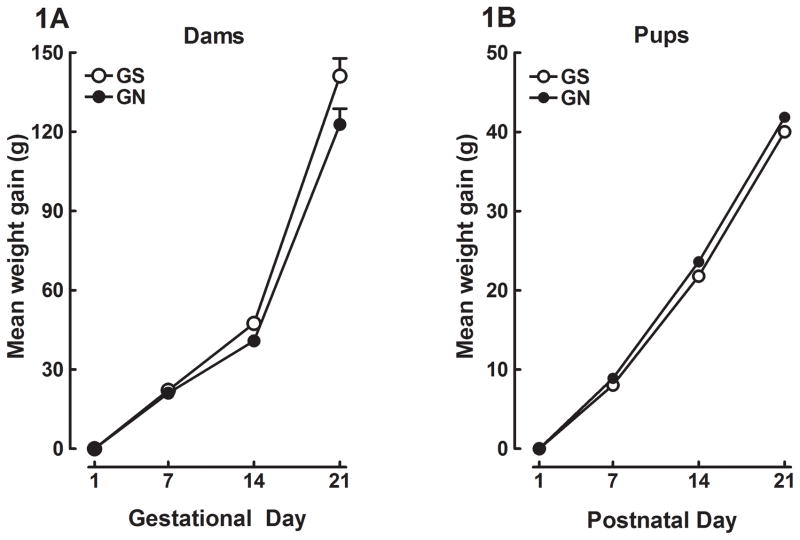
No significant effect of gestational treatment was observed for the total number of pups born to dams, the number of male to female pups, the righting reflex, negative geotaxis, or eye opening. There were also no differences in pup weight gain across PND 1, 7, 14, 21 (Figure 1A), or in maternal weight gain across GD 1, 7, 14, 21 (Figure 1B).
Figure 1.
Mean (±SEM) weight gain exhibited by the dams during gestational days 1–21 (1A). Dams were treated with IV saline or nicotine (0.05 mg/kg/injection) on gestational days 8–21. Mean (±SEM) weight gain of the pups across postnatal days 1–21 (1B). The offspring were prenatally exposed to IV gestational saline (GS) or gestational nicotine (GN).
The effect of gestational IV NIC on methamphetamine-induced locomotor activity in adolescent rats
Habituation
The gestational treatment × sex × day ANOVA conducted on the PND 25–27 data revealed a significant main effect of habituation day [F (2, 132) = 120.7, p<0.05], and there were no other significant main effects or interactions. The mean (± SEM) locomotor activity observed for all animals across the three habituation days were 9481.2 (± 251.1), 8123.3 (± 226.6), and 5817.0 (± 237.5). These data demonstrate that the highest number of total activity counts was recorded following the novel response to the chambers, on PND 25, and that habituation of the response was exhibited on PND 26 and 27, as a progressive decrease in locomotor activity. Neither gestational treatment nor sex was a significant factor during habituation to the activity chambers (data not shown).
Saline baseline
A gestation x adolescent treatment × sex univariate ANOVA was conducted on the saline baseline data (PND 28) to determine if there were any differences in locomotor activity following saline injection. The analysis revealed that there were no significant main effects or interactions, thus indicating no differences between groups during the saline baseline measurement.
Repeated methamphetamine treatment
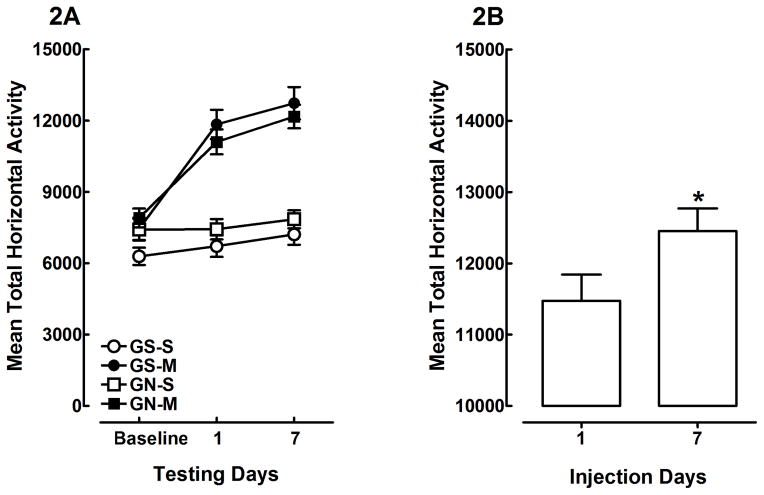
A gestational treatment × adolescent treatment × sex × test day ANOVA, which included the saline baseline day and methamphetamine/saline treatment days 1 and 7 (PND 29 and 35, respectively), revealed main effects of adolescent treatment [F (1, 62) = 76.2, p<0.001] and day [F (2, 124) = 70.0, p<0.001], and an adolescent treatment × test day interaction [F (2, 124) = 44.2, p<0.001]. There were no significant main effects of gestational treatment or sex, and moreover, there were no significant interactions between adolescent treatment and gestational treatment or sex. The adolescent treatment × test day interaction is shown in Figure 2A. These findings indicate that all groups exhibited similar behavior during the saline baseline measure, and that methamphetamine treatment increased locomotor activity after the first and seventh injections, whereas saline controls exhibited consistent activity across days one and seven. Methamphetamine produced a very robust acute psychomotor response, and repeated injection resulted in a greater increase in locomotor activity. Figure 2B shows the mean total horizontal activity means (±SEM) for day 1 and 7 of rats injected with methamphetamine. The sensitized behavioral response is a 9% increase over that of the acute response. Within-subjects comparison between injection days one and seven confirms that repeated methamphetamine injection produced a significant increase in locomotor activity [F (1, 35) = 7.6, p<0.01], which indicates the induction of methamphetamine-induced behavioral sensitization (Figure 2B). In contrast, saline controls did not show significant changes in activity across methamphetamine injection days one and seven [F (1, 33) = 1.8, p>0.05]. Taken together, these findings show that gestational nicotine treatment did not alter spontaneous locomotor activity in response to a novel environment, and did not alter habituation to the locomotor activity chambers across three days of exposure. Furthermore, no effect of gestational nicotine was observed on the induction of methamphetamine-induced behavioral sensitization.
Figure 2.
Mean (±SEM) total horizontal activity in offspring prenatally exposed to gestational saline (GS) or gestational nicotine (GN) and treated with saline (S) or methamphetamine (M; 0.3 mg/kg) during adolescence (2A). The adolescent treatment × testing day interaction [p<0.001] indicates that repeated methamphetamine injection significantly increased locomotor activity across testing days. Mean (±SEM) total horizontal activity in offspring injected with repeated methamphetamine (2B). Figure 2B is a detailed illustration of the significant methamphetamine-induced increase in locomotor activity following the 7th and final injection, collapsed across gestational nicotine treatment. * shows that animals treated with methamphetamine exhibited greater locomotor activity on day 7, relative to day 1, which is indicative of methamphetamine-induced behavioral sensitization (p<0.01).
The effects of IV GN and adolescent methamphetamine treatment on BDNF protein levels in the NAcc, Str, FC, and Hipp
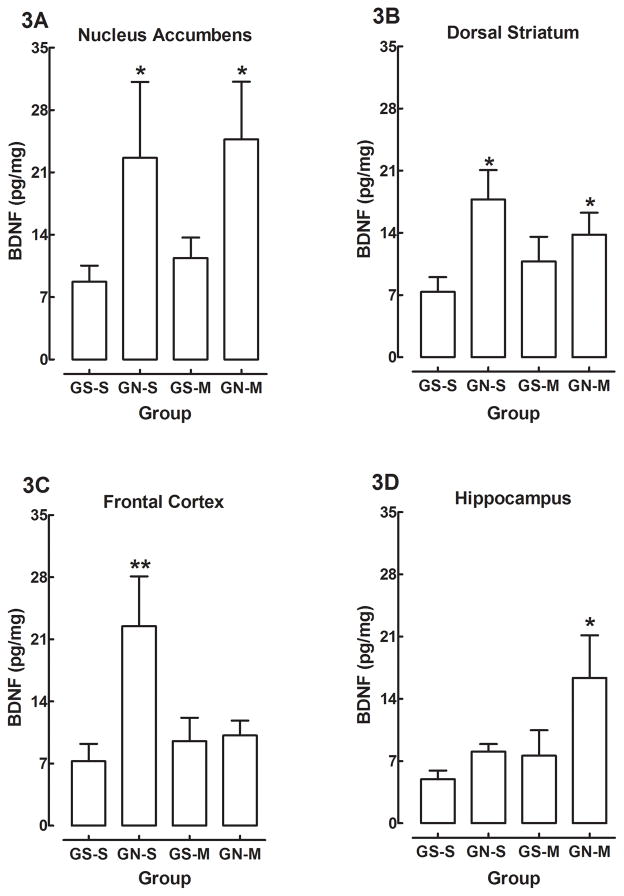
There were no main effects or interactions containing the factor of sex, and therefore the data are presented as collapsed across sex. The mean (±SEM) BDNF levels from the NAcc, Str, FC, and Hipp are shown in Figures 3A–D, respectively. The analysis of the NAcc revealed a significant main effect of gestational treatment [F (1, 37) = 5.13, p<0.03], but no other main effects or interactions were significant. Post hoc analyses confirmed that GN-S and GN-M groups exhibited significantly higher levels of NAcc BDNF relative to the GS-S controls (Figure 3A). The analysis of the Str resulted in a significant main effect of gestational treatment [F (1, 38) = 6.19, p<0.018], which indicates that BDNF protein was significantly higher in rats in the IV nicotine exposure groups (e.g., GN-S and GN-M) relative to rats in the IV saline controls. Comparisons show that the GN-S and the GN-M groups exhibited significantly higher levels of BDNF than the GS-S group (Figure 3B). Analysis of the FC revealed a significant main effect of gestational treatment [F (1, 38) = 5.42, p<0.026] and a significant gestational treatment × adolescent treatment interaction [F (1,38) = 4.58, p<0.039]. Interestingly, rats in the GN-S group demonstrated elevated BDNF in the FC, however, differently than the NAcc or Str analyses, rats in the GN-M group exhibited similar protein levels of BDNF as rats in the GS-S and GS-M groups (Figure 3C). This interaction indicates that although the gestational treatment elevated protein levels of BDNF like that of the NAcc and Str, adolescent methamphetamine treatment attenuated the apparent nicotine-induced elevation in cortical BDNF. Lastly, the analysis of the Hipp revealed effects of gestational treatment and adolescent treatment. There were significant main effects of gestational treatment [F (1,37) = 4.96, p<0.032] and adolescent treatment [F (1,37) = 4.26, p<0.047], but there was no gestational treatment × adolescent treatment interaction (Figure 3D). This result shows that IV nicotine exposure during the gestational period and methamphetamine treatment during adolescence elevated protein levels of BDNF, but the two factors did not interact. These findings suggest that gestational IV nicotine exposure and adolescent methamphetamine treatment altered BDNF in the Hipp; however, in contrast to the other regions, the BDNF levels in the Hipp were elevated by both prenatal nicotine and adolescent methamphetamine exposure, and this was most apparent in the GN-M group.
Figure 3.
Mean (±SEM) levels of BDNF from the nucleus accumbens (3A), dorsal striatum (3B), frontal cortex (3C), or hippocampus (3D) of gestational saline (GS) or gestational nicotine (GN) offspring treated with either saline (S) or methamphetamine (M) during adolescence. * indicates significant difference from GS-S (p<0.05); ** signifies a gestational treatment × adolescent methamphetamine treatment interaction (p<0.05).
Discussion
The present experiments show that IV nicotine exposure, administered 3 times per day on GD 8–21, resulted in elevated levels of BDNF protein in the striatum, nucleus accumbens, frontal cortex, and hippocampus of adolescent rats compared to saline-treated controls. The effects of prenatal IV nicotine treatment are consistent with the results of Wei et al. (2011), which showed increased BDNF mRNA expression in the nucleus accumbens and striatum of offspring exposed to continuous nicotine throughout gestation, indicating that GN administration alters the expression of BDNF mRNA and protein in the reward circuit of developing animals. These results are also generally in accord with previous research showing that adult animals administered nicotine exhibit increased BDNF protein and/or mRNA in the striatum and nucleus accumbens (Correll et al., 2009; Maggio et al., 1998). Adolescent exposure to repeated methamphetamine injection, however, did not alter levels of BDNF in the mesocorticolimbic system.
The effect of GN exposure to elevate BDNF levels in the frontal cortex was unique in relation to that shown in the dorsal and ventral striatum because the combination of prenatal nicotine and adolescent methamphetamine treatment interacted to produce an attenuation of the elevated levels of frontal cortical BDNF (i.e., gestational treatment × adolescent treatment interaction). Thus, although methamphetamine treatment alone during adolescence did not affect BDNF within the frontal cortex (i.e., Group GS-M), the combination of GN and adolescent methamphetamine exposure resulted in concentrations of BDNF similar to control levels (i.e., GN-M). It is not apparent how methamphetamine exposure attenuated the prenatal nicotine-induced increase of BDNF in the frontal cortex. Previous research, however, shows that d-amphetamine treatment results in decreased BDNF mRNA expression in the frontal cortex of rats (Banerjee et al., 2009). These findings indicate that the attenuation of the prenatal nicotine-induced enhancement in BDNF in the frontal cortex may be decreased by methamphetamine exposure during adolescent development.
The effect of prenatal IV nicotine exposure and adolescent methamphetamine treatment on hippocampal BDNF was complex, and unlike the findings with the frontal cortex, the interaction between these two factors was not significant. Rats that received GN treatment or adolescent methamphetamine exposure demonstrated an increase of hippocampal BDNF. The significant main effect of adolescent methamphetamine treatment is interesting given that this outcome was not observed in the nucleus accumbens, striatum, or the frontal cortex. The finding that methamphetamine increased hippocampal BDNF is in accord with previous research showing that repeated postnatal nicotine or continuous amphetamine treatment increased BDNF in the hippocampus of adult rats (French et al., 1999; Griesbach et al., 2008; Kenny et al., 2000). Our findings are in contrast to those of Banerjee et al. (2009), however, who reported that acute amphetamine injection decreased hippocampal BDNF of juvenile rats, as well as a relatively modest decrease in adults. These discrepancies between Banerjee et al. and other results, including the present findings, may be due to differences in age, route of drug administration, or frequency of injection.
Gestational IV nicotine exposure did not result in altered litter parameters or any of the postnatal tests of developmental milestones, such as eye opening, negative geotaxis, and the righting reflex, relative to controls. Moreover, prenatal nicotine exposure did not result in altered spontaneous locomotor activity or habituation to the activity chambers. Previous research investigating the effects of GN exposure on spontaneous locomotor activity reports no effect (Franke et al., 2007; Gaworski et al., 2004; Paulson et al., 1993; Shacka et al., 1997), hypoactivity (LeSage et al., 2006; Peters and Tang, 1982; Romero and Chen, 2004), or hyperactivity (Pauly et al., 2004; Paz et al., 2007; Peters et al., 1979; Richardson and Tizabi, 1994; Tizabi et al., 1997; Tizabi et al., 2000; Vaglenova et al., 2004), and there is no apparent correlation between route of exposure and the behavioral outcome (for review see Heath and Picciotto, 2009; LeSage et al., 2006). Our findings are consistent with the subset of experiments that report no effect of gestational nicotine exposure on spontaneous locomotor activity (Franke et al., 2007; Gaworski et al., 2004; Paulson et al., 1993; Shacka et al., 1997).
Repeated methamphetamine injection induced behavioral sensitization; however, neither IV GN treatment nor sex modulated the induction of sensitization. It should be noted that the present experiment did not investigate multiple dose levels of prenatal nicotine or postnatal methamphetamine. Dose-response data for both treatment regimens will be important to determine if IV gestational nicotine exposure alters spontaneous locomotor activity (LeSage et al., 2006; Pauly et al., 2004; Paz et al., 2007; Peters and Tang, 1982; Peters et al., 1979; Richardson and Tizabi, 1994; Romero and Chen, 2004; Tizabi et al., 1997; Tizabi et al., 2000; Vaglenova et al., 2004) and/or interacts with postnatal psychostimulant exposure to alter the induction or expression of behavioral sensitization (Franke et al., 2007). For example, the dose of methamphetamine used in the present experiment (0.3 mg/kg) may have produced a rapid acquisition of behavioral sensitization, because the sensitized response was exhibited as a 9% increase over that of the acute response. Thus, under such conditions the effect IV prenatal nicotine to alter sensitization may be more difficult to detect. Examining extended dose ranges of IV prenatal nicotine and methamphetamine will be important to determine the full effect of this treatment on spontaneous locomotor activity, as well as psychostimulant-induced behavioral sensitization. It will also be important for future experiments to determine if prenatal IV nicotine exposure enhances cue/contextual conditioning in offspring relative to saline-exposed controls, as has been reported with prenatal nicotine exposure in mice (Paz et al., 2007). The effect of context conditioning was explicitly minimized in the present experiment because it was of interest to assess the effects of prenatal nicotine on methamphetamine-induced sensitization without the addition of conditioned hyperactivity that would otherwise be elicited from the context. Conditional cues are an integral aspect of drug seeking behavior (Robinson and Berridge, 2003). Determining if IV gestational nicotine exposure alters cue learning will provide important information about the role of prenatal nicotine and vulnerability for drug dependence in offspring of maternal smokers.
It is noteworthy that although GN animals exhibited greater basal levels of BDNF than controls, there was no effect of gestation on either spontaneous locomotor activity or on methamphetamine-induced behavioral sensitization, suggesting that the increased levels of BDNF did not affect locomotor activity or sensitization to methamphetamine. This finding is in contrast to previous results showing that the administration of exogenous BDNF modulates spontaneous locomotor behavior and psychostimulant-induced activity (Martin-Iverson and Altar, 1996; Martin-Iverson et al., 1994). However, an obvious critical difference between these past studies and current results is that BDNF was given exogenously, whereas the increases in BDNF produced by gestational nicotine were endogenous. In addition, the differing results may also be related to differences in the developmental stages of the mesocorticolimbic DA system at the time of testing. Adolescent animals were investigated in the current experiments, whereas previous research used adult rats (Martin-Iverson and Altar, 1996; Martin-Iverson et al., 1994). Adolescence represents a profound change in dopaminergic tone throughout the mesocorticolimbic DA system, and the marked increase in DA turnover and DA receptor expression during adolescence subsides during adulthood (Andersen et al., 2000; Chambers et al., 2003; Spear, 2000). Thus, changes in locomotor activity during adulthood per elevated levels of BDNF may have been obfuscated by changes in DA turnover in adolescent rats.
The present research utilized a novel, intermittent IV exposure model, which administers 3x/day IV nicotine (0.05 mg/kg/injection) treatments on GD 8–21. There are advantages and disadvantages to all models, including the IV procedure used in the present experiments to model the effects of maternal smoking. An advantage to using the IV route of administration is that IV injection allows for 100% bioavailability of nicotine absorption and near instantaneous distribution; and as previously mentioned, it closely mimics the nicotine pharmacokinetics achieved through cigarette smoking. A second advantage is that this translationally relevant exposure method may be used to deliver less overall daily amounts of nicotine relative to other exposure models (Benowitz et al., 2009; Booze et al., 1999; Mactutus, 1989; Russell and Feyerabend, 1978). For example, Lacy et al.(2011) reported that the same IV exposure method used in the present experiments produced alterations in prepulse inhibition of the acoustic startle response that are consistent with that induced by a continuous GN exposure model (i.e., Popke et al., 1997). A disadvantage of the present model may be that it requires daily removal of the dam from the home cage to administer the saline or nicotine injections; alternatively, however, this method affords a unique opportunity to monitor the dams’ progression through pregnancy without adding what would otherwise be considered additional stress. Perhaps the most obvious disadvantage is that the present model uses nicotine to mimic maternal smoking although there are approximately 4,000 active compounds in cigarette smoke.
Together, these findings indicate that those who smoke a “low” number of daily cigarettes throughout their pregnancy may alter the expression of BDNF in brain circuitry that is responsible for organizing motivated behavior in developing offspring. Elevated levels of BDNF may produce effects on motivated behavior, such as sucrose-maintained responding or drug self-administration, which are also known to be mediated by the mesocorticolimbic dopaminergic system. Indeed, previous research suggests that continuous GN treatment alters operant responding for sucrose reward and for nicotine and cocaine self-administration, as well (Franke et al., 2008; Levin et al., 2006). Further research is needed to determine if the IV GN-induced elevations of BDNF throughout the mesocorticolimbic pathway modulates the rewarding properties of food and psychostimulant drugs of abuse in adolescent and adult animals. Such information will provide further insight into the neurobiological mechanisms that render offspring of maternal smokers more vulnerable to SUD.
Acknowledgments
The authors thank Lauren Ballina and Rachel Singleton for their technical expertise on this research. We also acknowledge the reviewers for their thoughtful insights and suggestions for the manuscript, as well as those of Charles Mactutus, PhD. This research was supported by National Institute for Drug Abuse grants DA 021287 (SBH) and DA 026721 (JZ).
References
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110(6):1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Gruber SH, El Khoury A, Tonali PA, Mathe AA. Chronic amphetamine treatment reduces NGF and BDNF in the rat brain. Eur Neuropsychopharmacol. 2007;17(12):756–762. doi: 10.1016/j.euroneuro.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Banerjee PS, Aston J, Khundakar AA, Zetterstrom TS. Differential regulation of psychostimulant-induced gene expression of brain derived neurotrophic factor and the immediate-early gene Arc in the juvenile and adult brain. Eur J Neurosci. 2009;29(3):465–476. doi: 10.1111/j.1460-9568.2008.06601.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;(192):29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Nicotine-conditioned locomotor sensitization in rats: assessment of the US-preexposure effect. Behav Brain Res. 2003;143(1):65–74. doi: 10.1016/s0166-4328(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Booze RM, Welch MA, Wood ML, Billings KA, Apple SR, Mactutus CF. Behavioral sensitization following repeated intravenous nicotine administration: gender differences and gonadal hormones. Pharmacol Biochem Behav. 1999;64(4):827–839. doi: 10.1016/s0091-3057(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry. 2003;160(11):1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- Button TM, Maughan B, McGuffin P. The relationship of maternal smoking to psychological problems in the offspring. Early Hum Dev. 2007;83(11):727–732. doi: 10.1016/j.earlhumdev.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180(2):258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, DeGenna N, Day NL. Smoking during teenage pregnancies: effects on behavioral problems in offspring. Nicotine Tob Res. 2007;9(7):739–750. doi: 10.1080/14622200701416971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll JA, Noel DM, Sheppard AB, Thompson KN, Li Y, Yin D, Brown RW. Nicotine sensitization and analysis of brain-derived neurotrophic factor in adolescent beta-arrestin-2 knockout mice. Synapse. 2009;63(6):510–519. doi: 10.1002/syn.20625. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84(1):30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol. 2010;5(3):393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch Gen Psychiatry. 1998;55(8):721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Franke RM, Belluzzi JD, Leslie FM. Gestational exposure to nicotine and monoamine oxidase inhibitors influences cocaine-induced locomotion in adolescent rats. Psychopharmacology (Berl) 2007;195(1):117–124. doi: 10.1007/s00213-007-0876-y. [DOI] [PubMed] [Google Scholar]
- Franke RM, Park M, Belluzzi JD, Leslie FM. Prenatal nicotine exposure changes natural and drug-induced reinforcement in adolescent male rats. Eur J Neurosci. 2008;27(11):2952–2961. doi: 10.1111/j.1460-9568.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- French SJ, Humby T, Horner CH, Sofroniew MV, Rattray M. Hippocampal neurotrophin and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Brain Res Mol Brain Res. 1999;67(1):124–136. doi: 10.1016/s0169-328x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Gaworski CL, Carmines EL, Faqi AS, Rajendran N. In utero and lactation exposure of rats to 1R4F reference cigarette mainstream smoke: effect on prenatal and postnatal development. Toxicol Sci. 2004;79(1):157–169. doi: 10.1093/toxsci/kfh083. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10(8):1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F, Sutton RL. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154(2):530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, Booze RM. Sex differences and repeated intravenous nicotine: behavioral sensitization and dopamine receptors. Pharmacol Biochem Behav. 2004;78(3):581–592. doi: 10.1016/j.pbb.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56(Suppl 1):254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19(10):4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Black MD, Ali SF. Effect of melatonin on methamphetamine- and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity and methamphetamine-induced behavioral sensitization. Neuropharmacology. 1998;37(6):781–791. doi: 10.1016/s0028-3908(98)00067-7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84(9):1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane VB, Fu Y, Matta SG, Sharp BM. Gestational nicotine exposure attenuates nicotine-stimulated dopamine release in the nucleus accumbens shell of adolescent Lewis rats. J Pharmacol Exp Ther. 2004;308(2):521–528. doi: 10.1124/jpet.103.059899. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, File SE, Rattray M. Acute nicotine decreases, and chronic nicotine increases the expression of brain-derived neurotrophic factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 2000;85(1–2):234–238. doi: 10.1016/s0169-328x(00)00246-1. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Mactutus CF, Harrod SB. Prenatal IV nicotine exposure produces a sex difference in sensorimotor gating of the auditory startle reflex in adult rats. Int J Dev Neurosci. 2011;29(2):153–161. doi: 10.1016/j.ijdevneu.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Gustaf E, Dufek MB, Pentel PR. Effects of maternal intravenous nicotine administration on locomotor behavior in pre-weanling rats. Pharmacol Biochem Behav. 2006;85(3):575–583. doi: 10.1016/j.pbb.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol Biochem Behav. 2006;85(3):669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mactutus CF. Developmental neurotoxicity of nicotine, carbon monoxide, and other tobacco smoke constituents. Ann N Y Acad Sci. 1989;562:105–122. doi: 10.1111/j.1749-6632.1989.tb21010.x. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Herman AS, Booze RM. Chronic intravenous model for studies of drug (Ab)use in the pregnant and/or group-housed rat: an initial study with cocaine. Neurotoxicol Teratol. 1994;16(2):183–191. doi: 10.1016/0892-0362(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Maggio R, Riva M, Vaglini F, Fornai F, Molteni R, Armogida M, Racagni G, Corsini GU. Nicotine prevents experimental parkinsonism in rodents and induces striatal increase of neurotrophic factors. J Neurochem. 1998;71(6):2439–2446. doi: 10.1046/j.1471-4159.1998.71062439.x. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Altar CA. Spontaneous behaviours of rats are differentially affected by substantia nigra infusions of brain-derived neurotrophic factor and neurotrophin-3. Eur J Neurosci. 1996;8(8):1696–1706. doi: 10.1111/j.1460-9568.1996.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Todd KG, Altar CA. Brain-derived neurotrophic factor and neurotrophin-3 activate striatal dopamine and serotonin metabolism and related behaviors: interactions with amphetamine. J Neurosci. 1994;14(3 Pt 1):1262–1270. doi: 10.1523/JNEUROSCI.14-03-01262.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, Muraoka S, Tomiyoshi R, Mimura Y, Kato H, Suzuki MR, Takigawa M. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Brain Res Dev Brain Res. 1997;102(1):117–126. doi: 10.1016/s0165-3806(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Eylers JP, Baker FE, Dobbins SS, Lappi SE, Slotkin TA. Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J Pharmacol Exp Ther. 1989;251(3):894–900. [PubMed] [Google Scholar]
- Park MK, Loughlin SE, Leslie FM. Gestational nicotine-induced changes in adolescent neuronal activity. Brain Res. 2006;1094(1):119–126. doi: 10.1016/j.brainres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Paulson RB, Shanfeld J, Vorhees CV, Sweazy A, Gagni S, Smith AR, Paulson JO. Behavioral effects of prenatally administered smokeless tobacco on rat offspring. Neurotoxicol Teratol. 1993;15(3):183–192. doi: 10.1016/0892-0362(93)90014-f. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Sparks JA, Hauser KF, Pauly TH. In utero nicotine exposure causes persistent, gender-dependant changes in locomotor activity and sensitivity to nicotine in C57Bl/6 mice. Int J Dev Neurosci. 2004;22(5–6):329–337. doi: 10.1016/j.ijdevneu.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Paz R, Barsness B, Martenson T, Tanner D, Allan AM. Behavioral teratogenicity induced by nonforced maternal nicotine consumption. Neuropsychopharmacology. 2007;32(3):693–699. doi: 10.1038/sj.npp.1301066. [DOI] [PubMed] [Google Scholar]
- Peters DA, Tang S. Sex-dependent biological changes following prenatal nicotine exposure in the rat. Pharmacol Biochem Behav. 1982;17(5):1077–1082. doi: 10.1016/0091-3057(82)90497-x. [DOI] [PubMed] [Google Scholar]
- Peters DA, Taub H, Tang S. Postnatal effects of maternal nicotine exposure. Neurobehav Toxicol. 1979;1(3):221–225. [PubMed] [Google Scholar]
- Popke EJ, Tizabi Y, Rahman MA, Nespor SM, Grunberg NE. Prenatal exposure to nicotine: effects on prepulse inhibition and central nicotinic receptors. Pharmacol Biochem Behav. 1997;58(4):843–849. doi: 10.1016/s0091-3057(97)98985-1. [DOI] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26(16):1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Ribary U, Lichtensteiger W. Effects of acute and chronic prenatal nicotine treatment on central catecholamine systems of male and female rat fetuses and offspring. J Pharmacol Exp Ther. 1989;248(2):786–792. [PubMed] [Google Scholar]
- Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol Biochem Behav. 1994;47(2):331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Romero RD, Chen WJ. Gender-related response in open-field activity following developmental nicotine exposure in rats. Pharmacol Biochem Behav. 2004;78(4):675–681. doi: 10.1016/j.pbb.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Russell MA, Feyerabend C. Cigarette smoking: a dependence on high-nicotine boli. Drug Metab Rev. 1978;8(1):29–57. doi: 10.3109/03602537808993776. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Fennell OB, Robinson SE. Prenatal nicotine sex-dependently alters agonist-induced locomotion and stereotypy. Neurotoxicol Teratol. 1997;19(6):467–476. doi: 10.1016/s0892-0362(97)00063-9. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction--developmental neurotoxicity of nicotine. Crit Rev Toxicol. 2005;35(8–9):703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285(3):931–945. [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198(2):132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stene-Larsen K, Borge AI, Vollrath ME. Maternal smoking in pregnancy and externalizing behavior in 18-month-old children: results from a population-based prospective study. J Am Acad Child Adolesc Psychiatry. 2009;48(3):283–289. doi: 10.1097/CHI.0b013e318195bcfb. [DOI] [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, Harold G, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry. 2003;160(11):1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270(5236):593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154(2):327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Popke EJ, Rahman MA, Nespor SM, Grunberg NE. Hyperactivity induced by prenatal nicotine exposure is associated with an increase in cortical nicotinic receptors. Pharmacol Biochem Behav. 1997;58(1):141–146. doi: 10.1016/s0091-3057(96)00461-3. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Russell LT, Nespor SM, Perry DC, Grunberg NE. Prenatal nicotine exposure: effects on locomotor activity and central [125I]alpha-BT binding in rats. Pharmacol Biochem Behav. 2000;66(3):495–500. doi: 10.1016/s0091-3057(00)00171-4. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150(1–2):159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Wei J, Wang J, Dwyer JB, Mangold J, Cao J, Leslie FM, Li MD. Gestational nicotine treatment modulates cell death/survival-related pathways in the brains of adolescent female rats. Int J Neuropsychopharmacol. 2011;14(1):91–106. doi: 10.1017/S1461145710000416. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38(7):892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Zhu J, Takita M, Konishi Y, Sudo M, Muramatsu I. Chronic nicotine treatment delays the developmental increase in brain muscarinic receptors in rat neonate. Brain Res. 1996;732(1–2):257–260. doi: 10.1016/0006-8993(96)00704-4. [DOI] [PubMed] [Google Scholar]