Abstract
The recently available guidelines on the management of advanced breast cancer (ABC) organized by Chinese Anti-Cancer Association, Committee of Breast Cancer Society (CACA-CBCS) do not elucidate ABC in details. To instruct clinicians in treatment of ABC, a Chinese expert consensus meeting on diagnosis and treatment of ABC was held in June 2014 and a consensus is developed. The following consensus provides the level of evidence and supporting documents for each recommendation, and introduces research topics to be urgently addressed. Notably, the consensus on diagnosis and treatment of ABC in China is developed to be applied nationwide. In different areas, multidisciplinary treatment (MDT) tailored to the each patient and the disease itself should be applied based on the basic principles of modern oncology.
Keywords: Advanced breast cancer (ABC), consensus, national, China
Introduction
Advanced breast cancer (ABC) patients not only suffer from the disease itself but also are under huge psychological and financial stress. They often feel frightened, desperate, and lost. They are not sure whether they should receive a specific therapy and whether it works. In addition, the poor communication between patient’s family and medical staff and the patients further harm the patients’ mental status (1). Treatment protocol selection and effectiveness evaluation can also be unique in ABC patients. Helping the patients make the right decision is a key challenge for each oncologist.
A large number of relatively mature treatment options with A-level evidences have been available for early breast cancer; for the treatment of ABC (especially after the failure of first-line treatment), however, no standard treatment protocol has been widely recognized. The overall median survival of ABC patients range 2-3 years, depending on the different molecular subtypes. For human epidermal growth factor receptor 2 (HER2)-positive ABC patients, the anti-HER2 drugs can significantly prolong the survival and improve the prognosis; however, the overall outcomes have not been markedly improved in patients with triple-negative ABC. For estrogen receptor (ER)—positive ABC, which is the most common type, no major breakthrough has been made since 1990s, and the patients’ overall survival (OS) remains unchanged (2-5). The Chinese Anti-Cancer Association, Committee of Breast Cancer Society (CACA-CBCS) already developed the CACA Guidelines and Norms on the Management of Breast Cancer and its updates, which has remarkably promoted the standardized management of early breast cancer; however, these guidelines do not elucidate ABC in details and therefore are less instructive for clinicians in this field. Therefore, steered by CACA-CBCS, dozens of Chinese experts on the diagnosis and treatment of breast cancer actively participated in the analysis, summarization, and discussion of domestic and foreign research data on the management of ABC. A Chinese expert consensus meeting on diagnosis and treatment of ABC was held in June 2014, during which the consensus on diagnosis and treatment of ABC in China was developed and issued.
This document summarizes the recommendations proposed in the expert consensus meeting, provides the level of evidence and supporting documents for each recommendation, and introduces research topics to be urgently addressed. Notably, the consensus on diagnosis and treatment of ABC in China is developed to be applied nationwide. In different areas, multidisciplinary treatment (MDT) tailored to the each patient and the disease itself should be applied based on the basic principles of modern oncology.
Methods
Before the holding of the Chinese expert consensus meeting on diagnosis and treatment of ABC, the organizers and the working group had prepared the draft document. The experts were grouped according to their subject, and the relevant parts were emailed to the group members for further discussion and revision. During the expert consensus meeting, the revised version was thoroughly discussed. Further revision was made in accordance with the comments made by the working group members. References were provided for the sources of each comment. After the meeting, the revised version was sent to each member for verification, and then the document was finalized.
Some recommendations in the Consensus apply to both locally ABC and metastatic breast cancer (MBC) (Table 1), whereas others only apply to MBC (Table 2).
Table 1. Principles of the guidelines.
| Recommendations | Evidence level |
|---|---|
| (I) The management of ABC is complicated and therefore urgently requires the involvement of experts from different departments (including but not limited to oncologists, radiologists, surgeons, imaging experts, pathologists, gynecologists, psychologists, social workers, nurses, and palliative care experts) | Expert opinions |
| (II) After the confirmation of ABC, the patients should be provided with reasonable psycho-social support, daily care, and symptomatic treatment, which should be included in the routine treatment protocol. The above treatment should follow the principles of individualized treatment to meet the demands of different patients | Expert opinions |
| (III) After detailed diseases assessment, the physicians should talk with the patients about the future treatment and nursing objectives. The patients should be informed that, although MBC is a non-curable disease, proper treatment can remarkably prolong the survival. Receptive language should be used during such a talk. Technical terms should be avoided. Meanwhile, the physician should respect the patients’ privacy and cultural differences and provide text message whenever possible | Expert opinions |
| (IV) Patients (and their family members and caregivers upon the patient’s permission) should be invited to participate in the treatment decision-making. Patients and their family members are encouraged to involve in treatment decision-making | Expert opinions |
| (V) Standard treatment for ABC is still very limited. After obtaining the informed content, the physician should encourage the patients to participate in well-designed prospective randomized clinical trials as early as possible | Expert opinions |
| (VI) The treatment cost should be taken into consideration. When making a decision on the ABC treatment, the physician must consider multiple aspects including the affordability, quality of life, life expectancy, and patient’s willingness | Expert opinions |
| (VII) The patients’ subjective experience often reflects both the severity of symptoms and the impacts of treatment on the quality of life. Therefore, it is important to accurately collect these information to inform the treatment and nursing | Expert opinions |
ABC, advanced breast cancer.
Table 2. Four levels of evidence.
| Recommendation level/description | Assessment of benefits and risks | Methodological quality of the supporting evidences | Note |
|---|---|---|---|
| 1A: Strongly recommended, based on high-quality evidences | Remarkably higher benefits than harms | RCTs without major defect or observational studies with strong evidences | Strongly recommend; apply for most patients |
| 1B: Strongly recommended, based on intermediate-quality evidences | Remarkably higher benefits than harms | RCTs with obvious limitations (defects in methods and results) or observational studies with relatively strong evidences | Strongly recommend; apply for most patients |
| 1C: Strongly recommended, based on low-quality evidences | Remarkably higher benefits than harms | Observational studies or case studies | Strongly recommend; may be adjusted if higher-quality evidence occurs |
| 2A: Weakly recommended, based on high-quality evidences | The benefits and harms are very close | RCTs without major defects or observational studies with strong evidences | Weakly recommend; adjusted based on the patients’ specific conditions |
| 2B: Weakly recommended, based on intermediate-quality evidences | The benefits and harms are very close | RCTs with obvious limitations (defects in methods and results) or observational studies with relatively strong evidences | Weakly recommend; adjusted based on the patients’ specific conditions |
| 2C: Weakly recommended, based on low-quality evidences | The benefits and harms are very close | Observational studies or case studies | Do not recommend; other alternative methods are available |
RCT, randomized controlled trial.
Principles of the guidelines
The concept of MDT, which was proposed at the end of the 20th century (6,7), is a major achievement in oncology. Based on this concept, the physicians need to provide individualized medical measures to each patient, and the active cooperation from staff in all relevant departments will help to establish a better treatment protocol for the patient. The establishment of the department of breast disease (8) is another breakthrough. In China, the first breast cancer center was established in 1990s and has been continuously improved in the past two decades. MDT and establishment of the department of breast disease are two milestones in breast disease management and have played key roles in the management of early breast cancer. However, MDT for ABC remains weak, especially for some specific metastatic sites (e.g., bone metastasis and brain metastasis) (Table 1).
Levels of evidence
Treatment protocol of ER-positive/HER-2-negative ABC (Table 2).
Imaging, tumor markers, and response evaluation
Diagnosis/staging-related examinations
These examinations should include history-taking, physical examination, blood tests (e.g., routine blood test, liver/kidney function tests, blood electrolytes, and tumor markers), and imaging of breast, chest, abdomen, and bone (including ECG). If trastuzumab treatment is scheduled, heart function test (ultrasound of the heart) should be added (1B). Tumor marker is an auxiliary indicator for response evaluation, in particular when no measurable lesion is available. However, the change of tumor marker is not an evidence for adjusting the treatment (2C).
Imaging examinations
Imaging examinations for staging
The main sites for the imaging examinations for staging include breast (ultrasound, mammography, and MRI), chest (chest CT if condition allows, or X-ray), abdomen (abdominal ultrasound, abdominal CT or MRI if necessary), and bone (bone scan) (1B). PET-CT may be considered if there is a need to determine whether the lesion is a relapse or there are multiple lesions (9,10) (2B). However, due to lack of high-level evidence, PET-CT is not routinely recommended (11-14).
Frequency of imaging examinations
The response evaluation for endocrine therapy (ET) should be performed every 2-3 months, and the response evaluation for chemotherapy (ChT) should be performed every 2-3 cycles. The intervals of response evaluation should be also based on disease progression speed, site and range of metastasis, and treatment mode. (Expert opinion) The interval of response evaluation should be shortened for patients with rapid disease progression. In some cases (e.g., the disease is relatively inert and progresses slowly), the interval of response evaluation can be appropriately prolonged. In patients with suspected progressive disease (PD) or with obvious disease-related symptoms, further examinations should be timely performed. Meanwhile, history-taking and physical examination should also be conducted.
Head imaging
Patients with obvious head and/or brain-related symptoms should receive head imaging (e.g., head MRI or CT). (Expert opinion) For patients with asymptomatic HER-2-positive or triple-negative breast cancer, head imaging can also be considered.
Biomarkers
If clinically applicable, biopsy of metastatic lesion is recommended to confirm the presence of metastasis (histological specimen is preferred), especially during the first diagnosis of metastasis. After a metastasis is confirmed, a second evaluation of the biomarkers [e.g., hormone receptor (HR), HER-2 and Ki67] of breast cancer should be performed at least once (15,16).
Tumor markers
Although the clinical values of tumor markers in predicting post-operative relapse/metastasis have not been demonstrated, it has been recognized that the dynamic changes of tumor markers can facilitate response evaluation, especially in MBC patients without measurable lesion (2C). However, again, the treatment protocol should not be changed only based on the change of tumor markers (2C);
The constant increase of post-operative tumor markers may be an early sign of tumor relapse. The increase of tumor markers during the treatment of ABC may be due to the following two reasons: (i) the anti-tumor is not effective; however, imaging examination should also be performed to confirm the judgment and thus decide whether the treatment protocol should be changed; (ii) the treatment is effective, but is associated with the transient increase of tumor markers. For patients without measurable lesion, increase of tumor markers alone can also be a sign of effective treatment. Thus, increase of tumor markers alone can not be a basis for changing the treatment protocol (17). Dynamic observation should be arranged, and a second examination should be arranged 1-2 months later;
The constant decrease of tumor markers may be an early sign of effective tumor treatment; however, imaging examination should also be performed to judge the treatment response and thus decide the next treatment protocol. The treatment protocol should not be changed only based on the decrease of tumor markers.
Frequency of response evaluation
Response evaluation for endocrine therapy should be performed every 2-3 months, and the response evaluation for chemotherapy should be performed every 2-3 cycles. The specific evaluation frequency should also be based on the disease progression speed, site and range of metastasis, and treatment mode;
Imaging of the targeted lesion is recommended for most patients; for patients with slow disease progression, the frequency of imaging can be lowered. For patients with suspected PD and those experienced obvious symptoms, examinations should be performed immediately. History-taking and physical examination should also be performed during each response evaluation.
General principles of the treatment of ABC
General principles
The treatment should be based on the following considerations: HR and HER-2 status, previous treatment (efficacy, toxicity, tolerance, etc.), disease-free interval, tumor load (metastasis site and number), age, general condition, menstrual status, and comorbidities. Also, it should be adjusted according to the patients’ symptom severity, needs for the rapid control of disease and/or symptoms, and the patients’ social, economic and psychological statuses;
If the detection results are not consistent between primary and metastatic lesions, if only one lesion (or one test) showed positive HR and/or HER-2, endocrine treatment and/or anti-HER-2 treatment can be selected based on this positive lesion (15,16);
For elderly patients, adequate and effective treatment should also be provided in accordance with the specific conditions (1B);
Patients with treatment-naive phase IV breast cancer in whom whether the resection of primary lesion can be beneficial remains controversial (18-21), some of them may receive palliative surgery. However, since all the currently available evidences are from retrospective studies with selection biases, they need to be further verified in prospective clinical trials.
Locally ABC
Single locally ABC should be treated by radical therapy including mastectomy, radical resection plus radiotherapy (RT), and radical resection plus local booster irradiation (22,23). If a local surgery is selected, efforts should be made to ensure the complete resection of the current tumor;
For unresectable locally recurrent ABC, systemic treatment remains the mainstream approach.
Metastatic breast cancer (MBC)
Systemic treatment is the first choice for MBC;
After the systemic treatment, local treatment may be applied for the purpose of alleviating symptoms or resolving complications (24).
Treatment of ER-positive/HER-2-negative ABC
Treatment principles
The purposes of the treatment of ER-positive/HER-2-negative ABC include restraining tumor progression, relieving symptoms, and prolonging survival. For patients who are sensitive to endocrine treatment, endocrine treatment not only can delay chemotherapy but also help the patient to maintain good quality of life. Therefore, endocrine therapy may be the preferred treatment in patients with HR-positive and/or progesterone receptor (PR)-positive, and HER-2-negative MBC that is limited in breast, bone, and soft tissue, asymptomatic, and with small tumor load. However, for patients who are resistant to endocrine treatment, with rapid tumor progression, diffuse visceral metastases, or obvious symptoms, and in whom a rapid decrease of tumor load is required, more effective treatment (e.g., chemotherapy) should be provided (25,26) (1A);
For patients who are responsive to the previous endocrine therapy (TTP is longer than 6 months), the subsequent endocrine therapy may still be able to effectively control tumor, no matter the patient has become menopausal. Other endocrine medications with different action mechanisms may be used after disease progression. Failure of three consecutive lines of endocrine therapy indicates endocrine resistance, and cytotoxic drug therapy should be used instead;
During the endocrine treatment, response evaluation should be performed every 2-3 months, and the treatment should be maintained for SD and PR patients. If tumor progresses, new endocrine treatment or chemotherapy should be used instead based on the specific disease conditions;
For patients who are not suitable for endocrine therapy, chemotherapy should be applied firstly, followed by the endocrine maintenance treatment after the disease has been effectively controlled. Although this treatment strategy has not been evaluated in randomized controlled trials, it has been widely applied in clinical settings (1C);
Three randomized controlled trials have compared the efficacy of the combination of two endocrine drugs and that of one endocrine drug. The FACT study compared the efficacy of fulvestrant combined with anastrozole and that of anastrozole monotherapy. It was found that the time to progress (TTP) was not superior in the combination group than in the monotherapy group. The S0226 study adopted the same protocol, although there were more tamoxifen-naive patients among the subjects; it was found that the combination group had longer progression-free survival (PFS) than the monotherapy group. In the second-line treatment, a phase III controlled study showed that fulvestrant combined with anastrozole or exemestane had similar efficacy with fulvestrant monotherapy. Thus, currently there is no definite evidence that supports the combined endocrine therapy;
Up to now, no clinical study has demonstrated that the concurrent application of chemotherapy and endocrine therapy can prolong the survival. Therefore, it is not recommended except in clinical trials (1B);
Since HR testing may result in false-negative result, ER/PR-negative MBC patients with slow tumor progression but without features such as long recurrence-free survival (RFS) or simple bone/soft tissue metastasis may still benefit from endocrine therapy. The National Comprehensive Cancer Network (NCCN) guidelines also indicate that endocrine therapy can be applied in these patients;
The incidence of drug resistance can be high after long-term endocrine therapy. Pre-clinical studies have shown that the drug resistance mechanism may be related with the activation of mammalian target of rapamycin (mTOR) signaling pathway. Clinical studies have also confirmed that, compared with endocrine therapy alone, everolimus combined with endocrine therapy can significantly prolong PFS in patients who failed to respond to the previous endocrine therapy.
Treatment protocol of ER-positive/HER-2-negative ABC
Treatment protocol of ER-positive/HER-2-negative ABC (Figure 1).
Figure 1.
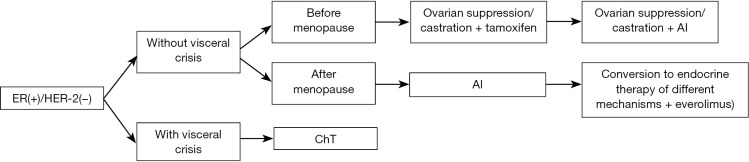
Treatment protocol of ER-positive/HER-2-negative ABC. ER, estrogen receptor; HER-2, HER-2-targeted therapy; ChT, chemotherapy; AI, aromatase inhibitor.
Drug selection in endocrine therapies
In premenopausal breast cancer patients with relapse/metastasis, ovarian suppression or castration combined with endocrine therapy is preferred (1A). If no tamoxifen is used in the adjuvant therapy or the withdrawal of tamoxifen has exceeded 12 months, tamoxifen combined with ovarian suppression or castration may be applied. After ovarian suppression or castration, the subsequent treatment is same as that in postmenopausal patients. For patients who have received tamoxifen in the adjuvant therapy, ovarian suppression and/or castration combined with aromatase inhibitor (AI) may be applied (1B);
The first-line endocrine therapy for postmenopausal patients is AI, and tamoxifen may also be described for some patients (1A). In areas and populations with limited economic condition, tamoxifen can also be used as the first-line therapeutic drug. When selecting endocrine therapy drugs for ABC patients, the physicians must consider the type and duration of endocrine drugs used during the adjuvant treatment stage (1A). No standard endocrine therapy protocol has been available for MBC patients who are unresponsive to the second-line endocrine therapy. The following drugs may be selected: tamoxifen, AI with different mechanisms, fulvestrant, and megestrol acetate. In patients with chemotherapy-induced amenorrhea, it is important to judge whether the patients have become menopausal, in particular when considering the use of AI, because young patients have a higher proportion of menstruation recovery after chemotherapy than the older ones (1A);
Targeted therapy after endocrine therapy resistance in ABC patients. Advances have been made in research on breast cancer drug resistance. Pre-clinical studies have demonstrated that the drug resistance mechanism may be associated with the activation of m-TOR signaling pathway. As shown in an early clinical study, in patients whose disease progresses after non-steroidal AI or tamoxifen treatment, endocrine drug in combination with everolimus, an m-TOR inhibitor, may improve the prognosis (27). As shown in BOLERO-2, a phase III randomized controlled clinical study, in HR-positive/HER-2-negative postmenopausal ABC patients who were unresponsive to non-steroidal AI, everolimus combined with exemestane could significantly prolong PFS by over 6 months (11 vs. 4.1 months, compared with exemestane monotherapy); accordingly, the combination group had relatively higher incidences of adverse reactions, which were controllable (28). Based on this study, the United States and the European Union approved the use of exemestane for the treatment of ABC patients who are unresponsive to non-steroidal aromatase inhibitor (NSAI) treatment (1A). Currently, this indication of exemestane has not been licensed in China. The physicians must balance the therapeutic benefits and the drug’s adverse reactions and meanwhile consider other factors including the drug availability and patient’s willingness.
Treatment of HER-2-positive ABC
Treatment principles
For HER-2-positive MBC, which is immunohistochemically shown as +++ or the presence of HER-2 gene proliferation under fluorescence in situ hybridization (FISH), anti-HER-2 treatment should be initiated as early as possible unless there are contraindications to its use (29-32) (1A);
For ER-negative/HER-2-positive ABC, monotherapy, combined chemotherapy, or chemotherapy combined with anti-HER-2 treatment may be selected according to disease condition (29,30,33) (1A);
For ER-positive/HER-2-positive ABC, the anti-HER-2 treatment combined with endocrine therapy has shown definite PFS benefits. Endocrine treatment combined with anti-HER-2 treatment can be used as the first- or second-line treatment according to the disease condition (34,35) (1A).
Application of anti-HER-2 treatment after disease progression
Continuous inhibition of HER-2 pathway may bring survival benefits. Therefore, the anti-HER-2 treatment should continue in patients who are unresponsive to the anti-HER-2 treatment. Currently, the best duration of anti-HER-2 treatment for MBC remains unclear. Therefore, if there is no disease progression or unacceptable toxicity, it is recommended that the initial treatment protocol should be maintained (36,37) (1B);
In patients whose disease progresses after the first-line treatment using trastuzumab combined with cytotoxic agents, it is still unclear whether the best option would be continuing the use of trastuzumab combined with cytotoxic agents or changing to lapatinib combined with capecitabine. Therefore, both of these two protocols can be used as the second-line options (38,39) (1A). In addition, some studies have shown that the combination of trastuzumab with cytotoxic drugs (trastuzumab-DM1, T-DM1) can bring more survival benefit in patients with trastuzumab resistance and therefore can be used as the second-line anti-HER-2 treatment (40,41) (1B). The dual-channel HER-2-inhibiting strategy such as trastuzumab + pertuzumab and trastuzumab + lapatinib can overcome trastuzumab resistance in some patients and may also be sued as a treatment option after the second-line treatment (42,43) (1B). Studies have shown that mTOR inhibitor everolimus has certain efficacy for ABC patients who have previously received trastuzumab (2A); in the case that T-DM1 is not available, it can also be used as a treatment option after the second-line treatment;
The optimal treatment for patients whose disease recurs after adjuvant treatment with trastuzumab remains unclear; however, the anti-HER-2 treatment should continue in all patients. It is recommended that anti-HER-2 treatment can be used as the second-line treatment in patients with an interval of ≤12 months between trastuzumab withdrawal and relapse. However, for patients with an interval of >12 months between trastuzumab withdrawal and relapse, anti-HER-2 treatment can be used as the first-line treatment.
Standard HER-2 detection and result judgment
For ABC patients with unknown HER-2 gene status, in situ hybridization (ISH, for determination of HER-2 gene copy number) or immunohistochemistry (IHC, for calculating the number of HER-2 receptor on cell membrane surface) can be applied to confirm the HER-2 gene status. However, mRNA detection and multiple gene sequence detection are not recommended (44);
HER-2 is judged as positive if HER-2 gene amplification is detected by ISH or HER-2 +++ is found by IHC. A IHC score of ++ is a threshold value, which should be further validated by FISH. Similarly, if the results of ISH is also within a threshold range (the range can be 1.8-2.0 HER-2 gene/chromosome/cells or >4 or <6 HER-2 genes/cell, based on the determination method), more cells should be counted or a second ISH should be performed; if the results are still within the threshold range, they should be verified by IHC (45).
Chemotherapy and biotherapy
Principles of chemotherapy
Most MBCs are non-curable. On the basis of ensuring the quality of life, the purposes of treatment include: controlling tumor; alleviating symptoms; prolonging tumor control time; and prolonging the patients’ survival whenever possible. Chemotherapy uses cytotoxic drugs to kill tumor cells. It has high efficacy and is faster than the endocrine therapy in exerting its effects. However, it is often accompanied with higher toxic effects, which deteriorates the patients’ quality of life. Thus, chemotherapy is often used in hormone receptor-negative patients. In hormone receptor-positive patients, if visceral crisis occurs, disease progresses rapidly, and/or the symptom is obvious or the patient develops endocrine treatment resistance, chemotherapy may be considered.
The selection of therapeutic protocol and the duration of treatment should be based on the effectiveness of treatment and the patient’s tolerance. If one protocol is effective, it should be continued until the disease progresses or intolerable toxicity develops.
Indications of chemotherapy
Include: (I) HR-negative; (II) with symptomatic visceral metastasis; (III) HR-positive patients who are unresponsive to third- or higher-line endocrine therapy.
The common chemotherapy drugs include anthracyclines (e.g., doxorubicin, epirubicin, pirarubicin, and pegylated liposomal doxorubicin); taxanes (e.g., paclitaxel, docetaxel, and albumin-bound paclitaxel), anti-metabolism drugs (e.g., capecitabine and gemcitabine), and non-taxane microtubule inhibitors (e.g., vinorelbine and airibulin). Other effective drugs include cyclophosphamide, cisplatin, and oral etoposide.
Principles of chemotherapy
Both combined therapy and monotherapy are reasonable options. Curing the disease is no longer a treatment objective for ABC patients. Instead, the patient’s quality of life should be ensured. Therefore, monotherapy is preferred (46-48). Based on the currently available data, combined chemotherapy may be applied in patients with rapid disease progression, with visceral crisis, or requiring treatment to rapidly resolve the symptoms and/or control disease progression;
In HER-2-negative MBC patients who had previously not received adjuvant therapy with anthracyclines or taxanes, anthracycline- or taxane-based protocols are often selected, and monotherapy or combined therapy may be selected for first-line treatment. Alternative drugs include capecitabine (47-49), vinorelbine (50), and gemcitabine;
In taxane-naive MBC patients who are resistant to anthracyclines or reaching the maximum cumulative dose of anthracycline or have developed the dose-limiting toxicity (e.g., cardiac toxicity) of anthracycline, the taxane-based protocols are often used for subsequent treatment, with taxane monotherapy being the preferred treatment. Alternative drugs include capecitabine, vinorelbine, and gemcitabine;
If taxanes have been used in the adjuvant therapy and tumor progresses 1 year later after the completion of taxane-based adjuvant therapy, the taxanes can still be used after relapse/metastasis;
The duration of each protocol (number of cycles) and the application of multi-line chemotherapy should be tailored according to the specific conditions of each patient. A meta-analysis (51) has shown that the long duration of first-line chemotherapy can prolong disease control time and may prolong the OS. Therefore, the first-line treatment can be maintained till the disease progresses or develops intolerable toxicity (the “intolerable toxicity” should be judged by both patients and physicians);
The endocrine therapy is preferred in HR-positive breast cancer patients, even if visceral metastasis occurs. Exceptions include endocrine resistance or conditions requiring the rapid resolvement of symptoms (25);
In HR-positive breast cancer patients who respond well to chemotherapy, maintenance therapy using either chemotherapy or endocrine therapy is a reasonable option;
During the maintenance therapy following an effective chemotherapy, either the initial protocol or one of the drugs can be constantly used for maintenance (52). Alternatively, oral chemotherapy drugs (e.g., capecitabine) can be applied for maintenance; however, it is still no clear whether capecitabine can bring survival benefit.
Biotherapy
The efficacy (in terms of OS) of bevacizumab in combination with taxanes has not been demonstrated in other subsequent clinical trials. No efficacy-predicting factor of bevacizumab has been found (53-55);
Comprehensive analysis of currently available clinical studies and a recent meta-analysis (56) have concluded that the use of bevacizumab in ABC patients can achieve limited PFS benefit but does not prolong OS;
-
Currently, the European Union has approved the combination of bevacizumab with taxanes as the first-line treatment for ABC. Bevacizumab combined with capecitabine can be another option;
-
Since bevacizumab has not been indicated for breast cancer in China, the selection of patients should be particularly cautious in clinical settings;
Other promising new drugs for biotherapy include cyclin-dependent kinase 4/6 (CDK4/6) inhibitors; however, none of them have been marketed in China.
-
Bone metastasis
Diagnosis
The risk of bone metastasis can be high in breast cancer patients. Clinical examinations for ruling out the possibility of bone metastasis can be considered as the routine examination items. Examinations for ruling out bone metastasis should be performed in patients with clinical manifestations including bone pain, pathological fractures, elevated alkaline phosphatase, spinal cord or spinal nerve root compression, or hypercalcemia. Emission computed tomography (ECT) is a screening tool for the initial diagnosis of bone metastasis, which can be further confirmed by X-ray, MRI, CT, or PET-CT (fluorine-18-deoxyglucose, FDG). Bone biopsy may be considered if necessary.
The diagnosis of the bone metastasis of malignant tumor should meet at least one of the following criteria:
The malignancy is diagnosed histopathologically or cytologically; or, the bone metastasis of malignant tumor is diagnosed by bone lesion biopsy or cytology;
The bone metastasis of malignancy is diagnosed by the X-ray, MRI, CT, or PET-CT (both FDG digestion and CT indicate the presence of bone metastasis) of the bone lesion.
The screening and examination of bone metastasis are mainly based on medical imaging (Figure 2) (57-63).
Figure 2.
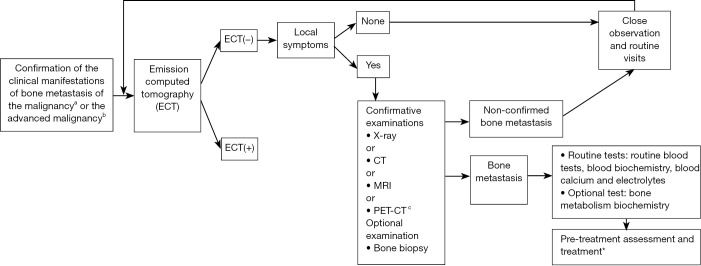
Screening and examination of bone metastasis. ECT, Emission computed tomography. a, Clinical manifestations of bone metastasis include bone pain, restricted activities, pathologic fractures, spinal cord and spinal nerve compression, and hypercalcemia; b, Advanced malignancies and malignant tumors at high risk of bone metastasis; c, PET-CT is not routinely recommended due to its high price; * see Figures 1,3.
Figure 3.
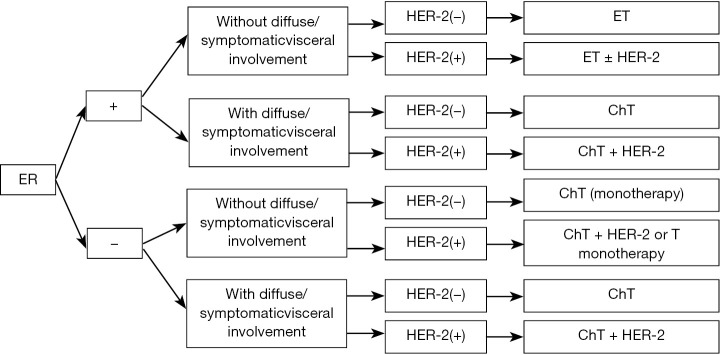
First-line treatment of ABC. ET, endocrine therapy; ChT, chemotherapy; HER-2, HER-2-targeted therapy; T, trastuzumab.
Treatment principles
The main targets of the MDT for the bone metastasis of breast cancer include: restoring functions and improving the quality of life; controlling tumor progression and prolonging survival; and preventing and treating skeletal related events (SREs) and relieving pain. SREs include pathologic fractures, spinal cord compression, and radiotherapy (RT) and surgery targeting bone metastases; in some literature, it may also include hypercalcemia;
Systemic therapy is the main treatment, with chemotherapy, endocrine therapy, and molecularly targeted therapy being the basic medications for ABC. Bone modulators (bisphosphonates and denosumab) can prevent and treat SREs, and therefore can be used as the essential drugs for treating the bone metastasis of breast cancer;
The criteria for judging the efficacy of a bone modulator is whether it can lower the incidence of SREs;
In patients with bone metastasis, a bisphosphonate may be added as early as possible before symptom (e.g., bone pain) occurs; it may be still used even when the systemic disease progresses (64,65) (1A);
The bisphosphonate should be constantly used until the patient cannot tolerate it or the general condition remarkably deteriorates. According to currently available evidences, administration of zoledronic acid (4 mg) every 3-4 weeks for 2 consecutive years is effective and safe;
No optimal dosing time and duration of a bone modulator has been determined for isolated bone metastasis;
It is difficult to evaluate the response of a metabolic bone lesion to the treatment, particularly in patients with simple bone metastasis. Among the approaches for evaluating the response, X-ray has a low sensitivity and MRI and PET-CT are not feasible for response evaluation. While CT scanning in combination with bone window is currently the best approach, attention should be paid to differentiate the osteoblastic bone metastases and the repair of metabolic bone lesion;
In patients with persistent or localized pain due to bone metastasis, imaging should be performed to decide whether there is (or will be) a pathologic fracture. For bone metastasis patients with (or at high risk of) long bone fracture, orthopedic assessment should be performed, and the subsequent treatment options include surgical fixation or RT. If there is no definite risk of bone fracture, RT may be a treatment option (1A);
In patients with suspected neurological symptoms and signs due to spinal cord compression, thorough evaluation should be performed as oncologic emergencies. Adequate imaging should be performed in the possibly involved areas and the adjacent areas of spine. MRI is the preferred examination. Emergency surgeries (neurosurgery and orthopedic surgery) may be needed for decompression. In case that there is no decompression/fixation method, RT may be applied (66,67) (1B).
Drug selection and precautions
Many randomized studies have supported the use of bisphosphonates in patients with bone metastasis from breast cancer (67-75). Compared with other bisphosphonates, the third-generation bisphosphonates (e.g., zoledronic acid) have improved action intensity and efficacy. Meta-analysis has shown that zoledronic acid can more effectively prevent SREs caused by bone metastasis;
Female patients with bone metastasis should receive intravenously injected bisphosphonates in combination with orally administered calcium and vitamin D;
Both bisphosphonates and denosumab can cause osteonecrosis of the jaw (ONJ), which has an incidence of 3‰ among breast cancer patients. The risk factors of ONJ include the baseline oral health status and the oral procedures during the treatment. Thus, dental examination is recommended before the intravenous injection of bisphosphonates or denosumab, and any dental surgery should be avoided during the treatment (76).
Plasma levels of calcium, creatinine, phosphorous, and magnesium should be monitored before the intravenous injection of bisphosphonates or subcutaneous injection of denosumab. Since hypophosphatemia and hypocalcemia may easily occur during the treatment, monitoring of the calcium, phosphorus, and magnesium levels should be enhanced.
Brain metastasis
Treatment principles
Diagnosis: after the confirmation of a primary breast cancer, the diagnosis of brain metastasis can be based on the brain enhanced MRI. However, imaging-guided biopsy or open biopsy/resection may be performed if a differential diagnosis from other brain tumors is needed. About 15% of ABC patients may develop central nervous system metastases (77). Among the solid tumors, breast cancer has a high incidence of brain metastasis, ranking the second place. It also has a trend to simultaneously involve multiple sites including meninges and brain parenchyma. It has the highest incidence of meningeal metastasis among all the solid tumors. The basic indicator for judging the prognosis of brain metastasis is the recursive partitioning analysis (RPA) (78); in addition, the Breast-Graded Prognostic Assessment (Breast-GPA) can also be applied (79) (2A);
Basic treatment principles for brain metastasis: once a diagnosis of brain metastasis is established, appropriate localized treatment and supporting treatment should be selected according to the patients’ general conditions, control of extracranial lesions, and number/location of metabolic brain lesions. In addition, reasonable systemic treatment should be selected based on the molecular type of primary tumor and the previous anti-tumor systemic treatment (2A);
-
Principles of the localized treatment for a single brain metastasis: among patients with good prognosis (based on brain metastasis prognosis indicators), based on the size, location and surgical risk of the tumors, the preferred treatment include: surgical resection + RT; compared with whole-brain RT alone, surgical resection + whole-brain RT can achieve higher local control rate, symptom control duration, and median survival (80). In patients with space-occupying mass, surgery can rapidly resolve the symptoms. Notably, surgery only benefits patients without extracranial metastases or with well controlled extracranial lesions. Surgery is not recommended for patients with other organ metastasis that has not been controlled. Compared with surgery alone, surgical resection + RT can increase the local control rate and decrease the increase of intracranial distant metastasis rate by two thirds (80) (2A);
Stereotactic radiotherapy (SRT) includes single session of stereotactic radiosurgery (SRS) or fractionated stereotactic radiotherapy (FSRT). SRT-based whole-brain RT cannot improve survival (81);
Principles of localized treatment for metastatic lesions (2-3 lesions, or 2-4 sites): patients with a good prognosis but with non-single brain metastases (no exceeding 3 lesions or 4 sites) with a maximal diameter of less than 3 or 4 cm, the optional protocols include: (i) SRS/FSRT ± whole-brain RT (81); (ii) for symptomatic lesions exceeding 3 or 4 cm, surgical resection can be performed to remove the larger lesions, followed by post-operative RT (whole-brain RT or SRS/FSRT) (82); and (iii) Whole-brain RT ± SRS/FSRT (83). Compared with SRT + whole-brain RT, simple SRT can decrease the neurocognitive impair caused by whole-brain RT (84). Whole-brain RT following SRT markedly decreases the incidence of intracranial recurrence but loses the protective effect of SRT alone on the cognitive function. Therefore, treatment decision should be based on the intracranial tumor status, the predicted survival, and the willingness of patients and their families. The range of SRS reference dose includes 24, 18, or 15 Gy. Dose selection is mainly based on the tumor size and RT mode (SRT alone or in combination with whole-brain RT) (2A);
Principles of localized treatment for multiple brain metastasis or for patients with poor general condition and/or with meningeal metastasis: Although whole-brain RT can be used as a localized treatment approach for all patients with brain metastases, whole-brain RT based on symptomatic supporting treatment including corticosteroids and dehydration is preferred for the following patients: (i) with a number of brain metastases exceeding 4; or (ii) with meningeal involvement; or (iii) although the number of brain metastases does not exceed 3/4, there is uncontrolled systemic spread of the disease and a Karnofsky performance status (KPS) score of less than 70. The ranges of whole-brain RT dose include 20-40 Gy in 5-20 fractions. More common options include 30 Gy in 10 fractions, 37.5 Gy in 15 fractions, and 40 Gy in 20 fractions. These doses and fractions have shown no significant difference in terms of local control rate and survival. In principle, patients with poor conditions tend to select short-course treatment (85,86). In patients with multiple brain metastases, whether a local booster irradiation should be applied after whole-brain RT should be decided based on the patient’s general condition and the features of the metastatic lesions. If the general condition is poor or the patient and his/her family refuse to receive whole-brain RT, symptomatic supporting treatment alone can be provided (2A);
Value of systemic treatment: In patients with brain metastasis, the anti-tumor systemic treatment should initially follow the molecular type of the primary tumor, in particularly patients who have undergone whole-brain RT because these patients have severer blood-brain barrier damage and thus are more likely to be benefited from the systemic treatment. The capability of the drugs passing through the blood-brain barrier should be adequately considered; in patients with meningeal metastasis, this capability should be fully considered during drug selection. HER-2-positive breast cancer is a special type of breast cancer. Although the incidence of central nervous system metastasis in patients with early HER-2-positive breast cancer is not higher than that in those with other molecular types, the incidence of brain metastasis may constantly increase along with disease progression; in patients with sufficiently long disease course, up to 50% of metastatic HER-2-positive breast cancer patients can develop brain metastasis (87,88). Retrospective data confirm that, in patients with HER-2-positive brain metastases, constant anti-HER-2 treatment on the basis of brain RT can effectively improve patients’ survival rate (89). The medical treatment of patients with HER-2-positive brain metastasis should follow the treatment principles for HER-2-positive ABC patients (90,91). In some special cases (e.g., disease progresses after whole-brain RT and/or SRS/FSRT), drugs (e.g., lapatinib) that can more easily pass the blood-brain barrier are preferred. A phase II clinical study on the combination of lapatinib and capecitabine showed that, in RT-naive HER-2-positive brain metastasis patients, the combined treatment achieved good volume-shrinking effect on the metastatic lesions, with an objective response rate (defined as the shrinking of tumor volume by 50% or more without increasing the steroid dose) of 65.9% and a median PFS of 5.5 months. Although 78% of initial treatment failure was still located at the central nerve system and the majority of the non-responsive patients receive the salvage RT, the whole group still obtained a median survival of 17 months. As shown in this study, although RT remains the standard treatment for HER-2-positive breast cancer patients with brain metastasis, it may be a cautious option to use the combination of lapatinib and capecitabine as the initial treatment (followed by RT as a salvage approach) for patients with small, asymptomatic tumors. Nevertheless, the premises include accurate disease assessment, adequate communication with patients, and availability of salvage RT (92) (2A);
Management of special conditions and new advances: Although research has shown that SRT may be preferred in patients with the number of metastatic lesions larger than 4 (temporarily not followed with whole-brain RT), the patients must be fully informed of the subsequent risk of relapse, especially the risk of relapse accompanied with neurological positioning signs (93) (2A);
Follow-up: Contrast-enhanced brain magnetic resonance imaging should be performed every 2-3 months within 1 year after the treatment for patients with brain metastasis, and then the frequency may be adjusted according to the disease stability (2A).
MBC in males
Breast cancer is a rare disease in males, accounting for about 1% of all breast cancer cases. Male MBC is even more rare. Essentially there is no randomized controlled trial on male MBC, and all the relevant data are from retrospective data analysis;
The positive rate of HR is roughly 90% in male breast cancer patients, which is higher than that in female patients.
Treatment
The treatment strategies of male MBC follow those for female breast cancer patients. Endocrine therapy is preferred in most ER-positive male MBC patients, except that the patients are suspected to be endocrine therapy-resistant or the symptoms need to be resolved rapidly;
For ER-positive male MBC patients, tamoxifen may be the treatment of choice, although its adverse reactions are more often than in female patients;
For male MBC patients requiring AI treatment, the combination with luteinising-hormone releasing hormone (LHRH) agonist or orchiectomy is needed (94), because AI treatment may increase the androgen and follicle-stimulating hormone (FSH) via the negative feedback mechanisms; in addition, some estrogen in male body is directly originated from the testicles. The decrease of male estrogen level by AI treatment alone (not combined with LHRHa) accounts for only 50-70%, which is far lower than that (over 95%) in females;
The positive rate of androgen receptor (AR) can reach 95%. Orchiectomy can be effective, with a response rate of 32-67%.
Conclusions
The treatment of ABC is a complicated process during which a variety of factors including the tumor itself, patient’s body condition, and currently available treatment must be considered. Due to the lack of high-level evidences, the current treatment strategies for ABC still have many limitations. The past decades have witnessed the fundamental changes in the adjutant treatment of breast cancer, which markedly change the previous treatment and drug resistance mechanisms of ABC patients. Thus, the conclusions of many previous studies may not suit the current treatment status.
Therefore, all the stakeholders including health care providers, pharmaceuticals, charities, and social entities are urged to be involved in well-designed high-quality clinical trials, so as to find the optimal treatment strategies and drug options (including dose, protocols, and efficacy prediction markers) for ABC. Meanwhile, joint efforts from clinical researchers and continued medical education workers are needed to disseminate the recent research findings to the clinical practices, so as to optimize the treatment of ABC and ultimately prolong the patients’ survival and improve their quality of life. The release of the consensus on diagnosis and treatment of ABC in China is a key step in realizing this goal. The more important task is to fully realize and implement the consensus and then achieve its correct application in clinical practices.
Acknowledgements
None.
This article is copublished in Translational Cancer Research and Annals of Translational Medicine.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cardoso F. Metastatic breast cancer patients: the forgotten heroes! Breast 2009;18:271-2. [DOI] [PubMed] [Google Scholar]
- 2.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol 2008;19:2012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre F, Slimane K, Bachelot T, et al. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol 2004;22:3302-8. [DOI] [PubMed] [Google Scholar]
- 4.Sundquist M, Eriksson Z, Tejler G, et al. Trends in survival in metastatic breast cancer. Eur J Cancer 2010;8:191. [Google Scholar]
- 5.Foukakis T, Fornander T, Lekberg T, et al. Age-specific trends of survival in metastatic breast cancer: 26 years longitudinal data from a population-based cancer registry in Stockholm, Sweden. Breast Cancer Research and Treatment 2011;130:553-60. [DOI] [PubMed] [Google Scholar]
- 6.Chirgwin J, Craike M, Gray C, et al. Does multidisciplinary care enhance the management of advanced breast cancer?: evaluation of advanced breast cancer multidisciplinary team meetings. J Oncol Pract 2010;6:294-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueno NT, Ito TD, Grigsby RK, et al. ABC conceptual model of effective multidisciplinary cancer care. Nat Rev Clin Oncol 2010;7:544-7. [DOI] [PubMed] [Google Scholar]
- 8.The requirements of a specialist breast unit. Eur J Cancer 2000;36:2288-93. [DOI] [PubMed] [Google Scholar]
- 9.Rosen EL, Eubank WB, Mankoff DA. FDG PET, PET/CT, and breast cancer imaging. Radiographics 2007;27 Suppl 1:S215-29. [DOI] [PubMed] [Google Scholar]
- 10.Eubank WB, Mankoff D, Bhattacharya M, et al. Impact of FDG PET on defining the extent of disease and on the treatment of patients with recurrent or metastatic breast cancer. AJR Am J Roentgenol 2004;183:479-86. [DOI] [PubMed] [Google Scholar]
- 11.Houssami N, Costelloe CM. Imaging bone metastases in breast cancer: evidence on comparative test accuracy. Ann Oncol 2012;23:834-43. [DOI] [PubMed] [Google Scholar]
- 12.Whitlock JP, Evans AJ, Jackson L, et al. Imaging of metastatic breast cancer: distribution and radiological assessment at presentation. Clin Oncol (R Coll Radiol) 2001;13:181-6. [DOI] [PubMed] [Google Scholar]
- 13.Costelloe CM, Rohren EM, Madewell JE, et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol 2009;10:606-14. [DOI] [PubMed] [Google Scholar]
- 14.Advanced breast cancer (update): Diagnosis and treatment. 2009. Available online: http://www.nice.org.uk/guidance/cg81
- 15.Arslan C, Sari E, Aksoy S, et al. Variation in hormone receptor and HER-2 status between primary and metastatic breast cancer: review of the literature. Expert Opin Ther Targets 2011;15:21-30. [DOI] [PubMed] [Google Scholar]
- 16.Pusztai L, Viale G, Kelly CM, et al. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist 2010;15:1164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cnota W, Sodowski K, Olesiak-Andryszczak M, et al. Program for early detection of ovarian cancer for women as prophylaxis provided at a municipal hospital. Wiad Lek 2004;57 Suppl 1:43-7. [PubMed] [Google Scholar]
- 18.Dominici L, Najita J, Hughes M, et al. Surgery of the primary tumor does not improve survival in stage IV breast cancer. Breast Cancer Res Treat 2011;129:459-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashaan ZM, Bastiaannet E, Portielje JE, et al. Surgery in metastatic breast cancer: patients with a favorable profile seem to have the most benefit from surgery. Eur J Surg Oncol 2012;38:52-6. [DOI] [PubMed] [Google Scholar]
- 20.Ly BH, Vlastos G, Rapiti E, et al. Local-regional radiotherapy and surgery is associated with a significant survival advantage in metastatic breast cancer patients. Tumori 2010;96:947-54. [PubMed] [Google Scholar]
- 21.Neuman HB, Morrogh M, Gonen M, et al. Stage IV breast cancer in the era of targeted therapy: does surgery of the primary tumor matter? Cancer 2010;116:1226-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Tienhoven G, Voogd AC, Peterse JL, et al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Eur J Cancer 1999;35:32-8. [DOI] [PubMed] [Google Scholar]
- 23.Cox CE, Furman BT, Kiluk JV, et al. Use of reoperative sentinel lymph node biopsy in breast cancer patients. J Am Coll Surg 2008;207:57-61. [DOI] [PubMed] [Google Scholar]
- 24.Hortobagyi GN. Multidisciplinary management of advanced primary and metastatic breast cancer. Cancer 1994;74:416-23. [DOI] [PubMed] [Google Scholar]
- 25.Wilcken N, Hornbuckle J, Ghersi D. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer. Cochrane Database Syst Rev 2003;(2):CD002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins MJ, Wolff AC. Therapeutic options in the management of metastatic breast cancer. Oncology (Williston Park) 2008;22:614-23; discussion 623, 627-9. [PubMed]
- 27.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pritchard KI, Burris HA, 3rd, Ito Y, et al. Safety and efficacy of everolimus with exemestane vs. exemestane alone in elderly patients with HER2-negative, hormone receptor-positive breast cancer in BOLERO-2. Clin Breast Cancer 2013;13:421-32. [DOI] [PubMed] [Google Scholar]
- 29.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [DOI] [PubMed] [Google Scholar]
- 30.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005;23:4265-74. [DOI] [PubMed] [Google Scholar]
- 31.Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol 2008;26:5544-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan Z, Xu B, Arpornwirat W, et al. Overall Survival Benefit Observed with Lapatinib (L) Plus Paclitaxel (P) as First-Line Therapy in Patients with HER2-Overexpressing Metastatic Breast Cancer (MBC). Cancer Res 2010;70:3-14-24.
- 33.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002;20:719-26. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 2009;27:5529-37. [DOI] [PubMed] [Google Scholar]
- 35.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009;27:5538-46. [DOI] [PubMed] [Google Scholar]
- 36.Pegram M, Liao J. Trastuzumab treatment in multiple lines: current data and future directions. Clin Breast Cancer 2012;12:10-8. [DOI] [PubMed] [Google Scholar]
- 37.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol 2009;27:1999-2006. [DOI] [PubMed] [Google Scholar]
- 38.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 2010;28:1124-30. [DOI] [PubMed] [Google Scholar]
- 39.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008;68:9280-90. [DOI] [PubMed] [Google Scholar]
- 41.Junttila TT, Li G, Parsons K, et al. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011;128:347-56. [DOI] [PubMed] [Google Scholar]
- 42.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 2010;28:1138-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 2012;30:2585-92. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Saboorian MH, Frenkel E, et al. Laboratory assessment of the status of Her-2/neu protein and oncogene in breast cancer specimens: comparison of immunohistochemistry assay with fluorescence in situ hybridisation assays. J Clin Pathol 2000;53:374-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlson RW, Moench SJ, Hammond ME, et al. HER2 testing in breast cancer: NCCN Task Force report and recommendations. J Natl Compr Canc Netw 2006;4 Suppl 3:S1-22; quiz S23-4. [PubMed]
- 46.Piccart-Gebhart MJ, Burzykowski T, Buyse M, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol 2008;26:1980-6. [DOI] [PubMed] [Google Scholar]
- 47.Stockler MR, Harvey VJ, Francis PA, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol 2011;29:4498-504. [DOI] [PubMed] [Google Scholar]
- 48.Carrick S, Parker S, Wilcken N, et al. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev 2005;(2):CD003372. [DOI] [PubMed] [Google Scholar]
- 49.Huang HY, Jiang ZF, Wang T, et al. Efficacy and safety of regimens of capecitabine-based chemotherapy in the treatment of advanced breast cancer. Zhonghua Zhong Liu Za Zhi 2011;33:850-3. [PubMed] [Google Scholar]
- 50.Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol 2011;29:264-71. [DOI] [PubMed] [Google Scholar]
- 51.Gennari A, Stockler M, Puntoni M, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol 2011;29:2144-9. [DOI] [PubMed] [Google Scholar]
- 52.Alba E, Ruiz-Borrego M, Margelí M, et al. Maintenance treatment with pegylated liposomal doxorubicin versus observation following induction chemotherapy for metastatic breast cancer: GEICAM 2001-01 study. Breast Cancer Res Treat 2010;122:169-76. [DOI] [PubMed] [Google Scholar]
- 53.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-76. [DOI] [PubMed] [Google Scholar]
- 54.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2010;28:3239-47. [DOI] [PubMed] [Google Scholar]
- 55.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 2011;29:1252-60. [DOI] [PubMed] [Google Scholar]
- 56.Rossari JR, Metzger-Filho O, Paesmans M, et al. Bevacizumab and Breast Cancer: A Meta-Analysis of First-Line Phase III Studies and a Critical Reappraisal of Available Evidence. J Oncol 2012;2012:417673. [DOI] [PMC free article] [PubMed]
- 57.Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med 2001;45:53-64. [PubMed] [Google Scholar]
- 58.Diel IJ, Kaufmann M, Bastert G. Metastatic Bone Disease: Fundamental and Clinical Aspects. Germany: Springer-Verlag Berlin Heidelberg, 1994:93-108. [Google Scholar]
- 59.Helms CA, Cann CE, Brunelle FO, et al. Detection of bone-marrow metastases using quantitative computed tomography. Radiology 1981;140:745-50. [DOI] [PubMed] [Google Scholar]
- 60.Steinborn MM, Heuck AF, Tiling R, et al. Whole-body bone marrow MRI in patients with metastatic disease to the skeletal system. J Comput Assist Tomogr 1999;23:123-9. [DOI] [PubMed] [Google Scholar]
- 61.Peterson JJ, Kransdorf MJ, O’Connor MI. Diagnosis of occult bone metastases: positron emission tomography. Clin Orthop Relat Res 2003;(415 Suppl):S120-8. [DOI] [PubMed] [Google Scholar]
- 62.Cook GJ, Houston S, Rubens R, et al. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol 1998;16:3375-9. [DOI] [PubMed] [Google Scholar]
- 63.Bury T, Barreto A, Daenen F, et al. Fluorine-18 deoxyglucose positron emission tomography for the detection of bone metastases in patients with non-small cell lung cancer. Eur J Nucl Med 1998;25:1244-7. [DOI] [PubMed] [Google Scholar]
- 64.Wong MH, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev 2012;2:CD003474. [DOI] [PubMed] [Google Scholar]
- 65.Van Poznak CH, Temin S, Yee GC, et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol 2011;29:1221-7. [DOI] [PubMed] [Google Scholar]
- 66.Dickinson F, Liddicoat A, Dhingsa R, et al. Magnetic resonance imaging versus radionuclide scintigraphy for screening in bone metastases. Clin Radiol 2000;55:653. [DOI] [PubMed] [Google Scholar]
- 67.George R, Jeba J, Ramkumar G, et al. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev 2008;(4):CD006716. [DOI] [PubMed] [Google Scholar]
- 68.Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol 1998;16:2038-44. [DOI] [PubMed] [Google Scholar]
- 69.Theriault RL, Lipton A, Hortobagyi GN, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol 1999;17:846-54. [DOI] [PubMed] [Google Scholar]
- 70.Rosen LS, Gordon DH, Dugan W, Jr, et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 2004;100:36-43. [DOI] [PubMed] [Google Scholar]
- 71.Diel IJ, Body JJ, Lichinitser MR, et al. Improved quality of life after long-term treatment with the bisphosphonate ibandronate in patients with metastatic bone disease due to breast cancer. Eur J Cancer 2004;40:1704-12. [DOI] [PubMed] [Google Scholar]
- 72.Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 1996;335:1785-91. [DOI] [PubMed] [Google Scholar]
- 73.Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer 2000;88:1082-90. [DOI] [PubMed] [Google Scholar]
- 74.McLachlan SA, Cameron D, Murray R, et al. Safety of oral ibandronate in the treatment of bone metastases from breast cancer: long-term follow-up experience. Clin Drug Investig 2006;26:43-8. [DOI] [PubMed] [Google Scholar]
- 75.Pecherstorfer M, Rivkin S, Body JJ, et al. Long-term safety of intravenous ibandronic acid for up to 4 years in metastatic breast cancer: an open-label trial. Clin Drug Investig 2006;26:315-22. [DOI] [PubMed] [Google Scholar]
- 76.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006;144:753-61. [DOI] [PubMed] [Google Scholar]
- 77.Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [DOI] [PubMed] [Google Scholar]
- 78.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [DOI] [PubMed] [Google Scholar]
- 79.Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510-4. [DOI] [PubMed] [Google Scholar]
- 80.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-9. [DOI] [PubMed] [Google Scholar]
- 81.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. [DOI] [PubMed] [Google Scholar]
- 82.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [DOI] [PubMed] [Google Scholar]
- 84.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [DOI] [PubMed] [Google Scholar]
- 85.Gaspar LE, Mehta MP, Patchell RA, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:17-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012;2:210-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol 2013;14:244-8. [DOI] [PubMed] [Google Scholar]
- 88.Olson EM, Abdel-Rasoul M, Maly J, et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol 2013;24:1526-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Q, Chen J, Yu X, et al. Systemic treatment after whole-brain radiotherapy may improve survival in RPA class II/III breast cancer patients with brain metastasis. J Neurooncol 2013;114:181-9. [DOI] [PubMed] [Google Scholar]
- 90.Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:2100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 2009;15:1452-9. [DOI] [PubMed] [Google Scholar]
- 92.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 2013;14:64-71. [DOI] [PubMed] [Google Scholar]
- 93.Serizawa T, Yamamoto M, Sato Y, et al. Gamma Knife surgery as sole treatment for multiple brain metastases: 2-center retrospective review of 1508 cases meeting the inclusion criteria of the JLGK0901 multi-institutional prospective study. J Neurosurg 2010;113 Suppl:48-52. [DOI] [PubMed] [Google Scholar]
- 94.Giordano SH, Hortobagyi GN. Leuprolide acetate plus aromatase inhibition for male breast cancer. J Clin Oncol 2006;24:e42-3. [DOI] [PubMed] [Google Scholar]


