Abstract
A cost-effective nutritional approach to improve postprandial glycaemia is attractive considering the rising burden of diabetes throughout the world. Whey protein, a by-product of the cheese-making process, can be used to manipulate gut function in order to slow gastric emptying and stimulate incretin hormone secretion, thereby attenuating postprandial glycaemic excursions. The function of the gastrointestinal tract plays a pivotal role in glucose homeostasis, particularly during the postprandial period, and this review will discuss the mechanisms by which whey protein slows gastric emptying and stimulates release of gut peptides, including the incretins. Whey protein is also a rich source of amino acids, and these can directly stimulate beta cells to secrete insulin, which contributes to the reduction in postprandial glycaemia. Appetite is suppressed with consumption of whey, due to its effects on the gut-brain axis and the hypothalamus. These properties of whey protein suggest its potential in the management of type 2 diabetes. However, the optimal dose and timing of whey protein ingestion are yet to be defined, and studies are required to examine the long-term benefits of whey consumption for overall glycaemic control.
Keywords: Whey protein, Postprandial glycaemia, Type 2 diabetes, Dietary intervention, Preload, Gastric emptying, Incretins, Gut hormones, Appetite, Amino acids
Core tip: Whey protein, a by-product of cheese-manufacture, shows promise in the dietary management of diabetes. Whey can slow gastric emptying, stimulate insulin and gut hormones including the incretins, and thereby reduce postprandial blood glucose, especially when consumed some minutes before a meal. Whey may also suppress appetite and reduce food intake. This review will summarise these properties of whey and examine what further evidence is needed before whey can be recommended in the management of type 2 diabetes.
INTRODUCTION
It is well established that the risk of microvascular, and to a lesser extent macrovascular complications of both type 1 and type 2 diabetes, is closely related to “average” glycaemic control as assessed by glycated haemoglobin (HbA1c). In people with type 2 diabetes who have relatively good glycaemic control, postprandial hyperglycaemia predominates over preprandial blood glucose in contributing to HbA1c[1,2]. Accordingly, focusing on postprandial glycaemia in patients with mild or moderate elevation of HbA1c is now appreciated as an important management strategy; indeed, achieving a “target” HbA1c of ≤ 7.0% is difficult without minimising postprandial glycaemic excursions[3,4]. The potential use of dietary manipulations to reduce postprandial glycaemia is intuitively appealing, particularly given the escalation in health care costs with the rising incidence of type 2 diabetes.
Whey, a by-product of cheese making, is gaining recognition as an important functional food[5]. Whey protein has been demonstrated to diminish postprandial glycaemia through various interrelated mechanisms including enhancement of insulin and incretin hormone secretion, slowing of gastric emptying, and reductions in appetite and energy consumption (Figure 1). These properties suggest the potential for whey in the management of type 2 diabetes. However, whey protein cannot be endorsed as a potential treatment until further studies show that it improves long-term glycaemic control without significant adverse outcomes.
Figure 1.
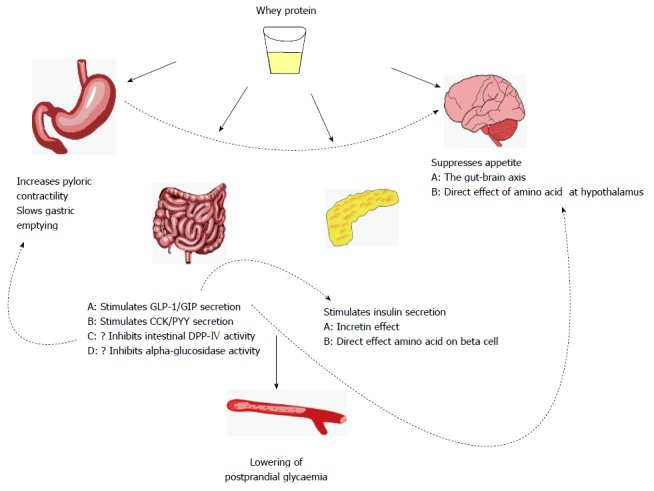
Mechanisms by which whey protein can reduce postprandial glycaemia. GLP-1: Glucagon-like-peptide-1; GIP: Glucose-dependent insulinotropic polypeptide; CCK: Cholecystokinin; PYY: Peptide YY; DPP-IV: Dipeptidyl peptidase-IV.
This review will explore the different forms of whey protein and compare the effects of whey with other sources of protein in reducing postprandial glycaemia. It will address the mechanisms by which whey lowers glycaemia, the factors that need to be considered for optimal use of whey, and the effects of long term consumption of whey protein on glycaemic control, together with its potential adverse effects.
COMPARISON OF WHEY AND CASEIN PROTEINS
Milk proteins are an important amino acid source for young mammals; they facilitate uptake of nutrients and trace elements[6] and provide a source of bioactive peptides with a range of physiological functions[6-8]. Cow’s milk contains about 3.5 g of protein per 100 mL, of which whey accounts for about 20% and casein 80%[9-11].
Whey consists of a heterogeneous group of proteins[12], including beta-lactoglobulin (35%), alpha-lactalbumin (12%), proteose peptone (12%), immunoglobulins (8%), and bovine serum albumin (5%)[11,13,14]. When chymosin is used in the cheese-making process, glycomacropeptide - which is high in branched chain amino acids - accounts for about 12% of total protein in whey[15]. Up to 1% of the total protein content of whey comprises “low abundance” proteins, including lactoferrin, and lactoperoxidase[14]. All these proteins have been reported to have nutritional and/or physiological functions[5].
Whey is seen as a more attractive protein for use as a dietary supplement compared to casein, due to differences in the amino acid composition and absorption kinetics between the two proteins[16]. Whey protein has a higher proportion of branched chain amino acids than casein[17], and is more soluble in the acidic environment of the stomach, leading to more rapid digestion[18] - hence it is termed a “fast” protein[19], while casein is a “slow” protein[16,20]. Using 13C-leucine-labelled whey and casein protein, Boirie et al[18] demonstrated in healthy subjects that whey protein results in more rapid appearance, and higher peak plasma concentrations of amino acid, when compared with casein, while Stanstrup et al[21] reported that levels of amino acids after a fat rich meal containing whey were substantially higher when compared to the same meal containing casein. As a result of greater solubility, more rapid digestion, and resultant higher plasma concentrations of amino acids, whey appears to be the more favourable protein to provide nutritional and functional benefits.
FORMS OF WHEY PROTEIN - ISOLATE, CONCENTRATE AND HYDROLYSATE
Whey protein is available in three forms: concentrate, isolate, and hydrolysate. Whey protein concentrate contains 35%-80% protein, with fat, lactose and minerals making up the remainder; whey protein isolate contains 85%-90% protein and very little fat or lactose[5,15,22]; and whey protein hydrolysate consists of proteins that have undergone hydrolysis by proteolytic enzymes[14]. Whey hydrolysates and isolates are more costly than whey concentrates, which is an important consideration if whey protein is to be used for a prolonged period of time in the management of type 2 diabetes. It is therefore important to consider the evidence that one form of whey protein is more “functional” than another.
Protein hydrolysates are usually more rapidly absorbed than the intact protein[23], but since intact whey is already a rapidly digested protein, any difference is likely to be minimal[24,25]. Some studies have suggested that whey hydrolysates may stimulate insulin and glucose-dependent insulinotropic polypeptide (GIP) secretion to a greater degree than the intact protein[26,27]. Mortensen et al[28] investigated the effects of adding 45 g of four different whey protein formulations (whey hydrolysate, whey isolate, alpha-lactalbumin enhanced whey, and caseinoglycomacropeptide enhanced whey) to a high fat/carbohydrate meal in subjects with type 2 diabetes, and reported that the first phase insulin response (as assessed by the incremental area under the curve (iAUC) up to 30 min) was enhanced after whey hydrolysate compared with the other three supplements, and that whey isolate and whey hydrolysate yielded a greater overall insulin response (iAUC at 480 min) than the other two supplements, without any difference between them. Whey proteins which have been hydrolysed are, however, usually less palatable[29], which detracts from their potential therapeutic use. There is no compelling evidence that one form of whey protein is significantly more potent than another, particularly in relation to reduction of postprandial glycaemia, so consideration of palatability and cost must also be taken into account.
ROLE OF THE INCRETIN HORMONES, GIP AND GLP-1, IN PROTEIN-INDUCED INSULIN SECRETION
The phenomenon by which insulin secretion is increased when glucose is given by the enteral route, when compared to an isoglycaemic intravenous glucose infusion, is called the “incretin effect”, and is attributed to the secretion of “incretin” hormones from the gut. The two known incretin hormones, glucagon-like-peptide-1 (GLP-1) and GIP, exert their insulinotropic actions through distinct G-protein-coupled receptors that are highly expressed on beta cells[30]. After oral glucose, about two thirds of the plasma insulin response can be attributed to the effects of GIP and GLP-1. The insulinotropic effects of both GIP and GLP-1 are glucose-dependent, requiring a substantial elevation of blood glucose (> 8 mmol/L) to be manifest[31]. Incretin based therapies, such as GLP-1 receptor agonists, are attractive for this reason, as insulin release is only triggered in the presence of elevated glucose concentrations, with consequently minimal risk of hypoglycaemia.
Incretin hormones may play an important role in protein-stimulated insulin release in health and type 2 diabetes[32]. GIP and GLP-1, when infused intravenously to mimic physiological increments after a meal, have been reported to potentiate the insulin secretory response to IV administration of an amino acid mixture[33]. In a study of oral administration of protein and amino acids in health, a whey drink resulted in a greater GIP response than a drink containing the essential amino acids found in whey, with an associated augmentation of the insulin response[34]. Additionally, the stimulation of insulin secretion from murine islets in vitro by whey was inhibited by GIP receptor antagonists[35]. The effects of the GLP-1 antagonist, exendin 9-39, on whey-induced insulin secretion have not been evaluated. However, it is clear that the insulintropic effects of whey, at least in part, involve the incretin axis.
In humans, fats and carbohydrates are reported to be the most potent stimuli for GLP-1 and GIP secretion[36], although the effects of protein on incretin secretion are less well studied than the other macronutrients[37]. Nevertheless, whey protein is reported to stimulate GLP-1 and GIP release[17,34,35,38-40]. Bowen et al[41] showed that plasma active GLP-1 concentrations were higher after intake of a whey protein beverage compared to a glucose or fructose drink, but the mechanisms mediating protein-induced incretin secretion remain largely unknown[37].
Although the capacity for GIP to stimulate insulin is markedly diminished in type 2 diabetes, at least in part due to the effects of chronic hyperglycaemia[42], GLP-1 retains much of its activity. As whey protein can augment incretin hormone secretion and enhance protein-stimulated insulin release, it seems reasonable to view whey as a potential therapeutic agent in the treatment of type 2 diabetes.
ROLE OF GASTRIC EMPTYING IN MEDIATING THE EFFECTS OF WHEY ON POSTPRANDIAL GLYCAEMIA
It is now well established that gastric emptying plays a major role in determining postprandial blood glucose concentrations, particularly the “early” glycaemic response, and that slowing gastric emptying can diminish postprandial glycaemic excursions in health and diabetes[43-46]. In healthy humans, the addition of protein to oral glucose lowers postprandial blood glucose concentrations acutely, probably predominantly by slowing gastric emptying[47]. Similarly, a “preload” of whey has been shown to slow gastric emptying of a subsequent meal in both health[17], and in type 2 diabetes[48].
The effects of whey on gastric emptying, postprandial glycaemia, and the secretion of incretin hormones, are interdependent. The incretins not only have major insulinotropic effects, but GLP-1 also slows gastric emptying, suppresses energy intake and has glucagonstatic effects to improve postprandial glycaemia[42]. Reports that GLP-1 secretion is impaired in longstanding type 2 diabetes[49,50] did not take potential differences in gastric emptying rates into account; furthermore, it has now been shown that in patients with type 2 diabetes managed by diet or metformin only, the GLP-1 response to an intraduodenal glucose challenge is apparently normal[46]. That GLP-1 secretion is intact in type 2 diabetes adds to the rationale for using a nutritional approach to enhance the secretion of endogenous GLP-1. Moreover, gastric emptying and appetite are inhibited by gut hormones other than the incretins, including cholecystokinin (CCK) and peptide YY (PYY)[51-53]. Stimulation of these hormones by nutritional supplements could also be beneficial in reducing postprandial glycaemia.
ANTROPYLORODUODENAL MOTILITY
Interactions between nutrients and the small intestine can induce feedback on gut function to suppress antral motility and stimulate pyloric contractions, with resultant slowing of gastric emptying[54]. In both healthy young and older humans, intraduodenal delivery of whey suppresses antral and duodenal waves and increases isolated pyloric pressure waves. Such changes in antropyloric motility in response to nutrient ingestion also appear to be independently related to subsequent energy intake in healthy young subjects[55]. Soenen et al[56] examined the effects of intraduodenal whey protein infusion on appetite and subsequent ad libitum energy intake in relation to antropyloroduodenal motility. They reported that energy intake at a buffet meal was inversely related to the number of isolated pyloric pressure waves, and positively related to the number of antral pressure waves, supporting a relationship between antropyloroduodenal motor activity and feeding behaviour.
POTENTIAL IMPACT OF WHEY ON DIPEPTIDYL PEPTIDASE-IV
The incretin hormones are rapidly degraded to inactive metabolites by dipeptidyl peptidase-IV (DPP-IV). More than 50% of the GLP-1 newly secreted from intestinal L cells is degraded before reaching the systemic circulation[57], mainly by DPP-IV present in the endothelium of the capillary bed in close proximity to the L cells[36,57]. Whey hydrolysates, produced using digestive enzymes such as pepsin and trypsin, have been found to inhibit the activity of DPP-IV in vitro[58-61]. For rodents in vivo, ingestion of whey protein can reduce DPP-IV activity in the proximal small bowel, thereby increasing intact incretin hormone concentrations[62]. Further in vivo studies, particularly in humans, are required to confirm this phenomenon, and establish its durability with long term ingestion of whey[63].
EFFECTS OF WHEY ON ALPHA-GLUCOSIDASE
Alpha glucosidase is an enzyme that hydrolyzes starch and disaccharides to enable absorption of glucose at the small intestinal brush border. In vitro studies have shown that whey protein hydrolysate has a modest effect to inhibit alpha-glucosidase[59], which may be clinically relevant given that alpha-glucosidase inhibitors, such as acarbose, are used widely in the management of type 2 diabetes to improve postprandial glycaemia. Human studies are required to further evaluate this mechanism and the magnitude of the glucose lowering effect attributable to it.
TIMING OF WHEY PROTEIN, “PRELOADS”, AND GASTRIC EMPTYING
The concept of a “preload” refers to administration of a small load of macronutrient at a fixed interval before a meal, so that the presence of nutrients in the small intestine induces the release of GLP-1 and GIP, and other gut peptides such as CCK and PYY, to slow gastric emptying and stimulate insulin secretion in advance of the main nutrient load. In health, whey protein preloads have been shown to slow gastric emptying, as assessed by the plasma concentrations of oral paracetamol given with the meal, and enhance post-prandial GLP-1 levels[64]. Similarly, whey given immediately before a meal, with or without additional amino acids, reduces the postprandial glycaemic response by over a third (iAUC 0-60 min), associated with an increase in the early postprandial plasma insulin and GLP-1 responses[65].
The capacity for a whey preload to stimulate incretin hormone secretion and slow gastric emptying has also been established in subjects with type 2 diabetes[48]. Ma et al[48] reported in type 2 patients that a 55 g whey protein preload, given 30 min before a meal, slows gastric emptying when compared to either a nutrient-free preload or ingestion of whey with the meal. In this study, gastric emptying was quantified using scintigraphy, which represents the “gold standard”. Whey protein markedly reduced postprandial glucose excursions (iAUC after whey preload about half that of control), and stimulated insulin and CCK, as well as GIP and GLP-1. Both the GLP-1 response and the reduction in postprandial glycaemia were greater when whey was given as a preload, when compared to ingestion with the meal. Accordingly, this study not only established that whey can slow gastric emptying substantially in type 2 diabetes, but that the timing of supplementation is pivotal to the stimulation of incretins and other gut hormones. These acute effects of whey preloads to improve postprandial glycaemia were recently confirmed in another study in type 2 patients[66]. While whey has been shown to slow gastric emptying acutely, it remains to be seen whether this effect is sustained with long term administration.
AMINO ACIDS AS A STIMULUS FOR INSULIN SECRETION
It has been established for many years that ingested protein stimulates insulin secretion[47,67], an effect observed in both healthy subjects and in those with type 2 diabetes. This effect is enhanced when protein is co-ingested with carbohydrates when compared with the ingestion of carbohydrate or protein alone, suggesting a synergy between oral protein and glucose[68-72]. In a recent comparison of four protein sources, the greatest postprandial insulin response was associated with whey compared to casein, gluten or cod, and was attributed to the more rapid appearance of amino acids in plasma when derived from whey[21].
Whey protein is a rich source of essential amino acids and branched chain amino acids known to have potent insulinotropic properties[73]. The branched chain amino acids - leucine, valine, and isoleucine - are more insulinogenic than other amino acids[40,74]. In the 1960s, Floyd et al[67,75,76] showed that amino acids, given either intravenously or orally, had the capacity to stimulate insulin secretion and reduce blood glucose concentrations. The insulinotropic effect of whey, at least in part, reflects a direct effect of amino acids to stimulate beta cells[35,77-80]; the underlying mechanisms are complex and involve mitochondrial metabolism[77].
Amino acids can stimulate insulin secretion in type 2 diabetes as well as in health. van Loon et al[81] reported that patients with long standing type 2 diabetes who co-ingested an amino acid/protein mixture (wheat protein hydrolysate) with a carbohydrate meal almost trebled their insulin response, when compared to ingestion of carbohydrate alone. This preserved stimulation of insulin by amino acids in type 2 diabetes contrasts with the diminished insulin response to carbohydrates, when compared with healthy controls. Similarly, addition of casein to carbohydrate has also been noted to potentiate insulin secretion in longstanding type 2 diabetes. That amino acids derived from ingested proteins remain a strong stimulus for insulin secretion, even in patients with long standing type 2 diabetes, supports their potential efficacy in the management of this condition[68].
ROLE OF GLUCAGON
Glucagon, secreted from the alpha cells of the pancreas, primarily acts on the liver to initiate glycogenolysis and gluconeogenesis, which then increases endogenous glucose production. Glucagon secretion is exaggerated in response to a meal in patients with type 2 diabetes[82], and ingested protein results in an increase in plasma glucagon levels[83]. It might therefore be expected that protein ingestion would increase blood glucose concentrations, but this is not necessarily the case.
Calbet et al[84] gave 6 healthy adults four tests meals containing glucose, cow’s milk solution, pea and whey peptide hydrolysates, and found that the glucagon response was linearly related to the increase in plasma amino acids. Despite this, plasma glucose levels after whey hydrolysates decreased by about 1.5 mmol/L from baseline to 180 min, most likely due to the effects of insulin, which is stimulated concurrently and is particularly effective at suppressing glycogenolysis.
IS WHEY PROTEIN EFFECTIVE IN REDUCING POSTPRANDIAL GLYCAEMIA IN TYPE 2 DIABETES?
Although it is clear that whey has an insulinotropic effect, it is less clear as to whether the magnitude of insulin stimulation is sufficient to reduce postprandial glycaemia in patients with type 2 diabetes, who tend to be insulin-resistant, and often exhibit hyperinsulinaemia[40,85-87]. Insulin sensitivity, assessed using a euglycaemic-hyperinsulinaemic clamp, impacts on the capacity for acute administration of protein to reduce blood glucose concentrations in healthy subjects[88], and this may explain why some studies of patients with type 2 diabetes reported no reduction in blood glucose despite stimulation of insulin after a protein meal[38,89].
Frid et al[39] evaluated the effect of adding whey protein to high glycaemic index meals taken at breakfast and lunch in patients with type 2 diabetes. Plasma insulin responses were higher after both breakfast (31%) and lunch (57%) with whey (27.6 g) when compared to lean ham or lactose. There was a reduction in blood glucose excursions after lunch but not breakfast, which might be related to either the differing meal content, or to higher insulin resistance seen in the fasting state[90] affecting responses after breakfast.
Conversely, other studies in type 2 diabetes have reported up to 3 or 4 fold increases in insulin responses to meals containing protein and carbohydrate, when compared to carbohydrate alone, with concomitant reductions in postprandial glycaemia[71,91]. Nuttall et al[70] evaluated nine male subjects with diet controlled type 2 diabetes and showed that the blood glucose response (AUC) to protein and glucose ingestion was one third lower than after glucose alone, and the mean insulin AUC was also considerably greater. While these studies used beef or casein, whey is also effective for both stimulating insulin secretion and reducing postprandial glycaemia in individuals with type 2 diabetes and/or insulin resistance[48,92].
IS THE DOSE OF WHEY IMPORTANT?
When assessing the magnitude of glycaemic responses after whey protein consumption, one should consider not only the timing of ingestion (e.g., whether giving as a preload), but also the dose, since the effects of whey on glycaemic responses, as well as appetite, appear to be dose-dependent[19,93]. Preloads of whey concentrate in doses of 5 g, 10 g, 20 g, and 40 g, and control, were given to 22 healthy individuals, followed 30 min later by a standardised pizza meal; the 20 g and 40 g whey preloads suppressed appetite more than control, or 5 g or 10 g whey protein, as assessed by visual analogue questionnaires[93]. In addition, whey protein reduced postprandial glucose in a dose-dependent manner. Poppit et al[94] gave 50 overweight women drinks containing 5 g, 10 g or 20 g whey, or control, 120 min after a standardized breakfast, and found that there was a tendency for hunger and fullness to be dose-related, although this did not reach statistical significance.
In healthy volunteers, whey protein taken with a meal increases insulin and reduces postprandial glycaemia in a dose-dependent manner[87]. Gunnerud et al[87] found that a drink containing 25 g glucose and either 4.5 g, 9 g or 18 g whey protein, reduced postprandial glycaemia (iAUC) by 25%, 37% and 46% respectively, compared to a 25 g glucose alone; the reductions with 9 g and 18 g whey were statistically significant. There was also a dose-dependent increase in insulin (iAUC 0 – 120 min), which reached statistical significance with the highest dose of whey.
While whey has convincing dose-dependent effects on glucose, insulin and appetite, the optimal dose for improving long-term glycaemic control in people with type 2 diabetes is yet to be determined.
WHEY AND APPETITE REGULATION
Reduction in energy expenditure and appetite may be achieved through manipulation of dietary macronutrient composition[95]. Protein has been shown to be more satiating than other macronutrients such as carbohydrate and fat[16,96], and has also been reported to increase satiety[97-99]. Whey protein, in particular, has been shown to enhance satiety and reduce food intake at the next meal in acute studies[93,100], and this effect is thought to be mediated by gut hormones[17,101], specifically by stimulation of CCK, PYY and GLP-1, and by suppression of the orexigenic hormone, ghrelin[16].
Bowen et al[95] reported prolonged postprandial suppression of ghrelin, and elevation of GLP-1 and CCK, after consumption of whey, gluten and soy based preloads compared with glucose, and this was associated with reduction of energy intake at an ad libitum meal. CCK is typically associated with satiation; however, in this study there was a trend for an inverse relationship between CCK and subsequent energy intake, which suggests that CCK can also contribute to satiety. Similarly, in a study where hunger scores were reduced after whey ingestion compared to casein, the CCK and GLP-1 responses were higher following whey, which may have contributed to its greater satiating effect[17]. Other studies have reported that PYY concentrations are higher after whey compared with other proteins, but with comparable CCK and ghrelin responses[64].
DIRECT EFFECTS OF AMINO ACIDS ON HUNGER
Elevation in plasma concentrations of amino acids after ingestion of whey may affect appetite[102,103] by hitherto poorly defined mechanisms, including vagal feedback and direct suppression of hunger at the level of the hypothalamus[104]. The greater suppression of hunger by whey, when compared to soy or casein, is associated with increased concentrations of the amino acids leucine, lysine, tryptophan, isoleucine, and threonine[105]. Furthermore, tryptophan is synthesised into serotonin, which itself is known to influence food intake[103,106].
EFFECT OF WHEY ON ENERGY EXPENDITURE
Energy expenditure from thermogenesis, which increases oxygen consumption and body temperature, is thought to induce feelings of satiety[107]. Of the macronutrients, dietary protein stimulates thermogenesis and satiety more than carbohydrate or fat[103]. Acheson et al[108] reported that whey protein elicits a greater thermic response than protein composed of either casein or soy, where protein accounted for 50% of the energy content of the meal. This may be because whey protein, as a “fast” protein, is rapidly digested to result in greater postprandial protein synthesis[18]. In particular, leucine, which is present in high concentrations in whey[109], has been shown to stimulate muscle protein synthesis[110] and may also increase postprandial energy expenditure[109].
EFFECTS OF LONG TERM CONSUMPTION OF WHEY PROTEIN ON GLYCAEMIC CONTROL
High protein diets induce weight loss and preserve lean mass[111]. However, there is a paucity of data relating to whether whey has the capacity to reduce glycated haemoglobin with ongoing treatment in patients with type 2 diabetes.
A 5-wk study in 8 men with type 2 diabetes showed that a diet containing 30% vs 15% of total energy derived from protein, with a corresponding decrease in carbohydrate content, was associated with a greater (by about 0.5%) decrease in glycated haemoglobin[112]. In another study, 72 non-diabetic obese men were randomised to receive supplements of either whey protein isolate, casein, or glucose (each 54 g/d), 30 min before breakfast and the evening meal for 12 wk. Improvements in fasting insulin and homeostasis model assessment of insulin resistance score of almost 10% were observed with whey compared to control, but there was no difference in the fasting serum glucose[113].
In considering the use of whey protein in the management of diabetes, it is also important to recognise the potential adverse effects of longer term supplementation. There have been concerns that high protein diets could potentially reduce bone density and impair renal function. However, a recent two year weight loss study in postmenopausal women found no clinically significant effect of a high protein diet on bone density[114]; nor was there any reduction in renal function in a one year weight loss study in patients with type 2 diabetes with microalbuminuria, assigned to a high protein diet (≥ 90 g protein/d)[111,115].
The effects of additional energy intake associated with protein supplements should also be considered if using this strategy over the long term. Subjects tend to compensate for the additional energy load by eating less at a subsequent ad libitum meal in acute and short term (5 d) studies[116,117]. This is supported by a 12-wk study in which overweight men received 54 g whey supplements per day, but showed no change in body composition[113]. Age may be an important determinant of this effect, however; Soenen et al[56] observed that older men (aged 68 to 81 years), had less capacity to compensate for the additional energy intake associated with whey administration when compared to young men.
Whey’s ability to slow gastric emptying is one of the main mechanisms by which postprandial glycaemia is reduced acutely after a meal. However, it is unknown whether the capacity for whey to slow gastric emptying is sustained with prolonged exposure, or whether there is an adaption to this macronutrient of the gut feedback mechanisms that control gastric emptying, as has been demonstrated for carbohydrates and fats[118]. It would therefore be important to establish whether slowing of gastric emptying induced by whey is sustained with prolonged exposure; this appears to be the case over four weeks in a small pilot study[119].
CONCLUSION
The acute effects of whey protein on postprandial glycaemic excursions appear promising, but the long term efficacy and optimal application in the management of type 2 diabetes remain to be determined.
Patients most likely to benefit from postprandial glucose lowering by whey protein are those with mild to moderate elevation of HbA1c, who have relatively well controlled fasting glucose, since this is the group of patients in whom postprandial glycaemia makes the greatest relative contribution to HbA1c. However, combining a dietary strategy with pharmacological agents in less well controlled patients should also be evaluated, such as the combination of insulin to control fasting glucose, together with whey protein to reduce postprandial glycaemia; such a concept has proven to be effective with the combination of basal insulin and short-acting GLP-1 receptor agonists[120]. Moreover, the combination of whey protein with a DPP-IV inhibitor should also be examined, given the potential to augment the stimulation of GLP-1[121].
The timing of protein ingestion is important when aiming to stimulate incretin secretion and suppress appetite in advance of the main meal[48], and this, together with the optimal dose of whey protein, requires further refinement.
Footnotes
Supported by Royal Adelaide Hospital Dawes Scholarship (Mignone LE), Royal Adelaide Hospital Research Committee Early Career Fellowship (Wu T), and National Health and Medical Research Council funding (No. APP1066835).
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 17, 2015
First decision: July 27, 2015
Article in press: October 19, 2015
P- Reviewer: Luo ZC, Ozdemir S S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
References
- 1.Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care. 2011;34:2508–2514. doi: 10.2337/dc11-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of hba(1c) Diabetes Care. 2003;26:881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 3.Monnier L. Is postprandial glucose a neglected cardiovascular risk factor in type 2 diabetes? Eur J Clin Invest. 2000;30 Suppl 2:3–11. [PubMed] [Google Scholar]
- 4.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smithers GW. Whey and whey proteins - from ‘gutter to gold’. Int Dairy J. 2008;18:695–704. [Google Scholar]
- 6.Sharma S, Singh R, Rana S. Bioactive peptides: A review. Int J BIOautomoation. 2011;15:223–250. [Google Scholar]
- 7.Nagpal R, Behare P, Rana R, Kumar A, Kumar M, Arora S, Morotta F, Jain S, Yadav H. Bioactive peptides derived from milk proteins and their health beneficial potentials: an update. Food Funct. 2011;2:18–27. doi: 10.1039/c0fo00016g. [DOI] [PubMed] [Google Scholar]
- 8.Pihlanto-Lappala A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci Technol. 2011;11:347–356. [Google Scholar]
- 9.Philanto A. Whey proteins and peptides: Emerging properties to promote health. NUTRA Foods. 2011;10:29–42. [Google Scholar]
- 10.McGregor RA, Poppitt SD. Milk protein for improved metabolic health: a review of the evidence. Nutr Metab (Lond) 2013;10:46. doi: 10.1186/1743-7075-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madureira AR, Tavares T, Gomes AM, Pintado ME, Malcata FX. Invited review: physiological properties of bioactive peptides obtained from whey proteins. J Dairy Sci. 2010;93:437–455. doi: 10.3168/jds.2009-2566. [DOI] [PubMed] [Google Scholar]
- 12.van Meijl LE, Vrolix R, Mensink RP. Dairy product consumption and the metabolic syndrome. Nutr Res Rev. 2008;21:148–157. doi: 10.1017/S0954422408116997. [DOI] [PubMed] [Google Scholar]
- 13.de Wit JN. Marschall Rhône-Poulenc Award Lecture. Nutritional and functional characteristics of whey proteins in food products. J Dairy Sci. 1998;81:597–608. doi: 10.3168/jds.s0022-0302(98)75613-9. [DOI] [PubMed] [Google Scholar]
- 14.Krissansen GW. Emerging health properties of whey proteins and their clinical implications. J Am Coll Nutr. 2007;26:713S–723S. doi: 10.1080/07315724.2007.10719652. [DOI] [PubMed] [Google Scholar]
- 15.Marshall K. Therapeutic applications of whey protein. Altern Med Rev. 2004;9:136–156. [PubMed] [Google Scholar]
- 16.Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C, Astrup A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials. Adv Nutr. 2013;4:418–438. doi: 10.3945/an.113.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89:239–248. doi: 10.1079/BJN2002760. [DOI] [PubMed] [Google Scholar]
- 18.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen BL, Ward LS, Bastian ED, Jenkins AL, Campbell J, Vuksan V. A whey protein supplement decreases post-prandial glycemia. Nutr J. 2009;8:47. doi: 10.1186/1475-2891-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahé S, Roos N, Benamouzig R, Davin L, Luengo C, Gagnon L, Gaussergès N, Rautureau J, Tomé D. Gastrojejunal kinetics and the digestion of [15N]beta-lactoglobulin and casein in humans: the influence of the nature and quantity of the protein. Am J Clin Nutr. 1996;63:546–552. doi: 10.1093/ajcn/63.4.546. [DOI] [PubMed] [Google Scholar]
- 21.Stanstrup J, Schou SS, Holmer-Jensen J, Hermansen K, Dragsted LO. Whey protein delays gastric emptying and suppresses plasma fatty acids and their metabolites compared to casein, gluten, and fish protein. J Proteome Res. 2014;13:2396–2408. doi: 10.1021/pr401214w. [DOI] [PubMed] [Google Scholar]
- 22.Walzem RL, Dillard CJ, German JB. Whey components: millennia of evolution create functionalities for mammalian nutrition: what we know and what we may be overlooking. Crit Rev Food Sci Nutr. 2002;42:353–375. doi: 10.1080/10408690290825574. [DOI] [PubMed] [Google Scholar]
- 23.Manninen AH. Protein hydrolysates in sports and exercise: a brief review. J Sports Sci Med. 2004;3:60–63. [PMC free article] [PubMed] [Google Scholar]
- 24.Baró L, Guadix EM, Martinez-Augustin O, Boza JJ, Gil A. Serum amino acid concentrations in growing rats fed intact protein versus enzymatic protein hydrolysate-based diets. Biol Neonate. 1995;68:55–61. doi: 10.1159/000244218. [DOI] [PubMed] [Google Scholar]
- 25.Boza JJ, Martínez-Augustin O, Baró L, Suarez MD, Gil A. Protein v. enzymic protein hydrolysates. Nitrogen utilization in starved rats. Br J Nutr. 1995;73:65–71. [PubMed] [Google Scholar]
- 26.Calbet JA, Holst JJ. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr. 2004;43:127–139. doi: 10.1007/s00394-004-0448-4. [DOI] [PubMed] [Google Scholar]
- 27.Power O, Hallihan A, Jakeman P. Human insulinotropic response to oral ingestion of native and hydrolysed whey protein. Amino Acids. 2009;37:333–339. doi: 10.1007/s00726-008-0156-0. [DOI] [PubMed] [Google Scholar]
- 28.Mortensen LS, Holmer-Jensen J, Hartvigsen ML, Jensen VK, Astrup A, de Vrese M, Holst JJ, Thomsen C, Hermansen K. Effects of different fractions of whey protein on postprandial lipid and hormone responses in type 2 diabetes. Eur J Clin Nutr. 2012;66:799–805. doi: 10.1038/ejcn.2012.48. [DOI] [PubMed] [Google Scholar]
- 29.Claessens M, Calame W, Siemensma AD, van Baak MA, Saris WH. The effect of different protein hydrolysate/carbohydrate mixtures on postprandial glucagon and insulin responses in healthy subjects. Eur J Clin Nutr. 2009;63:48–56. doi: 10.1038/sj.ejcn.1602896. [DOI] [PubMed] [Google Scholar]
- 30.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–E206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 32.Gannon MC, Nuttall FQ, Neil BJ, Westphal SA. The insulin and glucose responses to meals of glucose plus various proteins in type II diabetic subjects. Metabolism. 1988;37:1081–1088. doi: 10.1016/0026-0495(88)90072-8. [DOI] [PubMed] [Google Scholar]
- 33.Fieseler P, Bridenbaugh S, Nustede R, Martell J, Orskov C, Holst JJ, Nauck MA. Physiological augmentation of amino acid-induced insulin secretion by GIP and GLP-I but not by CCK-8. Am J Physiol. 1995;268:E949–E955. doi: 10.1152/ajpendo.1995.268.5.E949. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson M, Holst JJ, Björck IM. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr. 2007;85:996–1004. doi: 10.1093/ajcn/85.4.996. [DOI] [PubMed] [Google Scholar]
- 35.Salehi A, Gunnerud U, Muhammed SJ, Ostman E, Holst JJ, Björck I, Rorsman P. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on β-cells. Nutr Metab (Lond) 2012;9:48. doi: 10.1186/1743-7075-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 37.Wu T, Rayner CK, Jones K, Horowitz M. Dietary effects on incretin hormone secretion. Vitam Horm. 2010;84:81–110. doi: 10.1016/B978-0-12-381517-0.00003-5. [DOI] [PubMed] [Google Scholar]
- 38.Simpson RW, McDonald J, Wahlqvist ML, Atley L, Outch K. Macronutrients have different metabolic effects in nondiabetics and diabetics. Am J Clin Nutr. 1985;42:449–453. doi: 10.1093/ajcn/42.3.449. [DOI] [PubMed] [Google Scholar]
- 39.Frid AH, Nilsson M, Holst JJ, Björck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr. 2005;82:69–75. doi: 10.1093/ajcn.82.1.69. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson M, Stenberg M, Frid AH, Holst JJ, Björck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–1253. doi: 10.1093/ajcn/80.5.1246. [DOI] [PubMed] [Google Scholar]
- 41.Bowen J, Noakes M, Clifton PM. Appetite hormones and energy intake in obese men after consumption of fructose, glucose and whey protein beverages. Int J Obes (Lond) 2007;31:1696–1703. doi: 10.1038/sj.ijo.0803665. [DOI] [PubMed] [Google Scholar]
- 42.Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36:1396–1405. doi: 10.2337/dc12-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993;36:857–862. doi: 10.1007/BF00400362. [DOI] [PubMed] [Google Scholar]
- 44.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 45.Kojecky V, Bernatek J, Horowitz M, Zemek S, Bakala J, Hep A. Prevalence and determinants of delayed gastric emptying in hospitalised Type 2 diabetic patients. World J Gastroenterol. 2008;14:1564–1569. doi: 10.3748/wjg.14.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med. 1996;37:1643–1648. [PubMed] [Google Scholar]
- 47.Karamanlis A, Chaikomin R, Doran S, Bellon M, Bartholomeusz FD, Wishart JM, Jones KL, Horowitz M, Rayner CK. Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am J Clin Nutr. 2007;86:1364–1368. doi: 10.1093/ajcn/86.5.1364. [DOI] [PubMed] [Google Scholar]
- 48.Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, Clifton PM, Horowitz M, Rayner CK. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32:1600–1602. doi: 10.2337/dc09-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–613. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 50.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen NQ, Fraser RJ, Bryant LK, Chapman MJ, Wishart J, Holloway RH, Butler R, Horowitz M. The relationship between gastric emptying, plasma cholecystokinin, and peptide YY in critically ill patients. Crit Care. 2007;11:R132. doi: 10.1186/cc6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamagishi T, Debas HT. Cholecystokinin inhibits gastric emptying by acting on both proximal stomach and pylorus. Am J Physiol. 1978;234:E375–E378. doi: 10.1152/ajpendo.1978.234.4.E375. [DOI] [PubMed] [Google Scholar]
- 53.Allen JM, Fitzpatrick ML, Yeats JC, Darcy K, Adrian TE, Bloom SR. Effects of peptide YY and neuropeptide Y on gastric emptying in man. Digestion. 1984;30:255–262. doi: 10.1159/000199117. [DOI] [PubMed] [Google Scholar]
- 54.Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11:112–128. doi: 10.1038/nrendo.2014.202. [DOI] [PubMed] [Google Scholar]
- 55.Seimon RV, Lange K, Little TJ, Brennan IM, Pilichiewicz AN, Feltrin KL, Smeets AJ, Horowitz M, Feinle-Bisset C. Pooled-data analysis identifies pyloric pressures and plasma cholecystokinin concentrations as major determinants of acute energy intake in healthy, lean men. Am J Clin Nutr. 2010;92:61–68. doi: 10.3945/ajcn.2009.29015. [DOI] [PubMed] [Google Scholar]
- 56.Soenen S, Giezenaar C, Hutchison AT, Horowitz M, Chapman I, Luscombe-Marsh ND. Effects of intraduodenal protein on appetite, energy intake, and antropyloroduodenal motility in healthy older compared with young men in a randomized trial. Am J Clin Nutr. 2014;100:1108–1115. doi: 10.3945/ajcn.114.087981. [DOI] [PubMed] [Google Scholar]
- 57.Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–5363. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- 58.Tulipano G, Sibilia V, Caroli AM, Cocchi D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides. 2011;32:835–838. doi: 10.1016/j.peptides.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Lacroix IM, Li-Chan EC. Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin-treated whey proteins. J Agric Food Chem. 2013;61:7500–7506. doi: 10.1021/jf401000s. [DOI] [PubMed] [Google Scholar]
- 60.Lacroix IM, Li-Chan EC. Isolation and characterization of peptides with dipeptidyl peptidase-IV inhibitory activity from pepsin-treated bovine whey proteins. Peptides. 2014;54:39–48. doi: 10.1016/j.peptides.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Nongonierma AB, FitzGerald RJ. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides. 2013;39:157–163. doi: 10.1016/j.peptides.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Gunnarsson PT, Winzell MS, Deacon CF, Larsen MO, Jelic K, Carr RD, Ahrén B. Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology. 2006;147:3173–3180. doi: 10.1210/en.2005-1442. [DOI] [PubMed] [Google Scholar]
- 63.Drucker DJ. Enhancing the action of incretin hormones: a new whey forward? Endocrinology. 2006;147:3171–3172. doi: 10.1210/en.2006-0494. [DOI] [PubMed] [Google Scholar]
- 64.Akhavan T, Luhovyy BL, Panahi S, Kubant R, Brown PH, Anderson GH. Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J Nutr Biochem. 2014;25:36–43. doi: 10.1016/j.jnutbio.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Gunnerud UJ, Heinzle C, Holst JJ, Östman EM, Björck IM. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS One. 2012;7:e44731. doi: 10.1371/journal.pone.0044731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jakubowicz D, Froy O, Ahrén B, Boaz M, Landau Z, Bar-Dayan Y, Ganz T, Barnea M, Wainstein J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: a randomised clinical trial. Diabetologia. 2014;57:1807–1811. doi: 10.1007/s00125-014-3305-x. [DOI] [PubMed] [Google Scholar]
- 67.Floyd JC, Fajans SS, Conn JW, Knopf RF, Rull J. Insulin secretion in response to protein ingestion. J Clin Invest. 1966;45:1479–1486. doi: 10.1172/JCI105455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manders RJ, Hansen D, Zorenc AH, Dendale P, Kloek J, Saris WH, van Loon LJ. Protein co-ingestion strongly increases postprandial insulin secretion in type 2 diabetes patients. J Med Food. 2014;17:758–763. doi: 10.1089/jmf.2012.0294. [DOI] [PubMed] [Google Scholar]
- 69.Pallotta JA, Kennedy PJ. Response of plasma insulin and growth hormone to carbohydrate and protein feeding. Metabolism. 1968;17:901–908. doi: 10.1016/0026-0495(68)90156-x. [DOI] [PubMed] [Google Scholar]
- 70.Nuttall FQ, Mooradian AD, Gannon MC, Billington C, Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care. 1984;7:465–470. doi: 10.2337/diacare.7.5.465. [DOI] [PubMed] [Google Scholar]
- 71.Manders RJ, Wagenmakers AJ, Koopman R, Zorenc AH, Menheere PP, Schaper NC, Saris WH, van Loon LJ. Co-ingestion of a protein hydrolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am J Clin Nutr. 2005;82:76–83. doi: 10.1093/ajcn.82.1.76. [DOI] [PubMed] [Google Scholar]
- 72.Rabinowitz D, Merimee TJ, Maffezzoli R, Burgess JA. Patterns of hormonal release after glucose, protein, and glucose plus protein. Lancet. 1966;2:454–456. doi: 10.1016/s0140-6736(66)92767-x. [DOI] [PubMed] [Google Scholar]
- 73.Holmer-Jensen J, Hartvigsen ML, Mortensen LS, Astrup A, de Vrese M, Holst JJ, Thomsen C, Hermansen K. Acute differential effects of milk-derived dietary proteins on postprandial lipaemia in obese non-diabetic subjects. Eur J Clin Nutr. 2012;66:32–38. doi: 10.1038/ejcn.2011.142. [DOI] [PubMed] [Google Scholar]
- 74.van Loon LJ. Leucine as a pharmaconutrient in health and disease. Curr Opin Clin Nutr Metab Care. 2012;15:71–77. doi: 10.1097/MCO.0b013e32834d617a. [DOI] [PubMed] [Google Scholar]
- 75.Fajans SS, Knopf RF, Floyd JC, Power L, Conn JW. The experimental induction in man of sensitivity to leucine hypoglycemia. J Clin Invest. 1963;42:216–229. doi: 10.1172/JCI104708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Floyd JC, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45:1487–1502. doi: 10.1172/JCI105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Newsholme P, Brennan L, Rubi B, Maechler P. New insights into amino acid metabolism, beta-cell function and diabetes. Clin Sci (Lond) 2005;108:185–194. doi: 10.1042/CS20040290. [DOI] [PubMed] [Google Scholar]
- 78.van Loon LJ, Saris WH, Verhagen H, Wagenmakers AJ. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am J Clin Nutr. 2000;72:96–105. doi: 10.1093/ajcn/72.1.96. [DOI] [PubMed] [Google Scholar]
- 79.Blachier F, Mourtada A, Sener A, Malaisse WJ. Stimulus-secretion coupling of arginine-induced insulin release. Uptake of metabolized and nonmetabolized cationic amino acids by pancreatic islets. Endocrinology. 1989;124:134–141. doi: 10.1210/endo-124-1-134. [DOI] [PubMed] [Google Scholar]
- 80.Sener A, Blachier F, Rasschaert J, Mourtada A, Malaisse-Lagae F, Malaisse WJ. Stimulus-secretion coupling of arginine-induced insulin release: comparison with lysine-induced insulin secretion. Endocrinology. 1989;124:2558–2567. doi: 10.1210/endo-124-5-2558. [DOI] [PubMed] [Google Scholar]
- 81.van Loon LJ, Kruijshoop M, Menheere PP, Wagenmakers AJ, Saris WH, Keizer HA. Amino acid ingestion strongly enhances insulin secretion in patients with long-term type 2 diabetes. Diabetes Care. 2003;26:625–630. doi: 10.2337/diacare.26.3.625. [DOI] [PubMed] [Google Scholar]
- 82.Young A. Inhibition of glucagon secretion. Adv Pharmacol. 2005;52:151–171. doi: 10.1016/S1054-3589(05)52008-8. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed M, Nuttall FQ, Gannon MC, Lamusga RF. Plasma glucagon and alpha-amino acid nitrogen response to various diets in normal humans. Am J Clin Nutr. 1980;33:1917–1924. doi: 10.1093/ajcn/33.9.1917. [DOI] [PubMed] [Google Scholar]
- 84.Calbet JA, MacLean DA. Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans. J Nutr. 2002;132:2174–2182. doi: 10.1093/jn/132.8.2174. [DOI] [PubMed] [Google Scholar]
- 85.Wildová E, Dlouhý P, Kraml P, Rambousková J, Smejkalová V, Potočková J, Anděl M. Orally administered whey proteins have comparable effect on C-peptide secretion in healthy subjects as standard C-peptide stimulation tests. Physiol Res. 2013;62:179–186. doi: 10.33549/physiolres.932462. [DOI] [PubMed] [Google Scholar]
- 86.Gunnerud U, Holst JJ, Östman E, Björck I. The glycemic, insulinemic and plasma amino acid responses to equi-carbohydrate milk meals, a pilot- study of bovine and human milk. Nutr J. 2012;11:83. doi: 10.1186/1475-2891-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gunnerud UJ, Ostman EM, Björck IM. Effects of whey proteins on glycaemia and insulinaemia to an oral glucose load in healthy adults; a dose-response study. Eur J Clin Nutr. 2013;67:749–753. doi: 10.1038/ejcn.2013.88. [DOI] [PubMed] [Google Scholar]
- 88.Brand-Miller JC, Colagiuri S, Gan ST. Insulin sensitivity predicts glycemia after a protein load. Metabolism. 2000;49:1–5. doi: 10.1016/s0026-0495(00)90488-8. [DOI] [PubMed] [Google Scholar]
- 89.Tessari P, Kiwanuka E, Cristini M, Zaramella M, Enslen M, Zurlo C, Garcia-Rodenas C. Slow versus fast proteins in the stimulation of beta-cell response and the activation of the entero-insular axis in type 2 diabetes. Diabetes Metab Res Rev. 2007;23:378–385. doi: 10.1002/dmrr.698. [DOI] [PubMed] [Google Scholar]
- 90.Plat L, Byrne MM, Sturis J, Polonsky KS, Mockel J, Féry F, Van Cauter E. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am J Physiol. 1996;270:E36–E42. doi: 10.1152/ajpendo.1996.270.1.E36. [DOI] [PubMed] [Google Scholar]
- 91.Manders RJ, Koopman R, Sluijsmans WE, van den Berg R, Verbeek K, Saris WH, Wagenmakers AJ, van Loon LJ. Co-ingestion of a protein hydrolysate with or without additional leucine effectively reduces postprandial blood glucose excursions in Type 2 diabetic men. J Nutr. 2006;136:1294–1299. doi: 10.1093/jn/136.5.1294. [DOI] [PubMed] [Google Scholar]
- 92.Mortensen LS, Hartvigsen ML, Brader LJ, Astrup A, Schrezenmeir J, Holst JJ, Thomsen C, Hermansen K. Differential effects of protein quality on postprandial lipemia in response to a fat-rich meal in type 2 diabetes: comparison of whey, casein, gluten, and cod protein. Am J Clin Nutr. 2009;90:41–48. doi: 10.3945/ajcn.2008.27281. [DOI] [PubMed] [Google Scholar]
- 93.Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr. 2010;91:966–975. doi: 10.3945/ajcn.2009.28406. [DOI] [PubMed] [Google Scholar]
- 94.Poppitt SD, Proctor J, McGill AT, Wiessing KR, Falk S, Xin L, Budgett SC, Darragh A, Hall RS. Low-dose whey protein-enriched water beverages alter satiety in a study of overweight women. Appetite. 2011;56:456–464. doi: 10.1016/j.appet.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 95.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91:2913–2919. doi: 10.1210/jc.2006-0609. [DOI] [PubMed] [Google Scholar]
- 96.Clifton PM, Keogh J. Metabolic effects of high-protein diets. Curr Atheroscler Rep. 2007;9:472–478. doi: 10.1007/s11883-007-0063-y. [DOI] [PubMed] [Google Scholar]
- 97.Porrini M, Crovetti R, Testolin G, Silva S. Evaluation of satiety sensations and food intake after different preloads. Appetite. 1995;25:17–30. doi: 10.1006/appe.1995.0038. [DOI] [PubMed] [Google Scholar]
- 98.Poppitt SD, McCormack D, Buffenstein R. Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol Behav. 1998;64:279–285. doi: 10.1016/s0031-9384(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 99.Latner JD, Schwartz M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite. 1999;33:119–128. doi: 10.1006/appe.1999.0237. [DOI] [PubMed] [Google Scholar]
- 100.Zafar TA, Waslien C, AlRaefaei A, Alrashidi N, AlMahmoud E. Whey protein sweetened beverages reduce glycemic and appetite responses and food intake in young females. Nutr Res. 2013;33:303–310. doi: 10.1016/j.nutres.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 101.Luhovyy BL, Akhavan T, Anderson GH. Whey proteins in the regulation of food intake and satiety. J Am Coll Nutr. 2007;26:704S–712S. doi: 10.1080/07315724.2007.10719651. [DOI] [PubMed] [Google Scholar]
- 102.Mellinkoff SM, Frankland M, Boyle D, Greipel M. Relationship between serum amino acid concentration and fluctuations in appetite. J Appl Physiol. 1956;8:535–538. doi: 10.1152/jappl.1956.8.5.535. [DOI] [PubMed] [Google Scholar]
- 103.Veldhorst M, Smeets A, Soenen S, Hochstenbach-Waelen A, Hursel R, Diepvens K, Lejeune M, Luscombe-Marsh N, Westerterp-Plantenga M. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008;94:300–307. doi: 10.1016/j.physbeh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Fromentin G, Darcel N, Chaumontet C, Marsset-Baglieri A, Nadkarni N, Tomé D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr Res Rev. 2012;25:29–39. doi: 10.1017/S0954422411000175. [DOI] [PubMed] [Google Scholar]
- 105.Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav. 2009;96:675–682. doi: 10.1016/j.physbeh.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Beulens JW, Bindels JG, de Graaf C, Alles MS, Wouters-Wesseling W. Alpha-lactalbumin combined with a regular diet increases plasma Trp-LNAA ratio. Physiol Behav. 2004;81:585–593. doi: 10.1016/j.physbeh.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 107.Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr. 1999;53:495–502. doi: 10.1038/sj.ejcn.1600782. [DOI] [PubMed] [Google Scholar]
- 108.Acheson KJ, Blondel-Lubrano A, Oguey-Araymon S, Beaumont M, Emady-Azar S, Ammon-Zufferey C, Monnard I, Pinaud S, Nielsen-Moennoz C, Bovetto L. Protein choices targeting thermogenesis and metabolism. Am J Clin Nutr. 2011;93:525–534. doi: 10.3945/ajcn.110.005850. [DOI] [PubMed] [Google Scholar]
- 109.Jakubowicz D, Froy O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J Nutr Biochem. 2013;24:1–5. doi: 10.1016/j.jnutbio.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 110.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136:319S–323S. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- 111.Clifton P. Effects of a high protein diet on body weight and comorbidities associated with obesity. Br J Nutr. 2012;108 Suppl 2:S122–S129. doi: 10.1017/S0007114512002322. [DOI] [PubMed] [Google Scholar]
- 112.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78:734–741. doi: 10.1093/ajcn/78.4.734. [DOI] [PubMed] [Google Scholar]
- 113.Pal S, Ellis V, Dhaliwal S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr. 2010;104:716–723. doi: 10.1017/S0007114510000991. [DOI] [PubMed] [Google Scholar]
- 114.Jesudason D, Nordin BC, Keogh J, Clifton P. Comparison of 2 weight-loss diets of different protein content on bone health: a randomized trial. Am J Clin Nutr. 2013;98:1343–1352. doi: 10.3945/ajcn.113.058586. [DOI] [PubMed] [Google Scholar]
- 115.Jesudason DR, Pedersen E, Clifton PM. Weight-loss diets in people with type 2 diabetes and renal disease: a randomized controlled trial of the effect of different dietary protein amounts. Am J Clin Nutr. 2013;98:494–501. doi: 10.3945/ajcn.113.060889. [DOI] [PubMed] [Google Scholar]
- 116.Bertenshaw EJ, Lluch A, Yeomans MR. Dose-dependent effects of beverage protein content upon short-term intake. Appetite. 2009;52:580–587. doi: 10.1016/j.appet.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 117.Potier M, Fromentin G, Calvez J, Benamouzig R, Martin-Rouas C, Pichon L, Tomé D, Marsset-Baglieri A. A high-protein, moderate-energy, regular cheesy snack is energetically compensated in human subjects. Br J Nutr. 2009;102:625–631. doi: 10.1017/S0007114509236026. [DOI] [PubMed] [Google Scholar]
- 118.Cunningham KM, Daly J, Horowitz M, Read NW. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut. 1991;32:483–486. doi: 10.1136/gut.32.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma J, Jesudason DR, Stevens JE, Keogh JB, Jones KL, Clifton PM, Horowitz M, Rayner CK. Sustained effects of a protein ‘preload’ on glycaemia and gastric emptying over 4 weeks in patients with type 2 diabetes: A randomized clinical trial. Diabetes Res Clin Pract. 2015;108:e31–e34. doi: 10.1016/j.diabres.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 120.Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, Hoogwerf BJ, Rosenstock J. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: A randomized, controlled trial. Ann Intern Med. 2011;154:103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 121.Wu T, Bound MJ, Zhao BR, Standfield SD, Bellon M, Jones KL, Horowitz M, Rayner CK. Effects of a D-xylose preload with or without sitagliptin on gastric emptying, glucagon-like peptide-1, and postprandial glycemia in type 2 diabetes. Diabetes Care. 2013;Jul; 36:1913–1918. doi: 10.2337/dc12-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]


