Abstract
Cutaneous non-disseminated, non-tuberculous mycobacterial infections have been reported in both immunocompetent and immunocompromised subjects. Systemic Mycobacterium avium intracellulaire (MAI) have been reported in non-HIV patients with Idiopathic CD4 lymphocytopenia. We report a comprehensive immunological analysis in syndrome of selective IgM deficiency and T lymphocytopenia (both CD4+ and CD8+) with disseminated cutaneous MAI infection. Naïve (TN) and Central memory (TCM) subsets of both CD4+ and CD8+ T cells were decreased, whereas terminally differentiated effector memory (TEMRA) subset of CD4+ and CD8+ T cells were markedly increased. IFN-γ producing T cells were markedly decreased. Although CD14highCD16- proinflammatory monocytes were modestly increased, IFN-γR+ monocytes were markedly decreased. The expression of TLR3, TLR5, TLR7, and TLR9 on monocytes was decreased. Germinal center B cells (CD19+IgD-CD38+CD27lo) and B1 cells (CD20+CD27+CD43+CD70-) were markedly decreased. A role of immune alterations, including B cells and antibodies in disseminated cutaneous MAI infection is discussed.
Keywords: TLR, memory T cells, B1 cells, germinal center B cells, IFN-γ
Introduction
Non-tuberculous mycobacteria (NTM) were considered saprophytes until acquired immunodeficiency disease was discovered when Mycobacterium avium complex (MAC) species emerged as a major opportunistic infection in patients with HIV infection. The first case of Mycobacterium avium intracellular (MAI) infection of the lung in a non-HIV patient with CD4 lymphocytopenia was described in 1992 [1]. Later in the year, the Center for Disease Control and Disease Prevention coined the termed Idiopathic CD4+ lymphocytopenia (ICL) and defined as CD4+ depletion of < 300/ul or < 20% of the total lymphocytes on two separate times with a minimum of six weeks of time without any secondary causes of immunodeficiency or immunosuppression [2]. Since then, several reviews on ICL have been published [3-7]. We described a syndrome of T cell lymphocytopenia (shared by both CD4+ and CD8+ T cells) and selective IgM deficiency associated with systemic MAI infection [8]. This syndrome is different from ICL and selective IgM deficiency; ICL is not associated with selective IgM deficiency, and selective IgM deficiency is not associated with T cell lymphocytopenia or T cell defect functional defect [9,10]. Cutaneous NTM infections have been reported in both immunocompetent and immunocompromized hosts [11-15]. Although systemic MAI infections have been reported in patients with ICL, and in the syndrome of T cell lymphocytopenia and selective IgM deficiency, disseminated cutaneous MAI infection has not been reported in either conditions. The host immune responses to M. tuberculosis have been studied in detail; however, host immune responses to NTM are not completely understood. A role of macrophages and T cells in immune response to mycobacteria has recently been evaluated [16-18]. Here we present a comprehensive analysis of host immune responses in a patient with a syndrome of T cell lymphocytopenia and selective IgM deficiency with disseminated cutaneous MAI infection. This is the first report of comprehensive B cell subset analysis in mycobacterial infection. A possible role of B cell subsets and antibodies in mycobacterial defense is discussed.
Material and methods
Patient
In October 2012, the patient, a 53 year old man was involved in a motor vehicle accident where he fractured his collarbone. At that time he appreciated a small nodule on his right upper arm that began to grow. As time progressed, more lesions appeared on the medial aspect of upper right arm. A biopsy performed by a dermatologist was nonspecific. He then was referred to us for a second opinion. An immunological analysis and two biopsies were performed. His lesions at that time were two lesions that were 1 cm × 1 cm. He had no lymphadenopathy. The results of his immunological analysis are shown in Table 1, which revealed severe T cell lymphopenia that is shared by CD4+ and CD8+ T cells, selective IgM deficiency, and low NK cell functions. Similar phenotype has been reported in three patients with systemic MAI infection [8]. He was negative for HIV-1 and HIV-2, and delayed type hypersensitivity skin tests to Candida, tetanus toxoid, and PPD were negative. Biopsies were consistent with non-caseating granulomas with culture positive for Mycobacterium avium intracellulaire that was sensitive to ciprofloxacin, rifampin, ethambutol, streptomycin, amikacin, rifabutin, and clarithromycin. He was started on treatment in February 2013 with azithromycin 500 mg 3 times weekly, ethambutol 1500 mg/day and rifampin 600 mg 3 times weekly. Initially, his lesions responded to therapy, which was discontinued after 15 months. However, his lesions began to increase in size and now all four lesions were approximately 1.0 × 2.0 cm in size. He was resumed on same antimycobacterial regimen. However, his lesions continue to increase in size. Another biopsy was performed with culture positive for MAI. Moxifloxacin was added to his regimen. Lesions continued to increase in size. At the National Institutes of Health, he was started on IV amikacin as well as Interferon Gamma dosed at 50 mcg/m2 (1 million international units/m2) subcutaneously three times weekly. Within three months, his two forearm lesions completely resolved and his two proximal lesions markedly reduced in size. Later Amikacin was discontinued because of side effects. Patient continued to receive gamma interferon.
Table 1.
Immunological analysis of the Patient
| Test | Patient | Control (ranges) |
|---|---|---|
| Lymphocyte counts (/3mm) | ||
| Percentage | 13 | 14-44 |
| Absolute counts | 468 | 900-3000 |
| Serum Immunoglobulins (mg/dl) | ||
| IgG | 1,100 | 694-1,618 |
| IgA | 175 | 68-378 |
| IgM | 26 | 65-263 |
| IgE (IU/ml) | 7 | 10-150 |
| Lymphocyte subsets % (#)** | ||
| CD3+ | 9 (42) | 62-84 (619-1847) |
| CD3+CD4+ | 2 (9) | 31-61 (338-1194) |
| CD3+CD8+ | 4 (19) | 10-38 (85-729) |
| CD4/CD8 ratio | 0.47 | 0.9-3.7 |
| CD19+ | 51 (239) | 5-26 (51-473) |
| CD3-CD56+CD16+ | 38 (178) | 1-7 (12-349) |
| Delayed Type skin Hypersensitivity | ||
| Mumps | Negative | Positive |
| Tetanus | Negative | Positive |
| PPD | Negative | Positive* |
| Lymphocyte proliferation (counts per min) | ||
| PHA | 4,509 | 153,754-279,243 |
| ConA | 1,128 | 122,130-382,789 |
| PWM | 14,660 | 147,894-230,054 |
| NK cytotoxicity | ||
| Lytic unit | 4 | 8-40 |
| TB Quantiferon (IU/ml) | 0.01 | > 0.35 |
In subjects exposed to Mycobacterium or BCG vaccinated;
Lymphocyte subsets were performed at least on 4 separate occasions over 2 years period and were similar.
Antibodies and reagents
T cell subsets
CD4 PerCP and CD8 PerCP, CD45RA APC, CCR7 FITC, CD14 FITC, CD16 PE. IFN-γ R-PE all antibodies were from BD Pharmingen (San Jose, CA), and IL-12R PE from R&D Systems (Minneapolis, MN).
TLR expression
Antibodies to TLR2 Alexa647, TLR4 biotin + Streptidine PE (BD Pharmingen, San Jose, California), TLR5 Alexa488 and TLR7 FITC (R&D systems, Minneapolis, MN), TLR3 PE (e-biosciences San Diego, CA) TLR6 PE (Biolegand, San Diego, CA) were used.
B cell subsets
The following anti-human antibodies were uses: CD19 PerCP, CD20 PerCP anti-IgM APC, CD27 FITC, CD38 FITC, anti-IgD PE, CD21 PE, CD70 PE, CD27 APC, CD38 PE, all from BD Pharmingen (San Jose, CA), and CD43 APC from Biolegand (San Diego, CA).
Peripheral blood mononuclear cells (PBMNCs) were isolated from blood of patient and healthy subjects by Ficoll-hypaque density gradient. Protocol was approved by Human Subject Committee of the Institution Review Board of the University of California, Irvine.
Immunophenotyping
Whole blood was diluted with phosphate buffer saline (PBS), washed × 2, and then centrifuged. Cell pellet was diluted with 1 ml of PBS and stained with a panel of antibodies for various subsets of B cells and subsets of CD4+ and CD8+ T cell subsets (see below). After staining, RBC was lysed with 1 × lysing solution (BD Pharmingen, San Jose), washed with PBS, and analyzed. Flow cytometry was performed using FACSCalibur (Becton-Dickenson, San Jose, CA) equipped with argon ion laser emitting at 488 nm (for FITC, PE and PerCP excitation) and a spatially separate diode laser emitting at 631 nm (for APC excitation). Forward and side scatters were used to gate and exclude cellular debris. Ten thousand cells were acquired and analyzed using Flowjo software (Treestar, Ashland, OR). B cell subsets included naïve (CD19+IgM+IgD+CD27-), transitional (CD19+CD38+IgM+), mature (CD19+CD21+), marginal zone (CD19+CD27+IgD+), IgM memory (CD19+IgM+CD27+), class switched memory (CD19+CD27+IgD-), germinal center ((CD19+CD38+CD27lowIgD-), B1 cells (CD20+CD27+CD43+CD70-), and plasmablasts (CD19+CD38+IgM-). Subsets of CD4+ and CD8+ T cells included naïve (CD45RA+CCR7+), central memory (CD45RA-CCR7+), effector memory (CD45RA-CCR7-), and terminally differentiated effector memory/exhausted (CD45RA+CCR7-).
Antibody panel for 4-color B cell subsets phenotype (Table 2)
Table 2.
Antibody panel for 4-color B cell subsets phenotype
| Panel | FITC | PE | PerCP | APC |
|---|---|---|---|---|
| 1 | CD27 | anti-IgD | CD19 | anti-IgM |
| 2 | CD38 | CD21 | CD19 | anti-IgM |
| 3 | CD27 | CD70 | CD20 | CD43 |
| 4 | CD38 | IgD | CD19 | CD27 |
Antibody panel for subsets of CD4+ and CD8+ T cell phenotype (Table 3)
Table 3.
Antibody panel for subsets of CD4+ and CD8+ T cell phenotype
| Panel | FITC | PerCP | APC |
|---|---|---|---|
| 1 | CCR7 | CD4 | CD45RA |
| 2 | CCR7 | CD8 | CD45RA |
Detection of intracellular cytokines
2 × 106/ml peripheral blood mononuclear cells (PBMC) cells in RPMI-1640 medium were activated with 10 ng/ml Phorbol 12-myristate 13-acetate (PMA) + ionomycin 1 g/ml and 10 µg/ml Brefeldin A (BFA) (Sigma, St. Louis, MO) , and Incubate for 4 hours at 37°C in a 5% CO2 atmosphere. Cells were surface stained with CD4 PerCP for 30 min, fixed with 250 ul BD Cytofix/Cytoperm™ Buffer. Cells were washed by BD Perm/Wash™ Buffer, a permeabilization and wash buffer that maintain cellular permeability and facilitate intracellular staining. Activated and unactivated cells stained for Intracellular IFN, TNF and corresponding isotype controls. Ten thousand cells were acquired and analyzed with FACSCalibur.
Detection of TLRs
PBMCs were surface stained either CD14 FITC or PE and surface stained with antibodies toTLR 2, 4, 5 and 6 for 30 min, washed with PBS and acquire by FACSCalibur, TLR4 tube washed stained with additional streptidine PE for 30 min wash and acquired. TLR3, TLR7, and TLR9 tubes after CD14 surface staining were fixed and permeablized with BD Cytofix/Cytoperm™ Fixation/Permeablization Kit as per manufacturer instructions, and stained with antibodies to TLR3, TLR7, and TLR9, washed, and acquire by FACSCalibur. Corresponding isotypes were used as background. Data were analyzed by Flowjo software (Treestar, Ashland, OR).
Statistical analysis
Statistical analysis was performed using Graph Pad Prism. Differences between control and Patient sample were tested using one tail-paired t-tests. Values of P < 0.05 were considered significant.
Results
Naïve, central memory, and effector memory subsets of CD4+ and CD8+ T cells
CD4+ and CD8+ T cells have been classified into TN, TCM, TEM, and TEMRA. These subsets are phenotypically and functionally distinct [19-22]. Therefore, we examined these subsets with various monoclonal antibodies, using multicolor FACSCalibur. Figure 1 shows a marked increase in TEMRA CD4+ and CD8+ T cells; almost all CD8+ T cells are TEMRA, whereas TN and TCM subsets of both CD4+ and CD8+ T cells are decreased.
Figure 1.
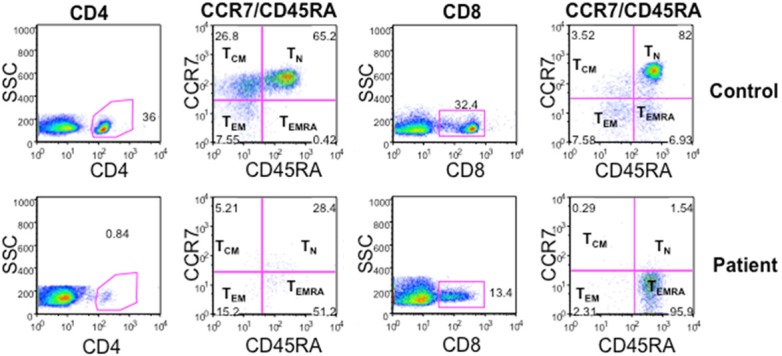
Naïve and memory subsets of CD4+ and CD8+ T cells. TN (CD54RA+CCR7+), TCM (CD45RA-CCR7+), TEM (CD45RA-CCR7-), and TEMRA (CD45RA+CCR7-) subsets of CD4 and CD8 T cell subsets were examined with FACSCalibur and analyzed with Flowjo. TN, and TCM, cells are markedly decreased as compared to controls. TEMRA of CD4 is increased, whereas majority of CD8+ T cells were TEMRA (95.9%).
Th1 cells are decreased
Th1 (IFN-γ) cells play an important role in defense against M. tuberculosis and M. leprae. More recently, it has been reported that IFN-γ and TNF-α are also important in defense against NTM [23,24]. Therefore, we examined CD4+ T cells with intracellular IFN-γ and TNF-α. A significant reduction in IFN-γ+ T cells was observed in the patient as compared to controls (Figure 2). In addition, serum quantiferon levels were undetectable (Table 1).
Figure 2.
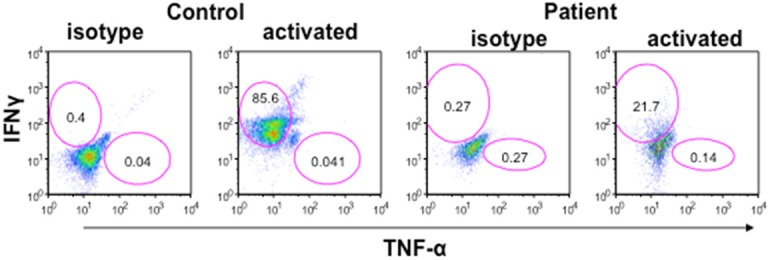
IFN-γ and TNF-α containing CD4+ (Th1) cells. Mononuclear cells were stimulated with PMA and Ionomycin and secretion of cytokine was blocked by brefelidine. Cells were stained for surface CD4, fixed and then stained for Intracellular IFN-γ and TNF-α with respective antibodies and isotype controls. Cells were gated on CD4+ T cells and then analyzed for IFN-γ+ and TNF-α+ cells by multicolor flow cytometry. IFN-γ+ cells were markedly decreased (21.7%) as compared to control (85.6%). TNF-α+ cells were comparable. Blue lines are for isotype control and red lines for specific antibodies.
Monocytes and TLR expression
When PBMNCs were isolated from whole blood CD14+ monocytes in the patient were markedly increased (26%) as compared to control (6%) Figure 3. Analysis of monocyte subsets using CD14 and CD16 antigens revealed a modest increase in “proinflammatory” CD14highCD16- monocytes (73.6%) as compared to control (62.8%); CD14+CD16+ “resident monocytes” were comparable. The innate immune system (monocytes and dendritic cells) utilizes pattern recognition receptors (PRR), including Toll-like receptors (TLR) for defense against mycobacterial infection [17]. Therefore, we examined the expression of TLR’s on CD14+ monocytes in the patient and control. The expression of TLR4 on monocytes was increased, whereas expression of TLR5, TLR7, and TLR9 on monocytes was decreased (Figure 4).
Figure 3.
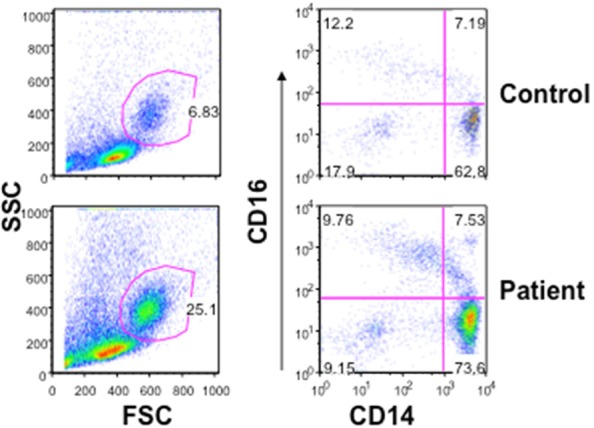
Monocytes and monocyte subsets. In mononuclear cells, isolated from peripheral blood, percentage of monocytes is increased in the patients (25.1%) as compared to control (6.83%). Monocyte subsets were analyzed using CD14 and CD16 antibodies. CD14++CD16- “proinflammatory” monocytes are marginally increased. CD14+CD16+ “resident” monocytes are comparable.
Figure 4.
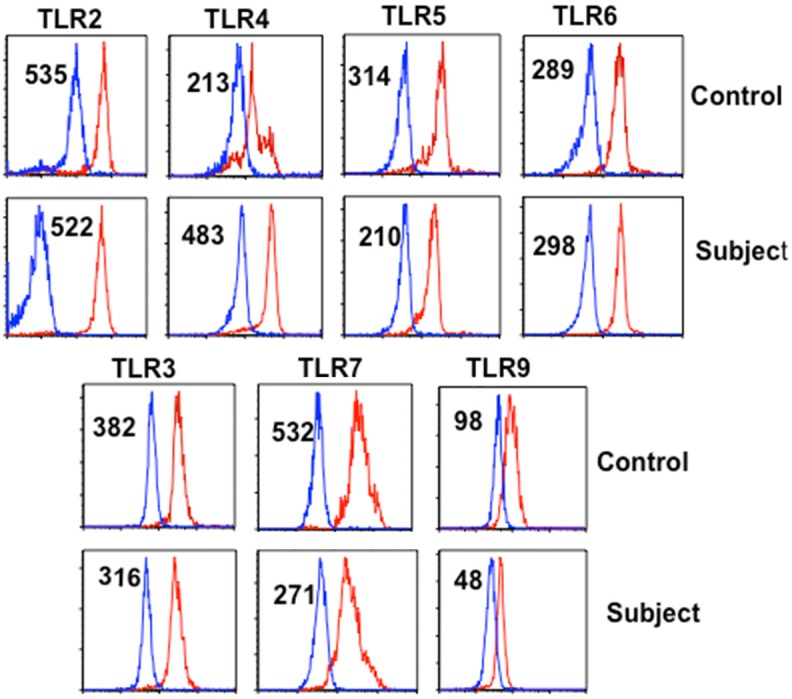
TLR expression on monocytes. TLR-2, -4, -5, and -6 are membrane bound, whereas TRL-3, -7, and -9 are intracellular and were analyzed following fixation with respective antibodies and isotype controls. Numbers in the parenthesis are mean fluorescence channel numbers (MFC#) as an indicator of density of molecules. TLR-4 expression is increased, whereas the expression of TLR-5, -7, and -9 is decreased.
IL-12-IFN-γ axis
IL-12 plays an important and critical role in Th1 polarization and depletion of IL-12 reduces resistance to Mycobacterium avium infection [25,26]. Therefore, we examined the expression of IL-12R and IFN-γR on monocytes and lymphocytes with receptor-specific antibodies and isotype controls using multicolor flow cytometry. IFN-γR+ monocytes were decreased; however, IL-12R+ monocytes were comparable to control (Figure 5). IL-12R+ and IFN-γR+ lymphocytes were also comparable between patient and control.
Figure 5.
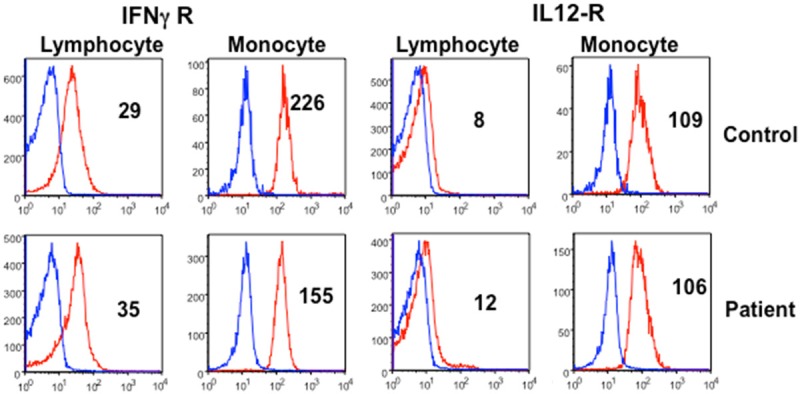
Expression of IL-12R and IFN-γR on lymphocytes and monocytes. Mononuclear cells were stained with monoclonal antibodies against IL-12R or IFN-γR and isotype controls. Cells were gated for lymphocytes and monocytes, and data were analyzed by Flowjo. Numbers represent MFC#. IFN-γR on monocytes is decreased. IL-12R on lymphocytes and monocytes, and IFN-γR on lymphocytes are comparable between patient and control.
B cell subsets
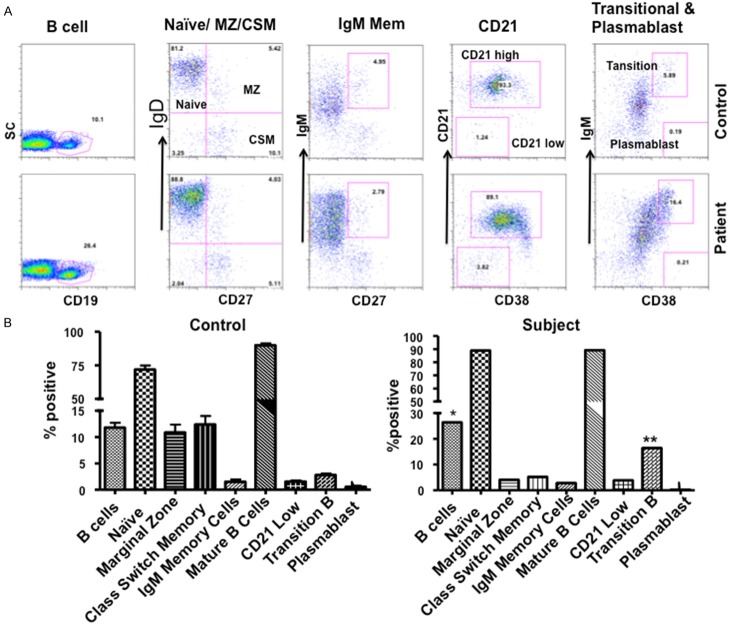
B cells have been divided into several subsets based upon their stage of maturation and differentiation [27]. Although a role of antibodies in defense against mycobacteria has not been fully explored, IgM has been shown to display specificity against PGL-A1 of M. leprae [28,29]. IgG antibodies against glycopeptidolipid (GPL) core antigen of MAC were present in a majority of patients with pulmonary MAC [30]. Therefore, we analyzed various subsets of B cells with a group of antibodies using multicolor flow cytometry. Transitional B cells (CD19+IgD+CD27+) were increased (Figure 6), whereas germinal center B cells (CD19+CD38+CD27lowIgD-) and B1 cells (CD20+CD27+CD43+CD70-) were decreased (Figure 7).
Figure 6.
B cell subsets. Total B cells, mature B cells, naïve B cells, IgM memory B cells, class-switched B cells, CD21lo B cells, marginal zone (MZ) B cells, and plasmablasts were analyzed using a variety of markers (see methods). A. Flow cytograph shows increased transitional B cells. B. Data of patient is compared with 20 healthy controls.
Figure 7.
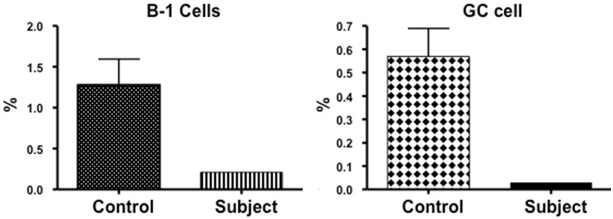
B1 and Germinal Center (GC) B cells. Both germinal center (CD19+CD38+CD27lowIgD-) and B1 cells (CD20+CD27+CD43+CD70-) are markedly decreased as compared to 20 healthy subjects.
Discussion
The incidence of NTM infection is increasing worldwide. In a very large study Hoefslooot and colleagues reported 91 different species of NTM in over 20,000 patients from 30 countries [31]. Systemic NTM infections in humans manifest as hypersensitivity pneumonitis, cavitary disease, and nodular bronchiectasis. Cutaneous NTM infections are uncommon. A solitary cutaneous MAI infection was reported in a young man who underwent allogeneic bone marrow transplantation [14]. Disseminated Mycobacterium chelonae infections, with or without cutaneous and osseous manifestation, have been reported [32,33]. Cutaneous MAI infections are rare and disseminated cutaneous MAI infection has not been reported. Detailed immunological analyses were not performed in any of these cases. Our case also highlights a possible role of B cells and antibodies, albeit minor, in defense against mycobacterial species. Initially, our patient responded to antimycobacterial therapy; however, later became resistant. Since our patient has markedly reduced IFN-γ producing CD4+ T cells and no quantiferon, IFN-γ was added to therapy, to which he responded. Patients with ICL and MAC infection have been successfully treated with IFN-γ and IL-2 therapy [34].
Host immune responses to M. tuberculosis and M. leprae have been studied in detail [35,36]. The host immune responses to NTM are similar to M. tuberculosis with some differences [18]. The major protective responses to mycobacteria are Th1 CD4 response and macrophages (Th1 CD4+ T cells produce TNF-α and IFN-γ); IFN-γ activates macrophages resulting in intracellular killing of mycobacteria [35]. IFN-γ deficiency has been considered as a major factor in the pathogenesis of MAC infection [35,36]. IL-12 plays an important role in defense against mycobacterial infection by polarizing Th0 cells to Th1 cells to produce IFN-γ, which then binds to macrophages via IFN-γR, and activating them to eliminate mycobacteria [37-39]. Our patient has a deficiency of interferon production (quantiferon), IFN-γ producing Th1 cells, and expression of IFN-γR on macrophages. A role of TNF-α in defense against M. tuberculosis has been demonstrated in both mice and humans [40,41], which is further supported by increased susceptibility to M. tuberculosis infection in patients receiving anti-TNF therapy. In our patient, TNF-α containing cells were comparable to control. It is possible that TNF-α plays a minor role if any, against NTM.
Toll-like receptors (TLRs) are a family of pattern recognition receptors that are capable of recognizing conserved pathogen-associated molecular patterns (PAMPs), including components of bacterial cell walls such as lipoproteins and lipoglycans present in mycobacteria species, and microbial nucleic acids [18,42-44]. M. tuberculosis has lipoprotein that interacts with TLR-2 to activate NF-κB and secrete IL-12 [43]. It also results in killing of intracellular M. tuberculosis. Other PAMPs of M. tuberculosis include mannose-capped lipoarabinomannan, which does not activate TLR2 or TLR4 and may activate other TLRs [45], and mannosylated phosphatidylinositol (PIM), a component of soluble tuberculosis factor (STF) that appears to activate TLR-2 and TLR-6. In our patient, TLR2 and TLR6 expression on monocytes was comparable to control. TLR-7 ligands induce autophagy in mycobacterial-infected macrophages [46]. Bakhru et al. [47] have demonstrated that BCG vaccine-mediated reduction in the expression of MHC II antigen on macrophages and dendritic cells is reversed by activation of TLR7 and TLR9. In our patient, expression of TLR7, and TLR9 was markedly decreased, and might contribute to MAI pathology.
Naïve T cells upon exposure to an antigen undergo a clonal expansion of effector cells, which after clearing the antigen undergo a phase of contraction when antigen-specific T cells undergo apoptosis, and then a small number of antigen-specific T cells stabilizes and retained as memory T cells [19-22]. These memory T cells differentially express adhesion molecules and chemokine receptors, which allow them to home in peripheral blood lymphoid tissues. Based upon the expression or lack of them, memory CD4+ and CD8+ T cells migrate to lymph nodes and spleen (central memory, TCM) or to extralymphoid tissue like lung and liver (effector memory; TEM). A small subpopulation of TEM cells re-acquires CD45RA, and is termed as TEMRA or terminally differentiated memory or exhausted T effector cells. TEM and TEMRA T cells T cells display poor proliferation, decreased telomere length, and are resistance to apoptosis [20], whereas TN and TCM cells proliferate and are antigen-dependent. Therefore, a deficiency of TN, and TCM cells and an expansion of TEMRA cells in our patient may be responsible for T cell functional defects contributing to increased susceptibility to disseminated cutaneous MAI infection. A deficiency of TN and TCM cells and expansion of TEMRA cells is also observed in aged human, which contributes to T cell immunosenescence [19].
Although a role of cell-mediated immunity is well established, a role of B cells and antibodies in defense against mycobacterial infection has not been investigated in detail. However, emerging evidence supports a role of B cells and antibodies in host defense against intracellular pathogens including M. tuberculosis [48,49]. Evidence suggests that [A] B cells can regulate both CD4+ and CD8+ T cell memory responses [50-52], [B] B cells by virtue of producing antibodies and cytokines can modulate the maturation of antigen-presenting cells; thereby regulating the adaptive immune response [53]. Natural antibodies bind to and alter the activity of co-stimulatory molecules B7 and CD40, thereby affecting antigen presentation [54]. Cytokines produce by B cells can polarize T cells [51,55]. [C] B cells can regulate the differentiation of macrophages into subsets. B1 cells promote polarization of macrophages into M2 subset [56]; macrophages are important in anti-mycobacterial defense. B1 cells spontaneously secrete natural IgM antibodies in the absence of exogenous antigenic stimulation and B1 and B2 cell-derived IgM antibodies play a protective role in intracellular microbe influenza virus infection [57-59]. One of the characteristics of B1 cells is the enrichment of their repertoire for poly- and self-reactive specificity [60]. In our patient, in addition to selective IgM deficiency, the numbers of B1 cells were markedly decreased. [D] Accumulating evidence suggest significant role of antibodies against intracellular pathogens including M. tuberculosis. Monoclonal antibodies specific for a number of mycobacterial components including arabinomannan, lipoarabinomannan, heparin-binding hemagglutinin, and 16kD-crystalin have been shown to protect against M. tuberculosis [61-65], and passive transfer of serum with polyclonal antibodies against M. tuberculosis is protective in relapse of tuberculosis in SCID mice [66]. Furthermore, IVIG in a mouse model of tuberculosis has been reported to be protective [67]. A role of antibodies in defense against mycobacterial defense is also supported by M. tuberculosis infections in patients with X-linked agammaglobulinemia [68,69], and M. tuberculosis and severe NTM infection in patients treated with Rituximab that deplete B cells [70]. [E] Finally, a role of antibodies in mycobacterial defense is supported by the presence of IgG antibodies against glycopeptidolipid (GPL) core antigen of MAC in 77% of patients with pulmonary MAC and none in pulmonary tuberculosis [29].
The granuloma in M. tuberculosis has aggregates of B cells, which has cellular markers of typical germinal centers [71]. Therefore, a deficiency of GC B cells may result in abnormal granulomatous reaction with exacerbated pathology. In our patient germinal center B cells were markedly decreased.
In summary, a deficiency of IFN-γ secretion, Th1 cells, and IFN-γ-R expression on monocytes, as well a deficiency of TN and TCM and accumulation of TEMRA T cell subsets likely play an important role in severe T cell deficiency and disseminated cutaneous MAI infection in the present patient. A deficiency of TLR7 and TLR9 via their effect on autophagy and MHC class II expression may also play a role in CD4+ T cell functions. A role of B cells and immunoglobulins in defense against M. tuberculosis is emerging. Selective IgM deficiency and B cell alterations in our patient suggest their possible role in defense against MAI and other NMT, and should be explored.
Acknowledgements
The work cited was supported by unrestricted funds from the Division of Basic and Clinical Immunology, University of California, Irvine.
Disclosure of conflict of interest
None.
References
- 1.Gupta S, Ribak CE, Gollapudi S, Kim CH, Salahuddin SZ. Detection of a human intracisternal retroviral particle associated with CD4+ nT-cell deficiency. Proc Natl Acad Sci U S A. 1992;89:7831–7835. doi: 10.1073/pnas.89.16.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control (CDC) Unexplained CD4+ T-lymphocyte depletion in persons without evident HIV infection--United States. MMWR Morb Mortal Wkly Rep. 1992;41:541–545. [PubMed] [Google Scholar]
- 3.Luo L, Li T. Idiopathic CD4 lymphocytopenia and opportunistic infection--an update. FEMS Immunol Med Microbiol. 2008;54:283–289. doi: 10.1111/j.1574-695X.2008.00490.x. [DOI] [PubMed] [Google Scholar]
- 4.Régent A, Autran B, Carcelain G, Cheynier R, Terrier B, Charmeteau-De Muylder B, Krivitzky A, Oksenhendler E, Costedoat-Chalumeau N, Hubert P, Lortholary O, Dupin N, Debré P, Guillevin L, Mouthon L French Idiopathic CDT Lymphocytopenia Study Group. Idiopathic CD4 lymphocytopenia: clinical and immunologic characteristics and follow-up of 40 patients. Medicine (Baltimore) 2014;93:61–72. doi: 10.1097/MD.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. An investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373–379. doi: 10.1056/NEJM199302113280601. [DOI] [PubMed] [Google Scholar]
- 6.Walker UA, Warnatz K. Idiopathic CD4 lymphocytopenia. Curr Opin Rheumatol. 2006;18:389–395. doi: 10.1097/01.bor.0000231908.57913.2f. [DOI] [PubMed] [Google Scholar]
- 7.Zonios DI, Falloon J, Bennett JE, Shaw PA, Chaitt D, Baseler MW, Adelsberger JW, Metcalf JA, Polis MA, Kovacs SB, Kovacs JA, Davey RT, Lane HC, Masur H, Sereti I. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287–294. doi: 10.1182/blood-2007-12-127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta SA, Agrawal S, Gollapudi S. Selective IgM deficiency with T cell defects and Mycobacterium avium complex (MAC) infection. The Open Immunol J. 2012;5:8–12. [Google Scholar]
- 9.Louis AG, Gupta S. Primary selective IgM deficiency: an ignored immunodeficiency. Clin Rev Allergy Immunol. 2014;46:104–111. doi: 10.1007/s12016-013-8375-x. [DOI] [PubMed] [Google Scholar]
- 10.Yel L, Ramanuja S, Gupta S. Clinical and immunological features in IgM deficiency. Int Arch Allergy Immunol. 2009;150:291–298. doi: 10.1159/000222682. [DOI] [PubMed] [Google Scholar]
- 11.Cox SK, Strausbaugh LJ. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794–796. [PubMed] [Google Scholar]
- 12.Hanke CW, Temofeew RK, Slama SL. Mycobacterium kansasii infection with multiple cutaneous lesions. J Am Acad Dermatol. 1987;16:1122–1128. doi: 10.1016/s0190-9622(87)80001-4. [DOI] [PubMed] [Google Scholar]
- 13.Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264–266. [PubMed] [Google Scholar]
- 14.Saruwatari H, Yoshifuku A, Kawai K, Kanekura T. Cutaneous Mycobacterium intracellulare infection in a bone marrow transplantation recipient. J Dermatol. 2010;37:185–187. doi: 10.1111/j.1346-8138.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Wang HS, Feng SY, Wang QL. Cutaneous Mycobacterium intracellulare infection in an immuno-competent person. Acta Derm Venereol. 2013;93:711–714. doi: 10.2340/00015555-1595. [DOI] [PubMed] [Google Scholar]
- 16.Bugault F, Benati D, Mouthon L, Landires I, Rohrlich P, Pestre V, Theze J, Lortholary O, Chakrabarti LA. Altered responses to homeostatic cytokines in patients with idiopathic CD4 lymphocytopenia. PLoS One. 2013;8:e55570. doi: 10.1371/journal.pone.0055570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krutzik SR, Modlin RL. The role of Toll-like receptors in combating mycobacteria. Semin Immunol. 2004;16:35–41. doi: 10.1016/j.smim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Orme IM, Ordway DJ. Host response to nontuberculous mycobacterial infections of current clinical importance. Infect Immun. 2014;82:3516–3522. doi: 10.1128/IAI.01606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S. Molecular mechanisms of apoptosis in the cells of the immune system in human aging. Immunol Rev. 2005;205:114–129. doi: 10.1111/j.0105-2896.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Bi R, Su K, Yel L, Chiplunkar S, Gollapudi S. Characterization of naive, memory and effector CD8+ T cells: effect of age. Exp Gerontol. 2004;39:545–550. doi: 10.1016/j.exger.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 23.Appelberg R. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology. 1994;191:520–525. doi: 10.1016/S0171-2985(11)80458-4. [DOI] [PubMed] [Google Scholar]
- 24.Winthrop KL, Daley CL, Griffith D. Nontuberuclous mycobacterial disease: updated diagnostic criteria for an under-recognized infectious complication of anti-tumor necrosis factor therapy. Nat Clin Pract Rheumatol. 2007;3:E1. doi: 10.1038/ncprheum0621. [DOI] [PubMed] [Google Scholar]
- 25.Bermudez LE, Wu M, Young LS. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect Immun. 1995;63:4099–4104. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castro AG, Silva RA, Appelberg R. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections in mice. J Immunol. 1995;155:2013–2019. [PubMed] [Google Scholar]
- 27.Kurosaki T. B-lymphocyte biology. Immunol Rev. 2010;237:5–9. doi: 10.1111/j.1600-065X.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 28.Bair JS, Wang CR, Sun CC, Chuang CY. The diagnostic value of IgM to natural trisaccharide phenylpropionyl bovine serum albumin in leprosy patients: a preliminary report from Taiwan. J Formos Med Assoc. 1991;90:1099–1102. [PubMed] [Google Scholar]
- 29.Chujor CS, Bernheimer H, Levis WR, Schwerer B. Serum IgA1 and IgM antibodies against Mycobacterium leprae-derived phenolic glycolipid-I: a comparative study in leprosy patients and their contacts. Int J Lepr Other Mycobact Dis. 1991;59:441–449. [PubMed] [Google Scholar]
- 30.Kobashi Y, Mouri K, Obase Y, Kato S, Oka M. Serological assay by use of glycopeptidolipid core antigen for Mycobacterium avium complex. Scand J Infect Dis. 2013;45:241–249. doi: 10.3109/00365548.2012.714904. [DOI] [PubMed] [Google Scholar]
- 31.Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, Beylis N, Boeree MJ, Cacho J, Chihota V, Chimara E, Churchyard G, Cias R, Daza R, Daley CL, Dekhuijzen PN, Domingo D, Drobniewski F, Esteban J, Fauville-Dufaux M, Folkvardsen DB, Gibbons N, Gómez-Mampaso E, Gonzalez R, Hoffmann H, Hsueh PR, Indra A, Jagielski T, Jamieson F, Jankovic M, Jong E, Keane J, Koh WJ, Lange B, Leao S, Macedo R, Mannsåker T, Marras TK, Maugein J, Milburn HJ, Mlinkó T, Morcillo N, Morimoto K, Papaventsis D, Palenque E, Paez-Peña M, Piersimoni C, Polanová M, Rastogi N, Richter E, Ruiz-Serrano MJ, Silva A, da Silva MP, Simsek H, van Soolingen D, Szabó N, Thomson R, Tórtola Fernandez T, Tortoli E, Totten SE, Tyrrell G, Vasankari T, Villar M, Walkiewicz R, Winthrop KL, Wagner D Nontuberculous Mycobacteria Network European Trials Group. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTMNET collaborative study. Eur Respir J. 2013;42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 32.Drabick JJ, Duffy PE, Samlaska CP, Scherbenske JM. Disseminated Mycobacterium chelonae subspecies chelonae infection with cutaneous and osseous manifestations. Arch Dermatol. 1990;126:1064–1067. [PubMed] [Google Scholar]
- 33.Ichihara A, Jinnin M, Fukushima S, Inoue Y, Ihn H. Case of disseminated cutaneous Mycobacterium chelonae infection mimicking cutaneous vasculitis. J Dermatol. 2014;41:414–417. doi: 10.1111/1346-8138.12459. [DOI] [PubMed] [Google Scholar]
- 34.Sternfeld T, Nigg A, Belohradsky BH, Bogner JR. Treatment of relapsing Mycobacterium avium infection with interferon-gamma and interleukin-2 in an HIV-negative patient with low CD4 syndrome. Int J Infect Dis. 2010;14(Suppl 3):e198–201. doi: 10.1016/j.ijid.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 36.Vankayalapati R, Wizel B, Samten B, Griffith DE, Shams H, Galland MR, Von Reyn CF, Girard WM, Wallace RJ Jr, Barnes PF. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J Infect Dis. 2001;183:478–484. doi: 10.1086/318087. [DOI] [PubMed] [Google Scholar]
- 37.Cooper AM, Solache A, Khader SA. Interleukin-12 and tuberculosis: an old story revisited. Curr Opin Immunol. 2007;19:441–447. doi: 10.1016/j.coi.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holscher C, Atkinson RA, Arendse B, Brown N, Myburgh E, Alber G, Brombacher F. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–6966. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 39.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 40.Mohan VP, Scanga CA, Yu K, Scott HM, Tanaka KE, Tsang E, Tsai MM, Flynn JL, Chan J. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001;69:1847–1855. doi: 10.1128/IAI.69.3.1847-1855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roach DR, Briscoe H, Saunders B, France MP, Riminton S, Britton WJ. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J Exp Med. 2001;193:239–246. doi: 10.1084/jem.193.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 43.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 44.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 45.Morris KR, Lutz RD, Choi HS, Kamitani T, Chmura K, Chan ED. Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infect Immun. 2003;71:1442–1452. doi: 10.1128/IAI.71.3.1442-1452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bakhru P, Sirisaengtaksin N, Soudani E, Mukherjee S, Khan A, Jagannath C. BCG vaccine mediated reduction in the MHC-II expression of macrophages and dendritic cells is reversed by activation of Toll-like receptors 7 and 9. Cell Immunol. 2014;287:53–61. doi: 10.1016/j.cellimm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozakiewicz L, Phuah J, Flynn J, Chan J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv Exp Med Biol. 2013;783:225–250. doi: 10.1007/978-1-4614-6111-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur J Immunol. 2009;39:676–686. doi: 10.1002/eji.200839148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 51.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 53.Sugimoto K, Ogawa A, Shimomura Y, Nagahama K, Mizoguchi A, Bhan AK. Inducible IL-12-producing B cells regulate Th2-mediated intestinal inflammation. Gastroenterology. 2007;133:124–136. doi: 10.1053/j.gastro.2007.03.112. [DOI] [PubMed] [Google Scholar]
- 54.Bayry J, Lacroix-Desmazes S, Donkova-Petrini V, Carbonneil C, Misra N, Lepelletier Y, Delignat S, Varambally S, Oksenhendler E, Levy Y, Debre M, Kazatchkine MD, Hermine O, Kaveri SV. Natural antibodies sustain differentiation and maturation of human dendritic cells. Proc Natl Acad Sci U S A. 2004;101:14210–14215. doi: 10.1073/pnas.0402183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 56.Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, Abastado JP, Lam KP, Biswas SK. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–2307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 57.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boes M. Role of natural and immune IgM antibodies in immune responses. Mol Immunol. 2000;37:1141–1149. doi: 10.1016/s0161-5890(01)00025-6. [DOI] [PubMed] [Google Scholar]
- 59.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baumgarth N. The double life of a B-1 cell: selfreactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 61.Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab’) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, Menozzi FD. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–194. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 63.Reljic R, Clark SO, Williams A, Falero-Diaz G, Singh M, Challacombe S, Marsh PD, Ivanyi J. Intranasal IFNgamma extends passive IgA antibody protection of mice against Mycobacterium tuberculosis lung infection. Clin Exp Immunol. 2006;143:467–473. doi: 10.1111/j.1365-2249.2006.03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, Bloom BR. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams A, Reljic R, Naylor I, Clark SO, Falero-Diaz G, Singh M, Challacombe S, Marsh PD, Ivanyi J. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111:328–333. doi: 10.1111/j.1365-2567.2004.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guirado E, Amat I, Gil O, Diaz J, Arcos V, Caceres N, Ausina V, Cardona PJ. Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect. 2006;8:1252–1259. doi: 10.1016/j.micinf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Roy E, Stavropoulos E, Brennan J, Coade S, Grigorieva E, Walker B, Dagg B, Tascon RE, Lowrie DB, Colston MJ, Jolles S. Therapeutic efficacy of high-dose intravenous immunoglobulin in Mycobacterium tuberculosis infection in mice. Infect Immun. 2005;73:6101–6109. doi: 10.1128/IAI.73.9.6101-6109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dogru D, Kiper N, Ozcelik U, Yalcin E, Tezcan I. Tuberculosis in children with congenital immunodeficiency syndromes. Tuberk Toraks. 2010;58:59–63. [PubMed] [Google Scholar]
- 69.Kawakami C, Inoue A, Takitani K, Kanegane H, Miyawaki T, Tamai H. X-linked agammaglobulinemia complicated with endobronchial tuberculosis. Acta Paediatr. 2011;100:466–468. doi: 10.1111/j.1651-2227.2010.02071.x. [DOI] [PubMed] [Google Scholar]
- 70.Lutt JR, Pisculli ML, Weinblatt ME, Deodhar A, Winthrop KL. Severe nontuberculous mycobacterial infection in 2 patients receiving rituximab for refractory myositis. J Rheumatol. 2008;35:1683–1685. [PubMed] [Google Scholar]
- 71.Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007;178:7222–7234. doi: 10.4049/jimmunol.178.11.7222. [DOI] [PubMed] [Google Scholar]