Abstract
The morphological consequences of retinal photoreceptor degeneration are well documented. Much less is known about changes in visual function during degeneration and whether central visual structures directly reflect changes in retinal ganglion cell (RGC) function. To address this, we compared changes in visual function of RGCs and cells in the superior colliculus (SC) in transgenic (Tg) P23H-1 rats, a model of retinitis pigmentosa (RP), and wild-type (WT) rats at postnatal days 35–50 (P35–50) and P300. RGCs were classified on the basis of their responses to light: onset (ON), offset (OFF), or both (ON-OFF). The distribution of ON, OFF, and ON-OFF RGCs is similar between WT and P35 Tg P23H-1 rats. By P300, many Tg P23H-1 RGCs are nonresponsive (NR). At this age, there is a sharp decline in ON and ON-OFF RGCs, and the majority that remain are OFF RGCs. Spontaneous rhythmic activity was observed in many RGCs at P300, but only in OFF or NR RGCs. In the SC, WT and P50 Tg P23H-1 responses are similar. At P300, Tg P23H-1 ON SC responses declined but OFF responses increased. We examined postsynaptic glutamate receptor expression located on the bipolar cells (BC), where the ON and OFF pathways arise. At P150, metabotropic glutamate receptor 6 (mGluR6) expression is lower than in WT, consistent with a decrease in ON RGC responses. GluR4 expression, an ionotropic glutamate receptor associated with OFF BCs, appears similar to that in WT. The loss of ON responses in Tg P23H-1 RGCs and in the SC is conserved and related to reduced mGluR6 signaling.
Keywords: retina, retinitis pigmentosa, P23H, superior colliculus, rhodopsin mutation
in humans and in animal models of retinitis pigmentosa (RP), rod photoreceptor degeneration occurs first, followed by cone photoreceptor degeneration (Fariss et al. 2000; Fei 2002; Strettoi and Pignatelli 2000). In addition to photoreceptor loss, late-stage changes occur in the inner retina. These include the partial loss of retinal ganglion cells (RGCs), bipolar cells (BCs), and amacrine cells, ectopic contacts, the loss of dendritic arbors, changes in synaptic receptor expression, and the formation of glial seals (Cuenca et al. 2004; Jones et al. 2003; Lu et al. 2013; Lund et al. 1998; Marc and Jones 2003; Marc et al. 2003). Whereas these morphological changes are well described, few studies have focused on alterations in visual function prior to the complete loss of visual responses (Lund et al. 1998; Pu et al. 2006; Puthussery et al. 2009; Sauve et al. 2001; Sekirnjak et al. 2011; Stasheff 2008; Stasheff et al. 2011). Across RP animal models, the most frequently reported changes are an increase in RGC spontaneous spiking activity, accompanied by the onset of rhythmic activity (Margolis et al. 2008; Sauve et al. 2001; Sekirnjak et al. 2011). Previous reports suggest signaling through the ON and OFF pathways may be differentially affected. For example, ON responses have been reported to be preferentially lost in rd1 and rd10 mice and in Royal College of Surgeons (RCS) rats (Pu et al. 2006; Puthussery et al. 2009; Sauve et al. 2001; Stasheff 2008). Consistent with this functional change, the expression of the metabotropic glutamate receptor 6 (mGluR6), required for signaling in ON BCs, declines in the transgenic (Tg) P23H-1 rat, as well as in the rd10 mouse (Cuenca et al. 2004; Puthussery et al. 2009). In contrast, ionotropic glutamate receptor (iGluR) expression (specifically types 1, 2, and 4) is retained in rd10 OFF BCs even when all visually evoked responses are absent (Puthussery et al. 2009).
The superior colliculus (SC) has been used to assess both changes and rescue of visual function in a variety of animal models of RP. These studies note a decline followed by a loss of light-evoked responses in the SC, as well as a reduction/loss in visually driven behavior (Lund et al. 1998; Sagdullaev et al. 2003; Sauve et al. 2001, 2004; Thomas et al. 2004; Woch et al. 2001). However, none of these studies directly addressed whether a correlation exists between changes in retinal function and SC function. For any therapeutic approach to be maximally effective, it should address all degenerative alterations, including those that occur more centrally. The purpose of the present study was to address this gap by using the same visual stimulus to compare light-evoked photopic (i.e., cone driven) responses of RGCs and SC neurons in WT rats and a rat model of RP. For this study, we chose the Tg P23H-1 rat model of RP, which carries a point mutation in the rhodopsin gene resulting in a proline-to-histidine mutation at position 23 in the rhodopsin protein. Pigmented hemizygous Tg P23H-1 rats show prolonged photoreceptor degeneration: outer nuclear layer thickness remains similar to that in WT up to approximately postnatal day 50 (P50) and then declines to ∼50% at P150 and to ∼12% at P300 (Sekirnjak et al. 2009). As a consequence of photoreceptor loss, ∼50% of RGCs are visually nonresponsive at P150, and all light responses are lost at ∼P550 (Sekirnjak et al. 2009). This prolonged degeneration allows us to assess time points that represent different stages of degeneration: no/very early stage (∼P50), early-mid stage (∼P150), and late stage when only cone-mediated light responses remain (∼P300). These time points are useful benchmarks to extrapolate changes that may occur in human RP patients at these different stages of disease. In this study, we compared visually evoked RGC responses to SC responses to determine if central changes simply reflect those of their presynaptic partners or if additional changes occur in this direct target of RGCs.
METHODS
Animals.
All experiments were carried out in accordance with the Association for Research in Vision and Ophthalmology “Statement for the Use of Animals in Ophthalmic and Visual Research” and with the approval of the Institutional Animal Care and Use Committee of the University of Louisville. Homozygous Tg P23H-1 rats were a gift from Dr. Matthew LaVail (University of California, San Francisco, CA). Pigmented hemizygous Tg P23H-1 rats were generated by breeding WT Long-Evans rats (Charles River Laboratories, Wilmington, MA) to homozygous Tg P23H-1 rats and were used for all experiments. Long-Evans rats were used as age-matched controls. We were able to record RGC activity on the multielectrode array (MEA) shortly after weaning (∼P30). Our stereotaxic setup used to record SC activity precluded recordings in very young pups. As a result, the youngest animals that we recorded in the SC were ∼P50.
MEA RGC recordings.
Spiking activity of RGCs was recorded in vitro using a 60-channel MEA recording system (Multi Channel Systems, Reutlingen, Germany). For MEA recordings, rats were anesthetized with an intraperitoneal injection of ketamine-xylazine (80 and 10 mg/kg, respectively). Rats were euthanized by cervical dislocation, and both eyes were enucleated and placed in light-tight chambers filled with oxygenated Ringer solution at 22°C. Under dim red light, the lens and cornea were removed, and the retina was dissected from the eyecup. Retinas were quartered, and one piece was placed RGC side down in the MEA chamber and perfused with oxygenated Ringer solution at 36°C for the duration of the experiment. For each eye, two to four quarters were recorded, although the retinal location was not noted. We recorded at least two quarters in every retina and combined the data across pieces to provide an overview of general retinal function. Data were recorded using MC_Rack software (Multi Channel Systems) and stored for offline analysis. Spike trains were sorted into single units with principal component analysis using Offline Sorter (Plexon, Dallas, TX) and further analyzed using NeuroExplorer (Nex Technologies, Madison, AL). Light-emitting diodes (LEDs) mounted in a Ganzfeld produced a full-field light stimulus (peak wavelength λ = 470 nm; 2.4 × 103 R*·cone−1·s−1) from a dark background. This configuration results in UV and M/L cone-driven (photopic) responses from RGCs. The stimulus consisted of 20 presentations of a light increment 2 s in duration with a 10-s interstimulus interval back to the dark background.
Extracellular recording from the SC.
The procedures for extracellular recordings from the SC have been described previously (DeMarco et al. 2007; Fransen 2014; Sagdullaev et al. 2003; Woch et al. 2001). Anesthesia was induced as described above and maintained throughout the experiments by infusion of diluted anesthesia (1:1 in Ringer solution) at ∼0.35 ml/h via a catheter inserted into the femoral vein. The animal was fixed in a stereotaxic frame, and a craniotomy was made just posterior to the coronal suture (∼1.5 mm2). The overlying cortex was aspirated to expose the left SC, and normal saline was used to keep the exposed surface moist. Multicellular activity was recorded in the SC using lacquer-coated tungsten electrodes (∼0.25–2 MΩ). Under light-adapted conditions, activity was systematically sampled across the dorsal surface of the SC. Recording sites were spaced 200 μm apart, and the entire map consisted of ∼54 recording sites. At each site, the electrode was lowered with the use of a hydraulic micromanipulator (model 2650; David Kopf Instruments, Tujunga, CA) until robust spontaneous activity was audible over a speaker. All sampling was limited to the first 400 μm below the SC surface. The light stimulus (described for MEA recording) was presented to the right eye. Evoked multicellular SC responses were recorded and analyzed offline using a Power1401 interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK). Custom scripts written in Spike2 analyzed the responses as follows. A threshold for significant responses was defined as a light-evoked response that exceeded 3 SE above the mean spontaneous activity. Response duration was calculated from the time the response reached threshold until it returned to threshold. The average spontaneous activity was subtracted from the peak firing rate to obtain the corrected peak response amplitude.
Immunohistochemistry.
Eyes from WT and Tg P23H-1 rats were fixed in a 4% paraformaldehyde solution dissolved in phosphate-buffered saline for 20 min, dissected from the eyecup, and cryoprotected in increasing concentrations of sucrose (5%, 10%, 15%, and 20%). Retinas were frozen in a mixture of O.C.T. freezing medium-20% sucrose solution (1:1), and 20-μm transverse sections were cut on a cryostat (CM1850; Leica Biosystems). WT and Tg P23H-1 sections were placed on the same slide and processed together for immunohistochemistry. Sections were blocked with 10% normal donkey serum for 1 h and then reacted with primary antibodies overnight. Primary antibodies included GluR4 (1:400; EMD Millipore, Billerica, MA), mGluR6 (1:500; Sigma-Aldrich, St. Louis, MO), and peanut agglutinin (PNA) conjugated to Alexa Fluor 568 (Life Technologies). Sections were washed in PBS and then incubated for 1.5 h at room temperature in secondary antibodies [donkey anti-rabbit (GluR4, Alexa Fluor 488) and donkey anti-sheep (mGluR6, Alexa Fluor 488; both from Sigma-Aldrich)]. Vectashield with DAPI (Vector Laboratories, Burlingame, CA) was used to label cell bodies. Slides were coverslipped and sealed with nail lacquer, and images were obtained on an Olympus laser scanning confocal microscope (Fluoview FV1000).
Statistics.
Statistical analyses were performed using Prism 5 (GraphPad Software, San Diego, CA) for experimental groups defined in Table 1. Distributions were tested for normality. Normally distributed data were analyzed using a one-way ANOVA test followed by Bonferroni's post hoc test, an unpaired t-test, or a χ2 test. Nonparametric statistical analyses were used when distributions failed the normality test; in particular, we used the Kruskal-Wallis test followed by Dunn's post hoc test. Differences at p ≤ 0.05 were considered significant. Values are means ± SE (in all figures, error bars represent ± SE).
Table 1.
Experimental group numbers
| Experimental Group | No. of Animals (RGCs) |
|
|---|---|---|
| MEA | SC | |
| WT (P60-300) | 4 (188) | 13 |
| Tg P23H-1 | 3 (94) | 0 |
| P35-45 | ||
| P50-90 | 0 | 5 |
| P120-150 | 0 | 5 |
| P300 | 4 (119) | 6 |
Values are the number of animals in wild-type (WT) group or postnatal day (P) age group of transgenic (Tg) P23H-1 rats recorded from retinal multielectrode array (MEA) or from superior colliculus (SC). Data for MEA groups also include the number of recorded retinal ganglion cells (RGCs) in parentheses.
RESULTS
Progressive loss of Tg P23H-1 rat ON RGC responses.
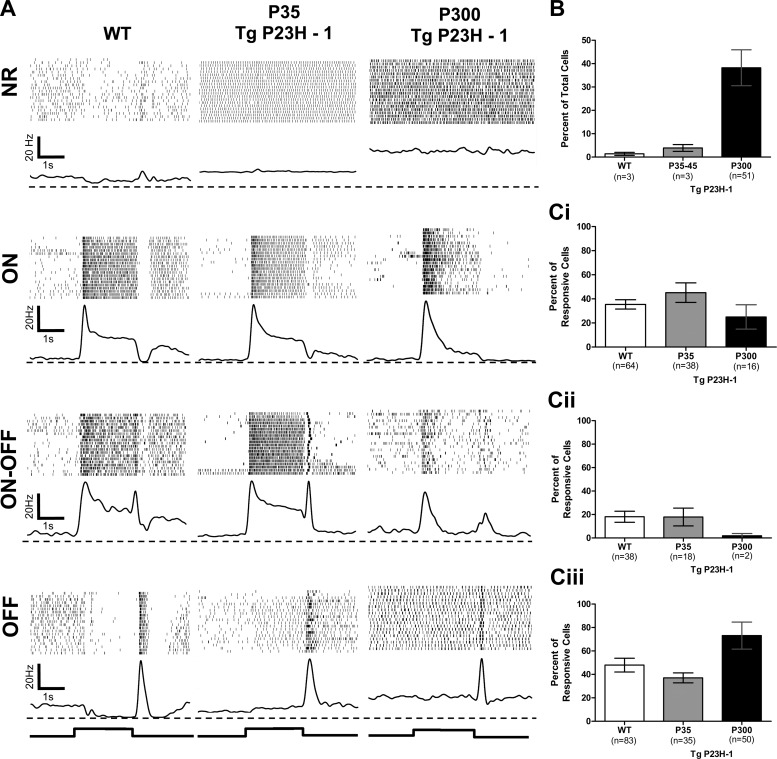
WT and Tg P23H-1 rat RGCs were initially separated into two groups: ones that were spontaneously active but lacked a visually evoked response (NR) and those with visually evoked responses. Visually responsive RGCs were further subdivided into functional classes based on their excitatory response to light onset (ON), offset (OFF), or both (ON-OFF; Fig. 1A). In our samples of both WT and Tg P23H-1 rats, the majority of ON RGCs were sustained and the OFF RGCs were transient (Fig. 1). We rarely encountered transient ON RGCs in either strain, similar to a previous report (Heine and Passaglia 2011). We only evoked transient responses in OFF RGCs as a result of our stimulus (a sustained intensity increase followed by a transient decrease to darkness). Almost all WT and P35 Tg P23H-1 RGCs were visually responsive (Fig. 1B). By P300, Tg P23H-1 NR RGCs were recorded much more frequently (n = 51/119, 42.8%) compared with WT (n = 3/188, 1.6%) or P35 Tg P23H-1 (n = 3/94, 3.19%; Fig. 1B; χ2, P < 0.001 for both comparisons). This increase in NR RGCs is consistent with the progressive loss of Tg P23H-1 photoreceptors and the decline in the electroretinogram and RGCs responses (Aleman et al. 2001; Cuenca et al. 2014; Machida et al. 2000). The distribution of visually responsive RGCs (Fig. 1, Ci–Ciii) was similar between WT and P35 Tg P23H-1 RGCs (ON: 35.4 vs. 45.2%; ON/OFF: 18.1 vs. 17.8%; and OFF: 46.5 vs. 37.0%, respectively; χ2, P = 0.465). At P300, the distributions of Tg P23H-1 RGCs differed significantly from that of both WT and P35 Tg P23H-1 RGCs (Fig. 1C; χ2, P < 0.0001 for both). The proportion of Tg P23H-1 ON and ON-OFF RGCs dropped to 23.5% and 3.0%, respectively (Fig. 1, Ci and Cii), and the majority of visually responsive cells were now OFF RGCs (73.5%; Fig. 1Ciii). This result is consistent with the preferential loss of RGCs with ON responses reported in rd1 and rd10 mice and the RCS rat (Pu et al. 2006; Puthussery et al. 2009; Stasheff 2008).
Fig. 1.
The distribution of retinal ganglion cell (RGC) classes is similar in wild-type (WT) and postnatal day 35–45 (P35-45) transgenic (Tg) P23H-1 rat RGCs, but both are significantly different from that in P300 Tg P23H-1 rat RGCs. A: representative responses from WT, P35, and P300 Tg P23H-1 RGCs to a 2-s full-field stimulus (750 cd/m2). Each line in the raster represents an individual trial, and each poststimulus time histogram (PSTH) is the average response for that RGC. The dashed line represents the zero point for the PSTHs. The onset and offset of the stimulus are indicated below the PSTHs. B: histogram of the percentage of nonresponsive (NR) RGCs for WT, P35, and P300 Tg P23H-1 rats. C: histograms of the percentage of each RGC class (ON, i; ON-OFF, ii; OFF, iii) in WT and Tg P23H-1 at P35 and at P300. The means represent the average across animals. The n value for each column indicates the total number of RGCs in each class for each group. A χ2 analysis shows that the distribution of functional RGC classes is similar between WT and P35 Tg P23H-1 RGCs, but the distribution for P300 Tg P23H-1 RGCs is significantly different from both (P < 0.0001 for both).
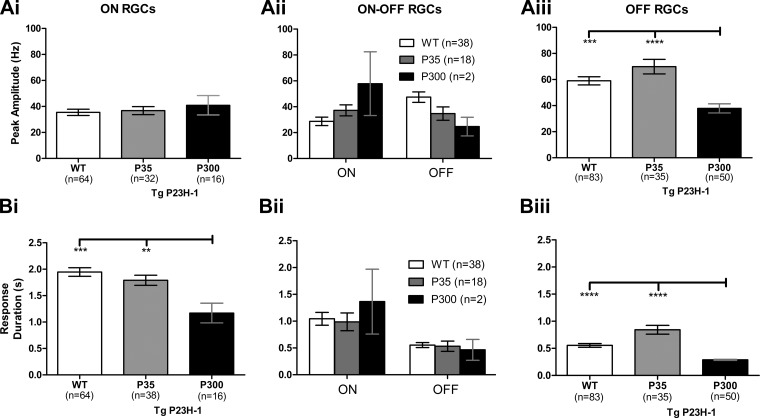
To probe specific changes in RGC responses, we quantified and compared the excitatory responses across age and genotype (Fig. 2). Regardless of age, the Tg P23H-1 ON RGCs that retained visual responses had peak amplitudes similar to those of WT (Fig. 2Ai). Similarly, P35 Tg P23H-1 ON-OFF and OFF RGC peak amplitudes were not significantly different from those of WT (Fig. 2, Aii and Aiii). In contrast, the peak amplitude of P300 Tg P23H-1 OFF RGCs differed from those of both WT and P35 Tg P23H-1 (Fig. 2Aiii; Kruskal Wallis, P < 0.05; Dunn's test: WT, P < 0.001 and P35 Tg P23H-1, P < 0.0001). There were too few ON-OFF Tg P23H-1 RGCs at P300 (n = 2) to make a statistical comparison. Similar to the peak amplitude, the response duration for P35 Tg P23H-1 RGCs was not significantly different from that in WT for any RGC class (Fig. 2B). For P300 Tg P23H-1 RGCs, the response duration for both ON and OFF RGCs was significantly reduced compared with that for both WT and P35 Tg P23H-1 RGCs (Fig. 2, Bi and Biii).
Fig. 2.
The OFF but not the ON Tg P23H-1 RGC response declines between P35 and P300. A: comparison of the peak response for ON (i), ON-OFF (ii), and OFF RGCs (iii) for WT, P35, and P300 Tg P23H-1 rats. Although there are more Tg P23H-1 RGCs with OFF responses at P300, their peak amplitudes are significantly lower than those of WT or Tg P23H-1 P35 responses (Kruskal-Wallis, P < 0.05; Dunn's post hoc, ***P < 0.001; ****P < 0.0001). B: comparison of the response duration for WT, P35, and P300 Tg P23H-1 ON (i), ON-OFF (ii), and OFF RGCs (iii). The response duration was significantly shorter for both Tg P23H-1 ON and OFF RGCs at P300 compared with WT as well as P35 Tg P23H-1 RGCs (Kruskal-Wallis, P < 0.05; Dunn's post hoc, **P < 0.01; ***P < 0.001; ****P < 0.0001).
Spontaneous activity increases and is rhythmic in many OFF and NR Tg P23H-1 RGCs.
Two common findings in RGCs of animal models of RP are an increase in overall spontaneous activity (Margolis and Detwiler 2011; Margolis et al. 2008; Stasheff 2008) and the presence of a rhythmic component in the spontaneous activity (Margolis et al. 2008, 2014; Menzler and Zeck 2011; Sauve et al. 2001; Stasheff 2008). Similar to previous reports, we found no evidence of an increase in spontaneous activity or rhythmic activity in WT RGCs (Borowska et al. 2011; Demas et al. 2006; Freeman et al. 2008; Margolis et al. 2008; Menzler and Zeck 2011; Stasheff 2008). As reported previously (Sekirnjak et al. 2011), we found no change in spontaneous activity of ON RGCs in Tg P23H-1 rats at either P35 (4.66 ± 0.89 Hz) or P300 (6.05 ± 0.95 Hz) compared with WT (ANOVA, P = 0.613), but spontaneous activity increased in OFF RGCs at both ages compared with WT (P35: 11.37 ± 9.07; P300: 12.05 ± 6.34; WT: 5.99 ± 0.80 Hz; ANOVA, P < 0.0001; Bonferroni post hoc, P < 0.0001 for both).
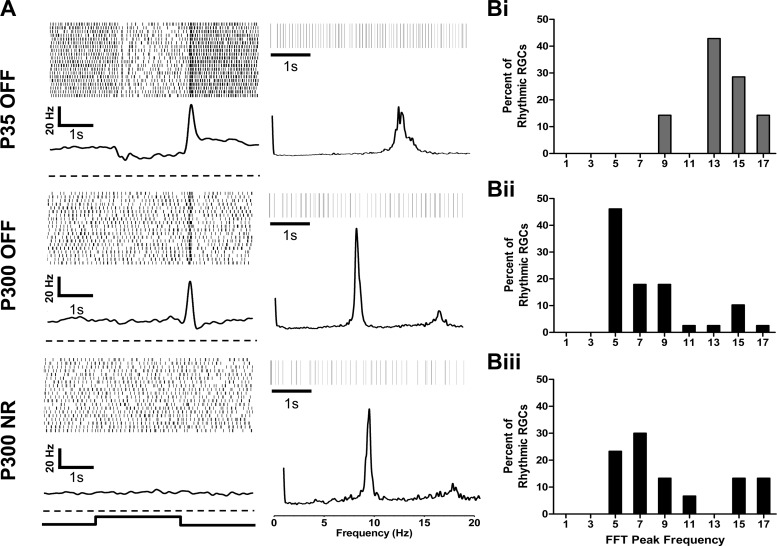
At both P35 and P300, Tg P23H-1 ON and ON-OFF RGCs lacked rhythmicity (data not shown). Among the P35 Tg P23H-1 OFF RGCs, 7.6% (7/91) had a significant peak in their fast Fourier transform (FFT), indicating a rhythmic component in their spontaneous activity (Fig. 3, A and Bi). At P300, 57.9% of all Tg P23H-1 recorded RGCs showed rhythmic spontaneous activity (n = 69/119 RGCs). These included the majority of the Tg P23H-1 OFF (Fig. 3Bii; n = 39/45 OFF RGCs) and NR RGCs (Fig. 3Biii; n = 30/51 NR RGCs). The mean fundamental frequency of P300 Tg P23H-1 NR RGCs (9.4 ± 0.61) was similar to P300 OFF RGCs (7.9 ± 0.5 Hz; unpaired t-test, P = 0.067). However, the mean fundamental frequency of rhythmic P35 Tg P23H-1 OFF RGCs (13.8 ± 0.82) was significantly higher than that of P300 OFF RGCs (unpaired t-test, P < 0.0001). This may indicate a change in the circuitry that generates this rhythmicity between P35 and P300.
Fig. 3.
Many OFF and NR Tg P23H-1 RGCs have a rhythmic component in their spontaneous activity. A, left: example PSTHs and raster plots for representative P35 and P300 Tg P23H-1 OFF and P300 NR RGCs that exhibit rhythmic spontaneous activity. The dashed line represents the zero point for each PSTH. Right, representative 5-s rasters of the spontaneous activity (top) and frequency distributions (bottom) resulting from a fast Fourier transform (FFT) analysis for each RGC shown at left. B: distributions of the peak frequency of the FFT for Tg P23H-1 OFF RGCs at P35 (P35 OFF, i; n = 7), Tg P23H-1 OFF RGCs at P300 (P300 OFF, ii; n = 39), and Tg P23H-1 NR RGCs at P300 (P300 NR, iii; n = 30). The majority of FFT peak frequencies among P35 Tg P23H-1 OFF RGCs were between 13 and 18 Hz. The majority of FFT peak frequencies among Tg P23H-1 P300 OFF and NR RGCs were between 4 and 8 Hz.
Glutamate receptor expression in Tg P23H-1 retina.
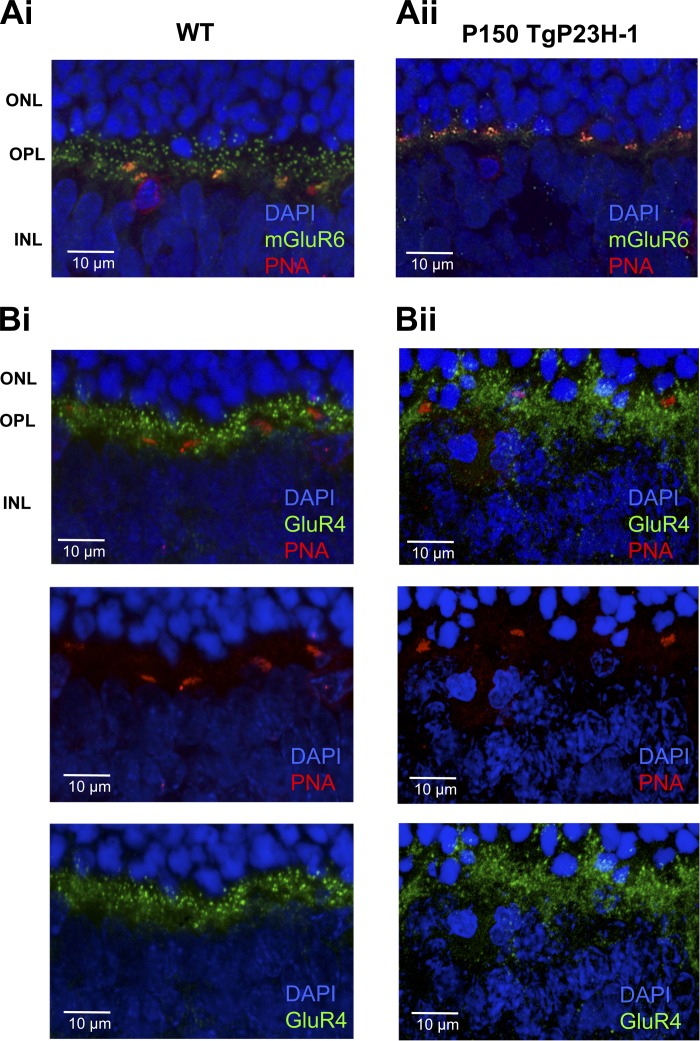
A loss of ON responses in RGCs is likely to reflect the elimination of their synaptic inputs from ON BCs or a lack of signaling in the ON BCs themselves. To address this question, we examined the expression of mGluR6, the glutamate receptor that initiates signaling in ON BCs to luminance increments. In WT rat retina, expression of mGluR6 was localized to the outer plexiform layer (OPL), where it displayed punctate expression (Fig. 4Ai) known to localize at the ON BC dendritic tips (Cuenca et al. 2004; Masu et al. 1995; Nomura et al. 1994; Peachey et al. 2012). A dense labeling was evident near the cone photoreceptor pedicles (PNA-positive profiles). In the Tg P23H-1 retina at P150, although many rod nuclei remain, the density of mGluR6 expression is reduced (Fig. 4Aii) and predominantly associated with the PNA-positive cone terminals. This result is consistent with the idea that the loss of RGCs with ON or ON-OFF responses results from the reduction/absence of signaling in rod and cone ON BCs. In the OPL of the WT rat, expression of GluR4, an iGluR associated with OFF BCs (Grunder et al. 2000; Hack et al. 2001; Peng et al. 1995), was robust (Fig. 4Bi), and expression remained in the OPL of the P150 Tg P23H-1 retina (Fig. 4Bii). Unlike mGluR6, GluR4 was not associated with PNA-positive cone terminals.
Fig. 4.
At P150, expression of metabotropic glutamate receptor 6 (mGluR6) is reduced in Tg P23H-1 retina, but GluR4 expression is similar to that in WT. A: WT (i) and P150 Tg P23H-1 (ii) retinal sections reacted with anti-mGluR6 and peanut agglutinin (PNA). The outer plexiform layer (OPL) in the P150 Tg P23H-1 retina shows a reduction in mGluR6 puncta. ONL, outer nuclear layer; INL, inner nuclear layer. B: WT (i) and P150 Tg P23H-1 (ii) retinal sections reacted with anti-GluR4 and PNA. The OPLs in the P150 Tg P23H-1 and WT retina show similar expression patterns. The individual channels showing PNA (red; middle) and GluR4 expression (green; bottom) are shown below the merged image (top).
ON SC responses reflect their altered retinal input, but OFF SC responses increase.
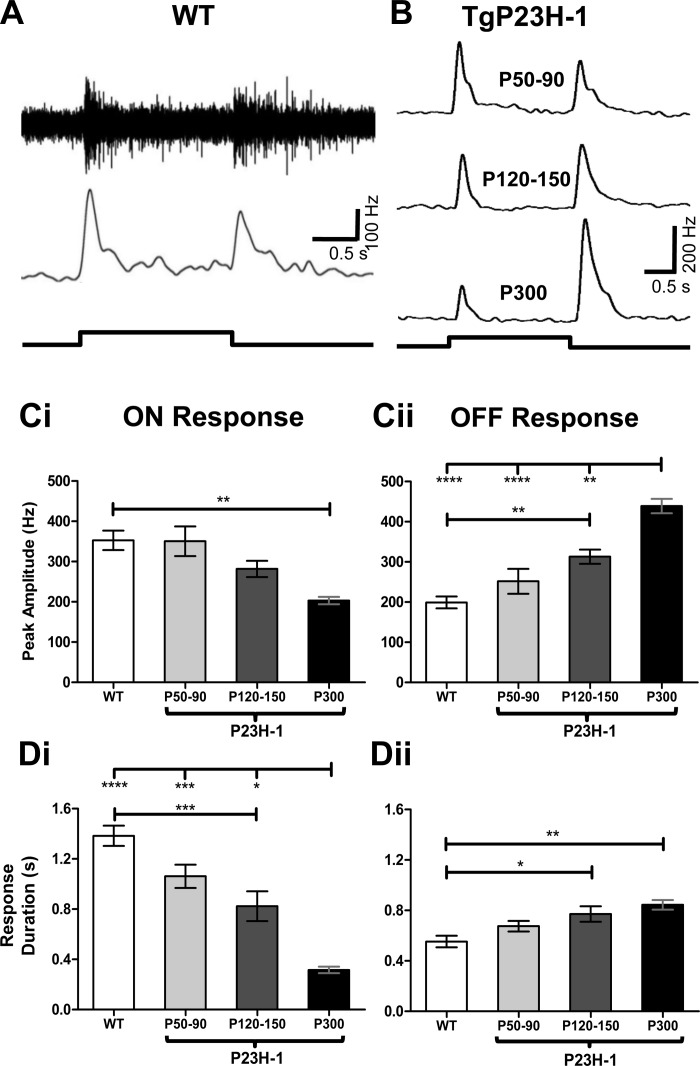
To examine whether the responses in the SC match their inputs from RGCs, we recorded visually evoked SC responses to the same stimulus used to characterize WT and Tg P23H-1 RGCs. We recorded visually evoked SC responses starting at P50, the earliest age that we could reliably record from rat SC, up to P300. All of the data and comparisons are summarized in Table 2. We reliably evoked responses across the entire dorsal surface of the SC, regardless of age or genotype and found no SC areas that were unresponsive to our photopic stimulus. At all ages tested, multiunit WT SC responses had both an ON and OFF component (Fig. 5A). Because WT SC responses were similar across age, we combined all WT data for comparison to Tg P23H-1 responses. Similar to our findings in Tg P23H-1 RGCs at P35, peak amplitude and duration were similar between WT and P50-90 Tg P23H-1 for both ON and OFF SC responses (Fig. 5, C and D). In contrast to WT rats, Tg P23H-1 SC ON responses progressively declined with age (Fig. 5B). This included a significantly reduced ON peak amplitude at P300 (Fig. 5Ci) and decreased ON response duration at P120-150 (Fig. 5Di). Surprisingly, the OFF SC response peak amplitude (Fig. 5Cii) and response duration (Fig. 5Dii) increased between P50 and P300.
Table 2.
SC data summary
| Experimental Group | Response Duration, s | Response Amplitude, Hz |
|---|---|---|
| WT ON | 1.38 ± 0.08 | 352.8 ± 24.1 |
| P50-90 ON | 1.06 ± 0.09 | 350.4 ± 36.7 |
| P120-150 ON | 0.82 ± 0.12c,e | 281.7 ± 20.0 |
| P300 ON | 0.32 ± 0.03d,f | 203.2 ± 9.0b |
| WT OFF | 0.55 ± 0.05 | 199.1 ± 14.67 |
| P50-90 OFF | 0.68 ± 0.04 | 251.7 ± 31.2 |
| P120-150 OFF | 0.77 ± 0.06a | 321.9 ± 17.7b |
| P300 OFF | 0.84 ± 0.04b | 438.8 ± 17.9d,g |
WT and Tg P23H-1 SC response properties were compared with one-way ANOVA. When the overall ANOVA was significant, a Bonferroni post hoc test was used to compare WT and Tg P23H-1 SC responses at different ages.
P < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001, significant differences between WT and Tg P23H-1. eP < 0.05; fP < 0.001; gP < 0.001, significant differences compared with P50-90 Tg P23H-1 responses.
Fig. 5.
Tg P23H-1 ON, but not OFF, superior colliculus (SC) responses decline with age. A: representative WT multiunit SC response (top) and smoothed PSTH (bottom) evoked by a full-field luminance increment (750 cd/m2) with a duration of 2 s. B: representative smoothed histograms of Tg P23H-1 SC responses evoked by the same stimulus as in A at P50–90, P120–150, and P300. C: the peak amplitude progressively declines in Tg P23H-1 ON SC responses (i), whereas the peak amplitude of the OFF SC response progressively increases with age. D: the duration of the response shows a similar change (i). The ON SC response duration decreases, whereas the OFF response duration (ii) increases. For C and D, Bonferroni post hoc, *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
DISCUSSION
This study compares visual dysfunction in the retina of the Tg P23H-1 rat model of RP to one of its major synaptic targets, the SC. The effects of photoreceptor degeneration on retinal (Lund et al. 1998; Margolis et al. 2008; Pu et al. 2006; Puthussery et al. 2009; Sauve et al. 2001; Sekirnjak et al. 2009, 2011; Stasheff et al. 2011) and SC function (Sagdullaev et al. 2003; Sauve et al. 2001; Thomas et al. 2004; Woch et al. 2001) have been separately evaluated in several RP animal models. This is the first study to use the same visual stimulation and evaluation approaches to compare how altered retinal output is reflected in visual responses in the SC. The prolonged degeneration in the Tg P23H-1 rat permits a detailed study of the changes in retinal pathways. We find that the proportion of RGCs that respond to light onset decline, but the responses of the remaining ON RGCs retain peak amplitudes and response durations similar to WT RGCs. In the SC, the excitatory responses to light onset are significantly reduced, consistent with a reduction in overall input from ON RGCs. Tg P23H-1 OFF RGCs are largely retained, but their peak amplitudes and response duration are significantly reduced compared with WT RGCs. Surprisingly, SC responses to light offset increase in duration and amplitude compared with WT. This change may reflect altered crossover interaction between the ON and OFF retinal pathways within the SC. The decline in ON RGC inputs may lower inhibition onto SC neurons, and this could increase the response amplitude and duration of OFF SC responses. Alternatively, an increase in convergence of OFF RGC inputs to the SC also could account for our observation, because SC neurons are known to receive synaptic connections from multiple RGCs (Wang et al. 2001, 2010).
Our results differ from findings of Sekirnjak et al. (2011), who reported a similar decline in ON and OFF RGCs. Using a full-field light stimulus, we find a significant decline in the proportion of ON RGCs with an increase in OFF RGCs. This difference could be related to the visual stimulation paradigms. We used a full-field stimulus, whereas Sekirnjak and colleagues used a white noise stimulus. The full-field stimulus evokes a response that reflects the summation of the receptive field (RF) center and surround. In contrast, a white noise stimulus (or spots confined within the RF center) evokes responses that are RF center dominated. A direct comparison of these stimuli would be needed to resolve this question. Regardless, our data indicate a differential sensitivity to RP within the retinal circuit between the ON and OFF pathways.
Some of the changes that we note in Tg P23H-1 rat RGCs have been described previously in this (Sekirnjak et al. 2009) and other models of RP, including the RCS rat (Pu et al. 2006; Sauve et al. 2001) and the rd1 (Stasheff 2008) and rd10 mouse (Puthussery et al. 2009). In many models there is an increase in spontaneous activity of RGCs that lack a light-evoked response (NR). The onset of this change is directly related to the time course of photoreceptor degeneration. In Tg P23H-1 rats, we find that an increase in spontaneous activity is evident in OFF RGCs, but not ON RGCs, as early as P35. These results are consistent with differential sensitivity of certain RGC classes or retinal pathways to photoreceptor degeneration.
A hallmark of many animal models of RP is the presence of a rhythmic component in the RGC spontaneous activity. We also observe that a rhythmic component is present in ∼8% of RGCs as early as P35, but only among OFF RGCs. At P300, this percentage increases (∼57%) and includes only OFF and NR RGCs. ON and ON-OFF RGCs lack a rhythmic component even as late as P300. These differences suggest a differential effect of photoreceptor degeneration on the ON and OFF retinal pathways. Published data in the rd1 mouse indicate that a large number of rhythmic NR RGCs have ON morphology (Yee et al. 2012). This suggests that in the Tg P23H-1 retina, both pathways likely have rhythmic spontaneous activity as the retina degenerates. The initial insult in RP occurs in rod photoreceptors, and they primarily influence the retina through the ON pathway. This implicates the AII amacrine cell in the emergence of rhythmic activity, consistent with previous reports (Borowska et al. 2011; Choi et al. 2014; Trenholm et al. 2012). Since other models of RP show rhythmic spontaneous activity, including the rd1 and rd10 mouse (Margolis et al. 2014; Pu et al. 2006; Stasheff 2008) as well as models of congenital stationary night blindness (Demas et al. 2006), deafferentation, either degenerative or due to a lack of required signaling complex proteins, appears to unmask rhythmic spontaneous activity. This is likely a crossover inhibitory mechanism between the ON and OFF pathways in the intact retina (Farajian et al. 2011; Nobles et al. 2012; Werblin 2010). We did not observe a rhythmic component in the SC multiunit activity. This suggests that if rhythmicity is transmitted from RGCs to SC neurons, the RGC rhythmicity is not synchronized and may have been masked by recording multiunit activity.
Some changes that occur in retinal function are directly correlated to changes in expression of the receptors that mediate synaptic transmission between the photoreceptor and the ON and OFF bipolar cells. Similar to a previous report (Cuenca et al. 2004), we also note a decline in mGluR6 receptor expression and pattern in the OPL. At P150, mGluR6 expression is primarily associated with cone pedicles, which is consistent with both the rapid decline in rod signaling and the decline in ON signaling in RGCs. Although we now know that kainate-type receptors mediate OFF BC responses (Borghuis et al. 2014; Puthussery et al. 2014), a lack of useful antibodies against kainate receptors precluded an immunohistochemistry investigation of expression levels directly related to OFF BC responses. However, GluR4, an iGluR associated with OFF BCs, is clearly less affected by photoreceptor degeneration in the Tg P23H-1 rat. This difference in glutamate receptor expression may be explained by activity dependence. The lack of glutamate signaling may lead to tonic depolarization of ON BCs, but not OFF BCs, due to the sign-inverting nature of mGluR6. In this scenario, downregulating mGluR6 may prevent excitotoxicity in ON BCs. This question would warrant further investigation to explore the mechanisms for receptor expression at the BC dendrites.
The detailed descriptions of phenotypic changes and their timing described in this study may have important implications for assessment of patients with RP. For example, the changes we observe may be useful for pinpointing the exact circuitry that is highly sensitive to early changes induced by RP. In addition, these changes may be important for evaluating approaches to maintain or restore glutamate receptor expression. For a patient with RP, these results may help define the timing of the earliest changes in RP and determine when therapeutic interventions are appropriate.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01 EY-018608 and R01 EY-140701 (to M. A. McCall), an unrestricted grant from Research to Prevent Blindness (Department of Ophthalmology & Visual Sciences, University of Louisville, KY), and NIH Institutional Training Grant 5 T32 HL-76138-09 (to J. W. Fransen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W.F. and M.A.M. conception and design of research; J.W.F., G.P., and I.S.P. performed experiments; J.W.F. and G.P. analyzed data; J.W.F., G.P., I.S.P., and M.A.M. interpreted results of experiments; J.W.F. prepared figures; J.W.F. drafted manuscript; J.W.F., G.P., I.S.P., and M.A.M. edited and revised manuscript; J.W.F. and M.A.M. approved final version of manuscript.
REFERENCES
- Aleman TS, LaVail MM, Montemayor R, Ying G, Maguire MM, Laties AM, Jacobson SG, Cideciyan AV. Augmented rod bipolar cell function in partial receptor loss: an ERG study in P23H rhodopsin transgenic and aging normal rats. Vision Res 41: 2779–2797, 2001. [DOI] [PubMed] [Google Scholar]
- Borghuis BG, Looger LL, Tomita S, Demb JB. Kainate receptors mediate signaling in both transient and sustained OFF bipolar cell pathways in mouse retina. J Neurosci 34: 6128–6139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowska J, Trenholm S, Awatramani GB. An intrinsic neural oscillator in the degenerating mouse retina. J Neurosci 31: 5000–5012, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Zhang L, Cembrowski MS, Sabottke CF, Markowitz AL, Butts DA, Kath WL, Singer JH, Riecke H. Intrinsic bursting of AII amacrine cells underlies oscillations in the rd1 mouse retina. J Neurophysiol 112: 1491–1504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N, Fernandez-Sanchez L, Sauve Y, Segura FJ, Martinez-Navarrete G, Tamarit JM, Fuentes-Broto L, Sanchez-Cano A, Pinilla I. Correlation between SD-OCT, immunocytochemistry and functional findings in an animal model of retinal degeneration. Front Neuroanat 8: 151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N, Pinilla I, Sauve Y, Lu B, Wang S, Lund RD. Regressive and reactive changes in the connectivity patterns of rod and cone pathways of P23H transgenic rat retina. Neuroscience 127: 301–317, 2004. [DOI] [PubMed] [Google Scholar]
- DeMarco PJ Jr, Yarbrough GL, Yee CW, McLean GY, Sagdullaev BT, Ball SL, McCall MA. Stimulation via a subretinally placed prosthetic elicits central activity and induces a trophic effect on visual responses. Invest Ophthalmol Vis Sci 48: 916–926, 2007. [DOI] [PubMed] [Google Scholar]
- Demas J, Sagdullaev BT, Green E, Jaubert-Miazza L, McCall MA, Gregg RG, Wong RO, Guido W. Failure to maintain eye-specific segregation in nob, a mutant with abnormally patterned retinal activity. Neuron 50: 247–259, 2006. [DOI] [PubMed] [Google Scholar]
- Farajian R, Pan F, Akopian A, Volgyi B, Bloomfield SA. Masked excitatory crosstalk between the ON and OFF visual pathways in the mammalian retina. J Physiol 589: 4473–4489, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariss RN, Li ZY, Milam AH. Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. Am J Ophthalmol 129: 215–223, 2000. [DOI] [PubMed] [Google Scholar]
- Fei Y. Cone neurite sprouting: an early onset abnormality of the cone photoreceptors in the retinal degeneration mouse. Mol Vis 8: 306–314, 2002. [PubMed] [Google Scholar]
- Fransen JW, Pangeni G, Pardue MT, McCall MA. Local signaling from a retinal prosthetic in a rodent retinitis pigmentosa model in vivo. J Neural Eng 11: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DK, Heine WF, Passaglia CL. The maintained discharge of rat retinal ganglion cells. Vis Neurosci 25: 535–548, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunder T, Kohler K, Guenther E. Distribution and developmental regulation of AMPA receptor subunit proteins in rat retina. Invest Ophthalmol Vis Sci 41: 3600–3606, 2000. [PubMed] [Google Scholar]
- Hack I, Frech M, Dick O, Peichl L, Brandstatter JH. Heterogeneous distribution of AMPA glutamate receptor subunits at the photoreceptor synapses of rodent retina. Eur J Neurosci 13: 15–24, 2001. [PubMed] [Google Scholar]
- Heine WF, Passaglia CL. Spatial receptive field properties of rat retinal ganglion cells. Vis Neurosci 28: 403–417, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM, Milam AH, Lavail MM, Marc RE. Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol 464: 1–16, 2003. [DOI] [PubMed] [Google Scholar]
- Lu B, Morgans CW, Girman S, Lund R, Wang S. Retinal morphological and functional changes in an animal model of retinitis pigmentosa. Vis Neurosci 30: 77–89, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund RD, Lawrence JM, Villegas-Perez MP, Litchfield TM, Sauve Y, Whiteley SJ, Coffey PJ. Retinal degeneration and transplantation in the Royal College of Surgeons rat. Eye (Lond) 12: 597–604, 1998. [DOI] [PubMed] [Google Scholar]
- Machida S, Kondo M, Jamison JA, Khan NW, Kononen LT, Sugawara T, Bush RA, Sieving PA. P23H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci 41: 3200–3209, 2000. [PubMed] [Google Scholar]
- Marc RE, Jones BW. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol 28: 139–147, 2003. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res 22: 607–655, 2003. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Detwiler PB. Cellular origin of spontaneous ganglion cell spike activity in animal models of retinitis pigmentosa. J Ophthalmol 2011: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Gartland AJ, Singer JH, Detwiler PB. Network oscillations drive correlated spiking of ON and OFF ganglion cells in the rd1 mouse model of retinal degeneration. PLoS One 9: e86253, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Newkirk G, Euler T, Detwiler PB. Functional stability of retinal ganglion cells after degeneration-induced changes in synaptic input. J Neurosci 28: 6526–6536, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, Takada M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 80: 757–765, 1995. [DOI] [PubMed] [Google Scholar]
- Menzler J, Zeck G. Network oscillations in rod-degenerated mouse retinas. J Neurosci 31: 2280–2291, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles RD, Zhang C, Muller U, Betz H, McCall MA. Selective glycine receptor alpha2 subunit control of crossover inhibition between the on and off retinal pathways. J Neurosci 32: 3321–3332, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell 77: 361–369, 1994. [DOI] [PubMed] [Google Scholar]
- Peachey NS, Ray TA, Florijn R, Rowe LB, Sjoerdsma T, Contreras-Alcantara S, Baba K, Tosini G, Pozdeyev N, Iuvone PM, Bojang P Jr, Pearring JN, Simonsz HJ, van Genderen M, Birch DG, Traboulsi EI, Dorfman A, Lopez I, Ren H, Goldberg AF, Nishina PM, Lachapelle P, McCall MA, Koenekoop RK, Bergen AA, Kamermans M, Gregg RG. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet 90: 331–339, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YW, Blackstone CD, Huganir RL, Yau KW. Distribution of glutamate receptor subtypes in the vertebrate retina. Neuroscience 66: 483–497, 1995. [DOI] [PubMed] [Google Scholar]
- Pu M, Xu L, Zhang H. Visual response properties of retinal ganglion cells in the royal college of surgeons dystrophic rat. Invest Ophthalmol Vis Sci 47: 3579–3585, 2006. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Gayet-Primo J, Pandey S, Duvoisin RM, Taylor WR. Differential loss and preservation of glutamate receptor function in bipolar cells in the rd10 mouse model of retinitis pigmentosa. Eur J Neurosci 29: 1533–1542, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthussery T, Percival KA, Venkataramani S, Gayet-Primo J, Grunert U, Taylor WR. Kainate receptors mediate synaptic input to transient and sustained OFF visual pathways in primate retina. J Neurosci 34: 7611–7621, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagdullaev BT, Aramant RB, Seiler MJ, Woch G, McCall MA. Retinal transplantation-induced recovery of retinotectal visual function in a rodent model of retinitis pigmentosa. Invest Ophthalmol Vis Sci 44: 1686–1695, 2003. [DOI] [PubMed] [Google Scholar]
- Sauve Y, Girman SV, Wang S, Lawrence JM, Lund RD. Progressive visual sensitivity loss in the Royal College of Surgeons rat: perimetric study in the superior colliculus. Neuroscience 103: 51–63, 2001. [DOI] [PubMed] [Google Scholar]
- Sauve Y, Lu B, Lund RD. The relationship between full field electroretinogram and perimetry-like visual thresholds in RCS rats during photoreceptor degeneration and rescue by cell transplants. Vision Res 44: 9–18, 2004. [DOI] [PubMed] [Google Scholar]
- Sekirnjak C, Hulse C, Jepson LH, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. Loss of responses to visual but not electrical stimulation in ganglion cells of rats with severe photoreceptor degeneration. J Neurophysiol 102: 3260–3269, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirnjak C, Jepson LH, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. Changes in physiological properties of rat ganglion cells during retinal degeneration. J Neurophysiol 105: 2560–2571, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasheff SF. Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. J Neurophysiol 99: 1408–1421, 2008. [DOI] [PubMed] [Google Scholar]
- Stasheff SF, Shankar M, Andrews MP. Developmental time course distinguishes changes in spontaneous and light-evoked retinal ganglion cell activity in rd1 and rd10 mice. J Neurophysiol 105: 3002–3009, 2011. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci USA 97: 11020–11025, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BB, Seiler MJ, Sadda SR, Aramant RB. Superior colliculus responses to light–preserved by transplantation in a slow degeneration rat model. Exp Eye Res 79: 29–39, 2004. [DOI] [PubMed] [Google Scholar]
- Trenholm S, Borowska J, Zhang J, Hoggarth A, Johnson K, Barnes S, Lewis TJ, Awatramani GB. Intrinsic oscillatory activity arising within the electrically coupled AII amacrine-ON cone bipolar cell network is driven by voltage-gated Na+ channels. J Physiol 590: 2501–2517, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Waleszczyk WJ, Benedek G, Burke W, Dreher B. Convergence of Y and non-Y channels onto single neurons in the superior colliculi of the cat. Neuroreport 12: 2927–2933, 2001. [DOI] [PubMed] [Google Scholar]
- Wang L, Sarnaik R, Rangarajan K, Liu X, Cang J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J Neurosci 30: 16573–16584, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis Neurosci 27: 1–8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woch G, Aramant RB, Seiler MJ, Sagdullaev BT, McCall MA. Retinal transplants restore visually evoked responses in rats with photoreceptor degeneration. Invest Ophthalmol Vis Sci 42: 1669–1676, 2001. [PubMed] [Google Scholar]
- Yee CW, Toychiev AH, Sagdullaev BT. Network deficiency exacerbates impairment in a mouse model of retinal degeneration. Front Syst Neurosci 6: 8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]