Abstract
Various PET studies, such as measurements of glucose, serotonin and oxygen metabolism, cerebral blood flow and receptor bindings are availabe for epilepsy. 18Fluoro-2-deoxyglucose (18F-FDG) PET imaging of brain glucose metabolism is a well established and widely available technique. Studies have demonstrated that the sensitivity of interictal FDG-PET is higher than interictal SPECT and similar to ictal SPECT for the lateralization and localization of epileptogenic foci in presurgical patients refractory to medical treatments who have noncontributory EEG and MRI. In addition to localizing epileptogenic focus, FDG-PET provide additional important information on the functional status of the rest of the brain. The main limitation of interictal FDG-PET is that it cannot precisely define the surgical margin as the area of hypometabolism usually extends beyond the epileptogenic zone. Various neurotransmitters (GABA, glutamate, opiates, serotonin, dopamine, acethylcholine, and adenosine) and receptor subtypes are involved in epilepsy. PET receptor imaging studies performed in limited centers help to understand the role of neurotransmitters in epileptogenesis, identify epileptic foci and investigate new treatment approaches. PET receptor imaging studies have demonstrated reduced 11C-flumazenil (GABAA-cBDZ) and 18F-MPPF (5-HT1A serotonin) and increased 11C-cerfentanil (mu opiate) and 11C-MeNTI (delta opiate) bindings in the area of seizure. 11C-flumazenil has been reported to be more sensitive than FDG-PET for identifying epileptic foci. The area of abnormality on GABAAcBDZ and opiate receptor images is usually smaller and more circumscribed than the area of hypometabolism on FDG images. Studies have demonstrated that 11C-alpha-methyl-L-tryptophan PET (to study synthesis of serotonin) can detect the epileptic focus within malformations of cortical development and helps in differentiating epileptogenic from non-epileptogenic tubers in patients with tuberous sclerosis complex. 15O-H2O PET was reported to have a similar sensitivity to FDG-PET in detecting epileptic foci.
Keywords: PET, Epilepsy, FDG, Neurotransmitter, receptor
Introduction
Epilepsy is an ancient Greek word which means to be taken, seized or attacked. Epilepsy is a central nervous system disorder characterized with recurring seizures caused by disrupted nerve cell activity. Almost 1%-2% of the population is affected by epilepsy. At least two unprovoked seizures are required for an epilepsy diagnosis. The etiologic classification of epilepsy includes idiopathic, symptomatic, and cryptogenic epilepsies. Idiopathic epilepsies are believed to have a genetic basis and generally start in childhood. Symptomatic epilepsies typically follow an identified brain insult such as head injuries, low oxygen during birth, brain tumors, infections, stroke, and abnormal blood levels of substances such as sodium or blood sugar. For cryptogenic epilepsies, the cause of the epilepsy is unknown but many presume that a cause could be identified with sufficient investigation.
Epileptic seizures are classified as partial and generalized seizures by International League Against Epilepsy (ILAE) [1]. Seizure that cannot be clearly diagnosed into one of the preceding categories should be considered uncalssified until further information allows their accurate diagnosis. Partial seizures (PS) are the most common form of epilepsies (approximately 50% of patients with epilepsy). PS can involve temporal, frontal, parietal and occipital lobes. Temporal lobe epilepsy (TLE) is the most common form of PS. Mesial TLE (mTLE) is the most frequent form of TLE. Mesial temporal sclerosis (MTS) is present in 60-70% of patients with mTLE. Mesial temporal structures include the hippocampus, amygdala, and parahippocampal gyrus. The frontal lobe is the second most common origin. Frontal lobe epilepsy (FLE) is featured by short seizures occuring several times a day and often during sleep.
The diagnosis of epilepsy includes a complete physical and neurological examination, extracranial or intracranial electroencephalogram (EEG), blood tests, magnetic resonance imaging (MRI), MR spectroscopy, single photon emission computed tomography (SPECT), and positron emission tomography (PET) studies. Treatment with medications usually controls seizures in majority of patients. However, nearly one third of patients with epilepsy become medically intractable and may require surgery. Surgery includes the local resection of the area of seizure. MTS is most often medically refractory, but surgical intervention is relatively effective in majority of the patients. Accurate localization of epileptic focus is important for a successful surgery. Brain perfusion SPECT imaging has been widely used in the detection of epileptic foci. Meta-analytic sensitivities of SPECT in patients with TLE were reported as 44% (interictal), 75% (postictal) and 97% (ictal) [2]. The sensitivities of SPECT in extra-TLE were 66% (ictal), and 40% (interictal) [3,4]. Ictal image is the most sensitive but not easy to obtain as the seizure lasts for few minutes only and the radiotracer must be readily available at the patient’s bedside at the time of seizure onset. PET, particularly PET/computed tomography (PET/CT) provides better quality and higher resolution images as compared to SPECT and allows quantitative measurements. There are various PET tracers to measure glucose, serotonin and oxygen metabolism, cerebral blood flow and receptor bindings in epilepsy. Imaging brain glucose metabolism with 18Fluoro-2-deoxyglucose (FDG) PET is the most commonly utilized technique in routine clinical practice. The other PET studies require well experienced staff as well as on-site equipment and cyclotron. Table 1 summarizes the PET findings in the area of seizure focus.
Table 1.
PET findings in the area of seizure focus in patients with epilepsy
| PET study | Findings |
|---|---|
| Interictal 18F-FDG | Usually reduced metabolism |
| Ictal 18F-FDG | Increased and decreased metabolism (complex pattern)* |
| Post-ictal 18F-FDG metabolism** | Complex pattern, increased or decreased |
| GABAA-cBZR receptor | Reduced binding |
| Opioid receptor | Increased mu and delta receptor bindings |
| Serotonin receptor | Reduced binding |
| Dopamine receptor | Reduced binding |
| 11C-alpha-methyl-L-tryptophan | Increased uptake |
| Interictal 15O-H2O | Usually reduced perfusion |
| Ictal 15O-H2O | Increased perfusion |
due to long brain uptake period of FDG;
depending on the time of injection after seizure.
FDG-PET imaging
FDG brain PET, particularly PET/CT, is a well established and widely available imaging technique. 18F-FDG is a radiolabeled glucose analog measuring glucose metabolism. Glucose is the major energy source for brain. Glucose metabolism is tightly connected to neuronal activity. 18F-FDG is transported from the blood into cells by glucose transporters, predominantly GLUT1. Once in the cell, FDG is phosphorylated by hexokinase to form FDG-6-phosphate. Further metabolism of FDG-6-phosphate is limited and it is essentially trapped in the cell.
FDG brain PET/CT imaging protocol is well described in Society of Nuclear Medicine (SNM) and European Association of Nuclear Medicine (EANM) guidelines [5,6]. Patients should be fasting for 4 to 6 hours prior to the study and avoiding caffeine, alcohol, or drugs that may affect cerebral glucose metabolism. Particularly sedatives, amphetamines, cocaine, narcotics, antipsychotic medications and corticosteroids alter cerebral metabolism. Environment (a quiet, dimly-lit room) should be stable for at least 30 minutes prior to FDG injection and in the subsequent uptake phase (at least 30 minutes). Blood glucose should be checked prior to FDG administration. If it is greater than 150-200 mg/dL, the patient is usually rescheduled. When hyperglycemia is present, high circulating insulin levels drive FDG into muscle and results in reduced uptake in the brain. In diabetic patients, best images are achieved in a euglycemic situation during stable therapeutic management. For preoperative evaluation of epilepsy, continuous EEG recording is recommended. Before intravenous injection of FDG, a low dose (10-30 mAs) CT attenuation correction (AC) images are obtained. FDG dose for adults is 185-740 MBq (5-20 mCi). Pediatric dose is 5.18-7.4 MBq (0.14-0.20 mCi). The dose is less for 3D scanners. Images are usually acquired in static technique. Most systems today use 3D acquisition. Static PET acquisition typically begins 30-60 minutes after FDG injection and lasts for 15-30 minutes. On a state of art PET/CT with high dose FDG (740 MBq, 20 mCi), imaging can be completed in 5 minutes. When absolute quantification of regional metabolic rates of glucose is needed dynamic images are acquired. Dynamic acquisition starts at the time of FDG administration and continues for 60-90 minutes (multipl 5-min frames). Filtered backprojection or iterative methods are used for image reconstruction. Measured AC method is used for AC of the images. Visual inspection with or without semiquantitative analysis is the standard method of interpretation.
In normal adult brain, there is high FDG uptake in cerebral and cerebellar cortices and sub cortical gray matter with mild FDG uptake in the white matter. FDG uptake in children varies with the patient age. Infants typically show diffusely low metabolic activity [7]. After 4 months of age there is a rapid increase in brain glucose metabolism reaching a peak level by about 4 years of age which is higher than adult brain activity [8,9]. There is decrease in cerebral metabolic rates with normal aging, primarily in lateral and medial frontal cortex, and anterior cingulate cortex [10-12]. Abnormal regions demonstrate increased, reduced or absent metabolic activity. Semiquantitative analysis helps to detect regional or global mild abnormalities which are not appearent on visual inspection. Semiquantitative analysis involves comparing FDG uptake in regions of interest over selected brain regions. Semiquantitative analysis can be performed manually or via automated software programs [13-15]. FDG-PET images co-registered to MR images or obtained on an integrated PET/MR scanner provide better structural and functional information. Quantitative analysis (absolute glucose metabolism) requires blood sampling (arterial or arterialized venous blood) with serial measurements. However, in the clinical setting most centers prefer simplified protocols based on static images. PET images are usually obtained in interictal phase. Interictal FDG-PET images usually demonstrate focal hypometabolism. Ictal PET studies are not easy to perform as majority of seizures are unpredictable and short lasting. Another limitation of ictal PET is long brain uptake period of FDG (30-45 minutes) after injection which causes complex pattern of increased and decreased metabolism. Postictal PET can demonstrate complex pattern of increased and decreased metabolism or only increased or decreased metabolism depending on the time of injection after seziure.
Although EEG continues to play a central role in the diagnosis and management of patients with seizure disorders, the sensitivity of EEG is relatively low, ranging between 25-56%. Subclinical seizures in small volumes or deep structures may not be detected with extracranial EEG. Intracranial cortical and particularly depth EEG is invasive and has complications. MRI is highly sensitive and specific for detecting hippocampal sclerosis as well as other structural lesions causing epilepsia but it fails to reveal any apparent abnormality in approximately 20% of the patients with medically refractory epilepsy. MRI may miss mild hippocampal sclerosis and subtle structural lesions. Advanced MRI techniques, such as MR spectroscopy, MR volumetry, diffusion tensor imaging, MR perfusion, and functional MR imaging may provide complementary information but they require high-performance MRI scanners. Magnetoencephalography (MEG), a non-invasive technique for investigating human brain activity, has limited value in identifying epileptic activities in deep-seated areas such as mesial temporal lobe.
Large number of studies have demonstrated that FDG-PET is highly sensitive for presurgical localization of epileptogenic foci in patients with medically refractory partial epilepsy who have noncontributory EEG and MRI. Figure 1 shows ictal SPECT and interictal FDG-PET images of a man with left complex partial seizures with false negative MRI [16]. The sensitivities of FDG-PET in TLE and extra-TLE were reported as 84% and 33%, respectively, in a meta analytic study in 1994 [3]. In the following years, some of the reported sensitivitities of PET for TLE and extra-TLE were 87-90% and 38–55%, respectively [14,17-19]. In 46 patients with PS and noncontributory EEG, FDG-PET demonstrated unilateral temporal hypometabolism in 26 patients and 18 of 23 were seizure-free after temporal lobectomy [20]. There was also unilateral frontotemporal hypometabolism in 5 patients and frontal hypometabolism in another patient. Interictal PET was able to lateralize the seizure focus in 95% of MRI positive, 69% of MRI equivocal and 84% of MRI negative patients [21]. PET helped in decision making in 53% of presurgical patients with normal or discordant MRI [22]. Extra-TLE is higher in children and MRI usually do not show a discrete lesion. In children with frontal lobe epilepsy, the sensitivity and specificity of FDG-PET were 92% and 62.5%, respectively [23].
Figure 1.
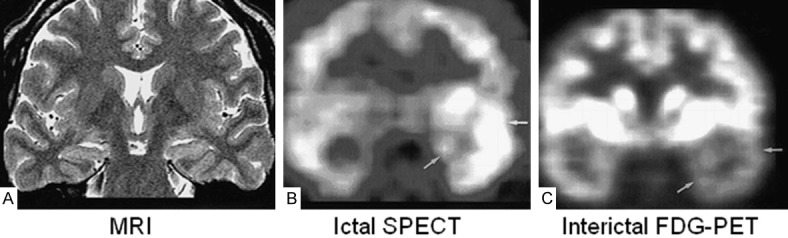
False-negative MR findings in a 30-year-old man with left complex partial seizures. Ictal activity was shown in left temporal area on video/EEG. A. Oblique coronal fast spin-echo T2-weighted MR image (4000/120/4) shows no abnormalities. B. Ictal SPECT scan shows hyperperfusion in left temporal lobe (arrows). Radioactivity ratio between right temporal cortex/left temporal cortex was 1:2.4. C. FDG-PET scan shows hypometabolism in left temporal lobe (arrows). Radioactivity ratio between right temporal cortex/left temporal cortex was 1:0.7. Invasive EEG showed ictal activity in left temporal lobe. After left anterior temporal lobectomy, pathologic diagnosis was hippocampal sclerosis of a mild degree associated with mild cortical dysplasia. Engel’s outcome was class II. Reprinted with permission from American Society of Neuroradiology [16].
In majority of FDG-PET studies, the images are obtained in interictal phase. Interictal FDG-PET was reported to be more sensitive than interictal perfusion SPECT [3]. Studies have demonstrated that there is more reduction in regional cerebral glucose metabolic rates than in regional cerebral perfusion in interictal period [24-27]. Interictal PET is a very sensitive technique for lateralization and general localization of epileptogenic focus, but it cannot precisely define the surgical margin as the area of hypometabolism usually extends beyond the epileptogenic zone. FDG-PET can not always differentiate mesial from lateral TLE as glucose hypometabolism may extend to the lateral aspect of the abnormal temporal lobe.
Studies have compared interictal FDG-PET to ictal SPECT [16,28-30]. In 117 patients who underwent surgery for intractable neocortical epilepsy, interictal PET and ictal SPECT correctly localized the lesions in 77.7%, and 70.3% of the patients, respectively [30]. In another study, interictal PET and ictal SPECT correctly lateralized the lesion in 85%, and 73% of patients, respectively [16]. Studies have also compared interictal PET with ictal subtraction SPECT (interictal SPECT fused, normalized and subtracted from ictal SPECT) [31,32]. In one report, interictal PET had 56% sensitivity and ictal subtraction SPECT had 87% in the detection of sezire foci [31]. In children, ictal subtraction SPECT coregistered to MR imaging (SISCOM) localized the epileptic focus in 67% of the patients. This was 57% with FDG-PET [32]. Ictal subtraction SPECT and SISCOM appear to increase the sensitivity of ictal SPECT, but their main limitation is to obtain two SPECT studies, one in interictal and one in ictal period.
Semiquantitative analysis of PET data and co-registration of PET image with MR increase the sensitivity of PET. Semiquantitative analysis can identify mild abnormalities which are not appearent on visual inspection. Drzezga et al. demonstrated that automated analysis of PET was more sensitive than visual analysis in patients with TLE and extra-TLE [14]. The benefits of PET and MR coregistration in presurgical evaluation of medically refractory epilepsy have also been demonstrated in several studies [33,34].
Studies have demonstrated that FDG-PET can predict surgical outcome. Greater severity of preoperative hypometabolism in the resected temporal lobe was associated with significantly better postoperative seizure control [35-37]. Severe extratemporal and bilateral hypometabolism was associated with a higher incidence of postoperative seizures [38,39]. Ipsilateral PET hypometabolism showed a predictive value of 86% for good outcome in meta-analysis of 46 studies [40].
There is limited data on ictal PET imaging [40-45]. Ictal PET studies were performed either in patients with status epilepticus (SE) or induced/provoked seizures [41-43]. SE is an epileptic seizure of greater than thirty minutes or more than one seizure within a thirty minute period without the person returning to normal between them. FDG-PET helped to establish the diagnosis in 8 patients when clinical features, MRI and EEG were incongruent regarding the origin of SE [41]. FDG-PET revealed hypermetabolism in the left orbitofrontal region in a patient suspicious for ongoing nonconvulsive frontal SE [42]. Subsequent FDG-PET following 5 days of oxcarbazepine therapy demonstrated resolution of the hypermetabolic focus in this patient. PET scan obtained in a patient exposed to seizure-eliciting music showed hypometabolism in the right lateral temporal lobe with an area of increased metabolism in the right anteromesial temporal lobe [44].
Postictal PET studies performed at different intervals following most recent seziure showed hyper or hypometabolism depending on the time of injection after seizure [46,47]. In early postictal phase (seizures within 15 min prior to FDG injection) PET revealed focal hypermetabolism in 3 pediatric patients [46]. The most severe regional hypometabolism occurred more than 48 hours after the seizure and the least severe hypometabolism was at 24-48 hours postictally [47]. The metabolism was intermediate in the first 24 hours. The authors have concluded that it may take longer than 24 hours after a partial seizure to return to its baseline state.
In addition to localizing epileptogenic focus, presurgical PET provides important information on the functional status of rest of the brain. Interictal FDG-PET is considered to be the best imaging technique to assess the functional deficit zone (FDZ). FDZ is defined as the brain area that shows abnormal functioning in the interictal period. Extratemporal hypometabolism is not uncommon in TLE and is associated with a poor seizure outcome after surgery. In forty seven patients with TLE, 18 patients had hypometabolism only in the ipsilateral temporal cortex; the remaining 29 patients had additional cortical hypometabolism confined to the ipsilateral, contralateral or bilateral cerebral cortex [48]. Bilateral temporal lobe hypometabolism (BTH) can be seen in TLE. Seizure-onset zone in patients with BTH may be unilateral or bilateral. BTH in unilateral TLE causes conflict in lateralizing the epileptogenic zone. It was recommended to perform PET scan more than 2 days after the last seizure to avoid this confliction [49]. Chronic epilepsy generally impairs cognition. Depression is common in TLE and after temporal lobectomy and associated with hypometabolism in the frontal lobe.
PET receptor imaging
There are alterations in the neurotransmitters and subreceptors in epilepsy. It is believed that there is abnormally high level of excitatory neurotransmitters increasing neuronal activity or abnormally low level of inhibitory neurotransmitters decreasing neuronal activity in epilepsy. Excitatory glutamatergic neurotransmission is responsible for the initiation and spread of seizure activity, particularly excessive activation of glutamate receptor 5 (mGluR5) [50,51]. One of the most-studied neurotransmitters that plays a role in epilepsy is γ-aminobutyric acid (GABA). GABA is an inhibitory neurotransmitter. GABA mediated synaptic inhibition is known to be critical in regulating epileptic activity [52]. Endogenous opioid peptides are also involved in epilepsy. Opioid peptides have anticonvulsant action and limit the spread of electrical activity [53]. Increased levels of serotonin have been observed in epileptogenic lesions. Serotonin exerts antiseizure effects in experimental models mediated by 5-HT1A receptors [54]. Alterations of different dopamine receptor subtypes, particularly D1 and D2, have been associated to different forms of epilepsy [55,56]. Multiple adenosine receptor subtypes are involved in epilepsy. In particular, adenosine A1 receptor subtype has a role to regulate seizure activity [57]. Histamine 3 receptor subtype, nicotinic and muscarinic acetylcholine receptors (nAChR and mAChR) are also believed to be involved in the pathophysiology of epilepsy.
PET receptor imaging studies have been utilized to understand the role of neurotransmitters in the epileptogenesis and spread of epileptic activity as well as to identify and localize epileptogenic regions and investigate new treatment approaches. There are large number of PET tracers for receptor imaging in human and animal models. Table 2 summarizes PET receptor imaging tracers used in patients with epilepsy. Table 3 lists the abbreviations for clinical terms.
Table 2.
PET tracers for receptor imaging in patients with epilepsy
| Receptors | PET tracer | Receptor subtypes |
|---|---|---|
| GABA | 11C flumazenil (FMZ)* | GABAA-cBZR |
| Opioid | 11C-carfentanil (CFN)** | mu |
| 11C-MeNTI* | delta | |
| 11C-diprenorphine (DPN)* | mu, delta, kappa | |
| 18F-cyclofoxy* | mu, kappa | |
| Serotonin | 18F-MPPF* | 5-HT1A |
| 11C-WAY-100635* | 5-HT1A | |
| 18F-FCWAY* | 5-HT1A | |
| Dopamine | 18F-fallypride* | D2/D3 |
| Acetylcholine | 18F-FA-85380 (2FA)** | nicotinic-α4β2 |
| 76Br-BDEX* | muscarinic |
Receptor antagonist;
Receptor agonist;
GABAA-cBZR: γ Aminobutyric acid A-central benzodiazepine receptor; MeNTI: N1’-methylnaltrindole MeNTI; MPPF: 2’-methoxyphenyl-(N-2’-pyridinyl)-p-fluoro-benzamidoethyipiperazine; 5-HT1A: 5-hydroxytryptamine 1A receptor WAY-100635: (3) H-(N-(2-(1-(4-(2-methoxyphenyl)-1-piperazinyl) ethyl)-N-(2-pyridyl) cyclohexane-carboxamide; FCWAY: N-{2-[4-(2-methoxyphenyl) piperazino]}-N-(2-pyridinyl) trans-4-fluorocyclohexanecarboxamide; FA-85380: fluoro-A-85380; BDEX: 4-bromodexetimide.
Table 3.
Abbreviations for clinical terms
| Abbreviation | Definition |
|---|---|
| FCD | Focal cortical dysplasia |
| HS | Hippocampalsclerosis |
| mTLE | Mesial temporal lobe epilepsy |
| MTS | Mesial temporal sclerosis |
| LTC | Lateral temporal cortex |
| PS | Partial seizures |
| SE | Status epilepticus |
| TLE | Temporal lobe epilepsy |
| TSC | Tuberous sclerosis complex |
| VNS | Vagus nerve stimulation |
11C-flumazenil (FMZ) PET studies targeting GABAA-central benzodiazepine receptor (GABAA-cBZR) complex have demonstrated reduced binding of tracer in epileptic foci in patients with partial epilepsy [58-63]. FMZ-PET has been found to be more sensitive and accurate than FDG-PET in the detection of cortical regions of seizure onset in patients with TLE and extra-TLE epilepsy [58,59,64]. The area of abnormality on FMZ-PET images was smaller and more circumscribed than the area of hypometabolism on FDG-PET images (Figure 2) [59,61,62,65]. In patients with mTLE, mesial temporal structures showed reduced FMZ binding and reduction of FMZ binding was restricted to the area of hippocampal sclerosis (HS) with no abnormalities being detected in the temporal neocortex or elsewhere in the neocortex [61,62]. The reduction in glucose metabolism was more widespread and often involved lateral temporal cortex (LTC) [61]. In one hundred epileptic patients undergoing pre-surgical evaluation, 73% of patients demonstrated FMZ-PET abnormality which was significantly higher in TLE (94%) than in other types of epilepsy (50%) [65]. Increased periventricular FMZ binding was reported to be associated with poor outcome and failure to become seizure free after surgery for TLE with HS [66,67]. Focal alterations of the BZD receptor density or affinity were not demonstrated in patients with generalized epilepsy [68].
Figure 2.
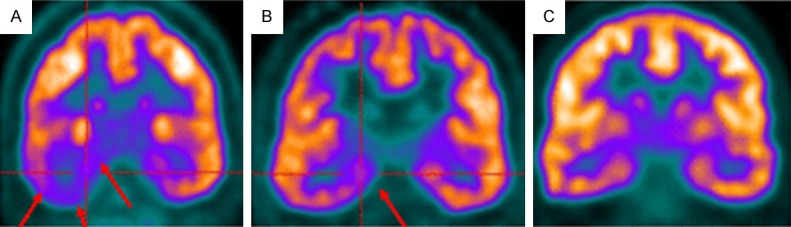
FDG-PET image shows extensive hypometabolism throughout right temporal lobe (arrows) (A). FMZ-PET image shows more restricted localization to mesial temporal region in same patient (arrows) (B). Symmetric FMZ distribution in control subject (C). Reprinted with permission from The Society of Nuclear Medicine and Molecular Imaging [62].
Opioid-receptor PET studies have supported the relationship between opioid peptide receptor availability and seizures. Increased mu and delta receptor bindings in the temporal cortex ipsilateral to the focus were reported in interictal PET studies with 11C-cerfentanil (CFN) and 11C-N1’-methylnaltrindole (MeNTI) [69-71]. Increases in opioid receptor bindings were smaller regionally than were decreases in FDG uptake [70]. Increased mu receptor binding were confined to the middle aspect of the inferior temporal cortex, whereas binding of delta receptors increased in the mid-inferior temporal cortex and anterior aspect of the middle and superior temporal cortex [70]. Various findings were reported with non-spesific (mu, delta, and kappa) opioid receptor agonist, 11C-diprenorphine (DPN). Interictal DPN-PET demonstrated no changes in non-spesific opiate receptor binding in TLE and idiopathic generalised epilepsy [71,72]. Reduced interictal DPN binding in medial temporal lobe and LTC was reported in 2 patients with mTLE [73]. Post-operatively, there was a further reduction of DPN binding in LTC in these patients. Within hours of spontaneous temporal lobe seizures, postictal binding of DPN was increased in the temporal pole and fusiform gyrus ipsilateral to the seizure focus which gradually returned to baseline [74]. Partial-volume effect (PVE)-corrected DPN-PET images showed post-ictal increases in ipsilateral fusiform gyri and lateral temporal pole which was not evident in uncorrected datasets [75]. After provocation of serial absence seizures, reduced DPN retention in the association cortex has been reported suggesting that endogenous opioids are released in the association cortex at the time of serial absences, lead to increased receptor occupancy, and may have an important role in the pathophysiology of generalised absences [76]. Ictal DPN binding to opioid receptors was reduced in the left parieto-temporo-occipital cortex (Brodmann area 37) in reading-epilepsy [77].
PET studies with various serotonin 5-HT1A receptor antagonists (18F-FCWAY, 11C-WAY-100635, and 18F-MPPF) demonstrated a reduced serotonin 5-HT1A receptor binding in the epileptogenic temporal lobe [78-86]. In addition to reduced binding in the mesial temporal structures, there was also involvement of rest of the temporal regions as well as other cortical and limbic regions. Figure 3 shows 11C-WAY-100635 PET images of a patient with with MRI-negative temporal lobe epilepsy [86]. Depression is the most frequent psychiatric disorder in epilepsy. A relationship between hippocampal 18F-FCWAY 5HT1A binding and depressive symptoms was reported in patients with TLE and symptoms of depression [87]. To evaluate 5-HT transport and 5-HT1A receptors in TLE and depression, patients were studied with 11C-DASB and 18F-FCWAY [88]. There was increased 11C-DASB asymmetry in insula and fusiform gyrus and relatively reduced transporter activity in subjects with both TLE and depression, as compared to subjects with TLE alone. PET studies in patients with mTLE have demonstrated that decrease in 5-HT1A receptor binding in temporal regions may play a role in memory impairment [89].
Figure 3.
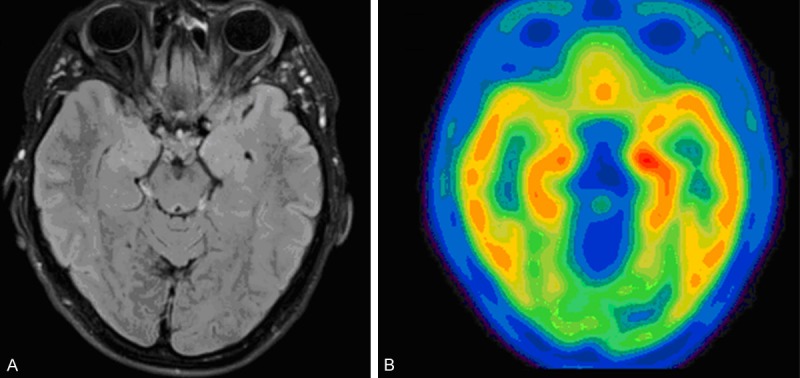
11C-WAY-100635 PET in a patient with MRI-negative temporal lobe epilepsy and a right temporal EEG focus. A. T1-weighted axial MRI reveals no structural abnormality. B. 11C-WAY-100635 PET imaging shows asymmetric binding with a decrease in both mesial and lateral structures of the right temporal lobe. Reprinted with permission from Elsevier Limited [86].
PET studies with various other receptor imaging tracers were also performed in patients and in animal models. PET imaging with a dopamine receptor antagonist, 18F-fallypride, revealed reduced D2 and D3 receptor bindings at the pole and in lateral aspects of the epileptogenic temporal lobe in patients with mTLE and HS [90]. Patients with juvenile myoclonic epilepsy showed a reduction in D2 and D3 receptor bindings restricted to the bilateral posterior putamen, suggesting a specific alteration of the dopaminergic system [91]. Glutamate receptor subtype mGluR5 is an attractive target in epilepsy. Changes of mGluR5 were evaluated using 11C-ABP688 PET during the epileptogenesis in a pilocarpine-induced epilepsy rat model [92]. In chronic epilepsy, 11C-ABP688 binding was reduced in hippocampus and amygdala, whereas in acute period after SE mGluR5 binding was reduced in the whole brain. In subacute period, mGluR5 binding was restored in caudate-putamen, while it was still lower in the rest of the brain. In chronic period, global mGluR5 binding was normalized except in hippocampus and amygdala. PET study with nicotinic AChR agonist, 18F-FA-85380, demonstrated a regional nAChR density decrease in the prefrontal cortex in patients with autosomal dominant nocturnal frontal lobe epilepsy [93]. Reduced 76Br-BDEX (muscarinic AChR antagonist) concentration was reported in the temporal lobe ipsilateral to the seizure focus in patients with mTLE [94]. To determine N-methyl-D-aspartate (NMDA) receptor changes, 11C-(S)-[N-methyl] ketamine PET was performed in patients with mTLE [95]. There was 9-34% reduction of tracer radioactivity in the temporal lobes of ictal onset.
Other PET studies
11C-alpha-methyl-L-tryptophan (AMT) is a radiolabeled tryptophan analogue to study synthesis of serotonin in the brain. Tryptophan is the precursor of serotonin. Interictal PET studies have demonstrated a focal increased uptake of AMT in the epileptogenic area in patients with TLE, cortical dysplasia, cryptogenic partial epilepsy, tuberous sclerosis complex (TSC), and cortical developmental malformations (Figure 4) [96-100]. Majority of patients with TSC have seizures. AMT-PET helped in differentiating epileptogenic from non-epileptogenic tubers in patients with TSC [100]. PET localization was mostly seen in patients with frequent interictal abnormalities on the EEG [97]. Studies of brain tissue subsequent to epilepsy surgery in patients with TSC implicated the kynurenine pathway of tryptophan metabolism as a primary mechanism of increased brain tissue retention of AMT in epileptogenic brain regions, rather than alterations in serotonin synthesis [101]. AMT-PET can detect the epileptic focus within malformations of cortical development. Focal increase of cortical AMT uptake in children was less sensitive but more specific for the lobe of seizure onset than corresponding FDG-PET hypometabolism, and it was often associated with epileptogenic cortical developmental malformations [102]. In children with intractable, neocortical epilepsy with and without malformations of cortical development, the specificity of AMT-PET for detecting seizure onset lobe was equally high in lesional (97%) and nonlesional groups (100%), whereas sensitivity was higher in the lesional than the nonlesional group (47% versus 29%) [99].
Figure 4.
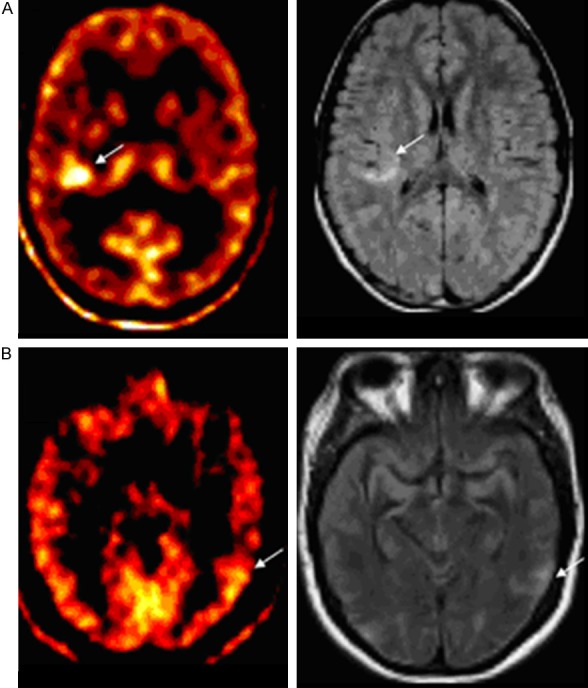
Increased AMT uptake in cortical tubers (arrows). A. Clearly MRI hyperintense right perisylvian tuber in a patient. B. Subtly MRI hyperintense left temporal tuber in another patient. Reprinted with permission from John Wiley and Sons [96].
Oxygen-15 water (15O-H2O) is an inert, diffusible flow tracer. It allows quantitative measurement of rCBF. The physical half-life of 15O is approximately 2 minutes and its production requires an on-site cyclotron. 15O-H2O PET was performed for both identification of the language dominant hemisphere and in the lateralization of the epileptic focus in preoperative patients with complex partial seizures (CPS) [103]. Intracarotid amytal procedure was discordant with PET language mapping in 1 out of 24 cases. For epileptic focus lateralization, 15O-H2O PET was highly sensitive (87%) and specific (100%). Figure 5 demonstrates 15O-H2O PET images of a patient with bilateral temporal lobe epilepsy [103]. In the analysis of 35 patients who had an anterior temporal lobectomy for medically intractable seizures FDG and 15O-H2O were highly correlated in demonstrating the epileptic focus [104]. FDG and 15O-H2O PET showed significant asymmetries in 83% and 77% of cases, respectively. Using 15O-H2O PET, Henry et al. evaluated acute blood flow changes and efficacy of vagus nerve stimulation (VNS) in partial epilepsy [105]. Seizure-frequency changes ranged from 71% decrease to 12% increase during VNS. Only the right and left thalami showed significant associations of rCBF change with seizure-frequency change. Increased right and left thalamic CBF correlated with decreased seizures. PET with 15O steady state or bolus inhalation technique was used to provide quantitative values of regional CBF, oxygen consumption (CMRO2) and oxygen extraction ratio (OER) in patients with CPS during the interictal state and in patients during SE [106,107]. Interictal scans showed zone(s) of hypoperfusion without significant variation of the OER in approximately 80% of patients [106]. In all cases, ictal scans revealed a focal or multifocal increase in CBF and CMRO2. Significant interictal changes in rCMRO and rCBF were demonstrated in patients with CPS [107]. While the hemisphere containing the abnormal focus showed the more marked changes, particularly with respect to rCMRO2 the contralateral hemisphere was also abnormal with respect to normal aged matched controls.
Figure 5.
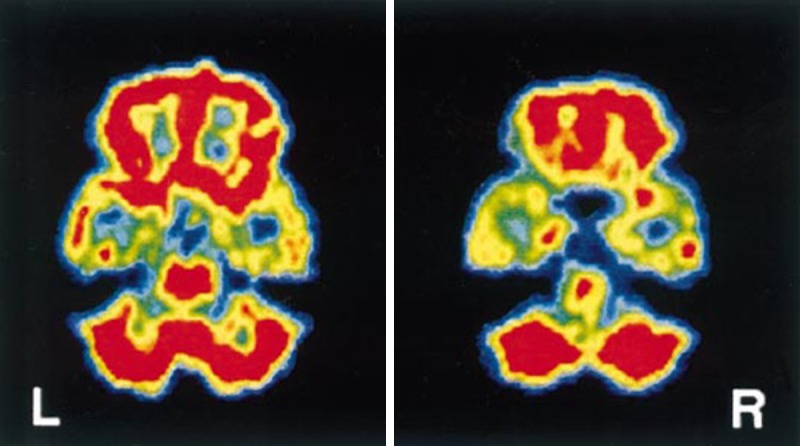
Interictal 15O-H2O PET images in a patient with bilateral temporal lobe epilepsia. Note relative hypoperfusion of temporal lobes compared to the whole brain. Reprinted with permission from John Wiley and Sons [103].
In some TLE patients with amygdala enlargement, an increased 11C-methionine (MET) uptake (a radiolabeled amino acid measuring protein synthesis) was observed in the enlarged amygdala [108]. Focal cortical dysplasia (FCD), a neuronal migration disorder, has been recognized as a cause of intractable epilepsy. MET-PET was useful for identifying FCD as a high uptake area [109].
Conclusion
Interictal FDG-PET is more sensitive than interictal SPECT and has similar sensitivity to ictal SPECT for presurgical localization of epileptic foci in patients with noncontributory EEG and normal MR or MR findings discordant with the EEG findings. In addition to localizing epileptic focus, FDG-PET provides additional important information on the functional status of the rest of the brain. The main limitation of interictal FDG-PET is that it cannot precisely define the surgical margin as the area of hypometabolism usually extends beyond the epileptogenic zone. FDG-PET can not always differentiate mesial from lateral TLE as glucose hypometabolism may extend to the lateral aspect of the abnormal temporal lobe.
PET studies have demonstrated reduced binding of specific tracers to GABAA-cBDZ and 5-HT1A serotonin receptors as well as increased binding to mu and delta opiate receptors in the area of seizure. FMZ-PET (GABAA-cBDZ receptor binding) has been reported to be more sensitive than FDG-PET for identfying epileptic foci. The area of abnormality on GABAA-cBDZ and opiate receptor binding images is usually smaller and more circumscribed than the area of hypometabolism on FDG images which is important for precise definition of surgical margin. 5-HT1A serotonin receptor binding can be reduced not only in mesial temporal structures but also in the rest of the temporal regions as well as other cortical and limbic regions. Depression is common in patients with epilepsy. 5-HT1A PET serotonin receptor imaging studies can help to understand the association between epilepsy and depression. Studies have demonstrated that AMT-PET (to study synthesis of serotonin) can detect the epileptic focus within malformations of cortical development and helps in differentiating epileptogenic from non-epileptogenic tubers in patients with TSC. A focus of increased AMT uptake is highly specific for epileptic focus. 15O-H2O PET perfusion imaging has high sensitivity in demonstrating the epileptic focus. Despite their many advantages, majority of non-FDG brain PET studies are not widely available and performed in limited centers only as they require well experienced staff with on-site radiochemistry equipment and cyclotron.
Disclosure of conflict of interest
None.
References
- 1.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshé SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 2.Devous MD Sr, Thisted RA, Morgan GF, Leroy RF, Rowe CC. SPECT brain imaging in epilepsy: a meta-analysis. J Nucl Med. 1998;39:285–93. [PubMed] [Google Scholar]
- 3.Spencer SS. The relative contributions of MRI SPECT and PET imaging in epilepsy. Epilepsia. 1994;35:S72–S89. doi: 10.1111/j.1528-1157.1994.tb05990.x. [DOI] [PubMed] [Google Scholar]
- 4.Weil S, Noachtar S, Arnold S, Yousry TA, Winkler PA, Tatsch K. Ictal ECD-SPECT differentiates between temporal and extratemporal epilepsy: confirmation by excellent postoperative seizure control. Nucl Med Commun. 2001;22:233–7. doi: 10.1097/00006231-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Society of Nuclear Medicine Procedure Guideline for FDG PET Brain Imaging Version 1.0. approved February 8, 2009. [Google Scholar]
- 6.Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, Darcourt J, Kapucu OL, Tatsch K, Bartenstein P, Van Laere K European Association of Nuclear Medicine Neuroimaging Committee. EANM procedure guidelines for PET brain imaging using [18F] FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–10. doi: 10.1007/s00259-009-1264-0. [DOI] [PubMed] [Google Scholar]
- 7.Stanescu L, Ishak GE, Khanna PC, Biyyam DR, Shaw DW, Parisi MT. FDG PET of the brain in pediatric patients: imaging spectrum with MR imaging correlation. Radiographics. 2013;33:1279–303. doi: 10.1148/rg.335125152. [DOI] [PubMed] [Google Scholar]
- 8.Chugani HT, Phelps ME. Imaging human brain development with positron emission tomography. J Nucl Med. 1991;32:23–6. [PubMed] [Google Scholar]
- 9.Kennedy C, Sokoloff L. An adaptation of the nitrous oxide method to the study of the cerebral circulation in children: normal values for cerebral blood flow and cerebral metabolic rate in childhood. J Clin Invest. 1957;36:1130–1137. doi: 10.1172/JCI103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frölich L, Schönknecht P, Ito K, Mielke R, Kalbe E, Zündorf G, Delbeuck X, Pelati O, Anchisi D, Fazio F, Kerrouche N, Desgranges B, Eustache F, Beuthien-Baumann B, Menzel C, Schröder J, Kato T, Arahata Y, Henze M, Heiss WD. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–16. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 11.Loessner A, Alavi A, Lewandrowski KU, Mozley D, Souder E, Gur RE. Regional cerebral function determined by FDG-PET in healthy volunteers: normal patterns and changes with age. J Nucl Med. 1995;36:1141–9. [PubMed] [Google Scholar]
- 12.Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, Grady C, Pietrini P, Eidelberg D. The metabolic topography of normal aging. J Cereb Blood Flow Metab. 1996;16:385–98. doi: 10.1097/00004647-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ferrie CD, Marsden PK, Maisey MN, Robinson RO. Visual and semiquantitative analysis of cortical FDG-PET scans in childhood epileptic encephalopathies. J Nucl Med. 1997;38:1891–4. [PubMed] [Google Scholar]
- 14.Drzezga A, Arnold S, Minoshima S, Noachtar S, Szecsi J, Winkler P, Römer W, Tatsch K, Weber W, Bartenstein P. 18F-FDG PET studies in patients with extratemporal and temporal epilepsy: evaluation of an observer-independent analysis. J Nucl Med. 1999;40:737–46. [PubMed] [Google Scholar]
- 15.Hikima A, Mochizuki H, Oriuchi N, Endo K, Morikawa A. Semiquantitative analysis of interictal glucose metabolism between generalized epilepsy and localization related epilepsy. Ann Nucl Med. 2004;18:579–84. doi: 10.1007/BF02984579. [DOI] [PubMed] [Google Scholar]
- 16.Won HJ, Chang KH, Cheon JE, Kim HD, Lee DS, Han MH, Kim IO, Lee SK, Chung CK. Comparison of MR imaging with PET and ictal SPECT in 118 patients with intractable epilepsy. AJNR Am J Neuroradiol. 1999;20:593–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Gaillard WD, Bhatia S, Bookheimer SY, Fazilat S, Sato S, Theodore WH. FDGPET and volumetric MRI in the evaluation of patients with partial epilepsy. Neurology. 1995;45:123–126. doi: 10.1212/wnl.45.1.123. [DOI] [PubMed] [Google Scholar]
- 18.Knowlton RC, Laxer KD, Ende G, Hawkins RA, Wong ST, Matson GB, Rowley HA, Fein G, Weiner MW. Presurgical multimodality neuroimaging in electroencephalographic lateralized temporal lobe epilepsy. Ann Neurol. 1997;42:829–837. doi: 10.1002/ana.410420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YK, Lee DS, Lee SK, Chung CK, Chung JK, Lee MC. (18)F-FDG PET in localization of frontal lobe epilepsy: comparison of visual and SPM analysis. J Nucl Med. 2002;43:1167–74. [PubMed] [Google Scholar]
- 20.Theodore WH, Sato S, Kufta CV, Gaillard WD, Kelley K. FDG-positron emission tomography and invasive EEG: seizure focus detection and surgical outcome. Epilepsia. 1997;38:81–6. doi: 10.1111/j.1528-1157.1997.tb01081.x. [DOI] [PubMed] [Google Scholar]
- 21.Gok B, Jallo G, Hayeri R, Wahl R, Aygun N. The evaluation of FDG-PET imaging for epileptogenic focus localization in patients with MRI positive and MRI negative temporal lobe epilepsy. Neuroradiology. 2013;55:541–50. doi: 10.1007/s00234-012-1121-x. [DOI] [PubMed] [Google Scholar]
- 22.Rathore C, Dickson JC, Teotónio R, Ell P, Duncan JS. The utility of 18F-fluorodeoxyglucose PET (FDG PET) in epilepsy surgery. Epilepsy Res. 2014;108:1306–14. doi: 10.1016/j.eplepsyres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 23.da Silva EA, Chugani DC, Muzik O, Chugani HT. Identification of frontal lobe epileptic foci in children using positron emission tomography. Epilepsia. 1997;38:1198–208. doi: 10.1111/j.1528-1157.1997.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 24.Leiderman DB, Balish M, Sato S, Kufta C, Reeves P, Gaillard WD, Theodore WH. Comparison of PET measurements of cerebral blood flow and glucose metabolism for the localization of human epileptic foci. Epilepsy Res. 1992;13:153–7. doi: 10.1016/0920-1211(92)90071-z. [DOI] [PubMed] [Google Scholar]
- 25.Fink GR, Pawlik G, Stefan H, Pietrzyk U, Wienhard K, Heiss WD. Temporal lobe epilepsy: evidence for interictal uncoupling of blood flow and glucose metabolism in temporomesial structures. J Neurol Sci. 1996;137:28–34. doi: 10.1016/0022-510x(95)00323-t. [DOI] [PubMed] [Google Scholar]
- 26.Zubal IG, Avery RA, Stokking R, Studholme C, Corsi M, Dey H, Seibyl JP, Spencer SS. Ratioimages calculated from interictal positron emission tomography and single-photon emission computed tomography for quantification of the uncoupling of brain metabolism and perfusion in epilepsy. Epilepsia. 2000;41:1560–6. doi: 10.1111/j.1499-1654.2000.001560.x. [DOI] [PubMed] [Google Scholar]
- 27.Buch K, Blumenfeld H, Spencer S, Novotny E, Zubal IG. Evaluating the accuracy of perfusion/metabolism (SPET/PET) ratio in seizure localization. Eur J Nucl Med Mol Imaging. 2008;35:579–88. doi: 10.1007/s00259-007-0550-y. [DOI] [PubMed] [Google Scholar]
- 28.Ho SS, Berkovic SF, Berlangieri SU, Newton MR, Egan GF, Tochon-Danguy HJ, McKay WJ. Comparison of ictal SPECT and interictal PET in the presurgical evaluation of temporal lobe epilepsy. Ann Neurol. 1995;37:738–45. doi: 10.1002/ana.410370607. [DOI] [PubMed] [Google Scholar]
- 29.Bouilleret V, Valenti MP, Hirsch E, Semah F, Namer IJ. Correlation between PET and SISCOM in temporal lobe epilepsy. J Nucl Med. 2002;43:991–8. [PubMed] [Google Scholar]
- 30.Hwang SI, Kim JH, Park SW, Han MH, Yu IK, Lee SH, Lee DS, Lee SK, Chung CK, Chang KH. Comparative analysis of MR imaging, positron emission tomography, and ictal single-photon emission CT in patients with neocortical epilepsy. Am J Neuroradiol. 2001;22:937–46. [PMC free article] [PubMed] [Google Scholar]
- 31.Desai A, Bekelis K, Thadani VM, Roberts DW, Jobst BC, Duhaime AC, Gilbert K, Darcey TM, Studholme C, Siegel A. Interictal PET and ictal subtraction SPECT: sensitivity in the detection of seizure foci in patients with medically intractable epilepsy. Epilepsia. 2013;54:341–50. doi: 10.1111/j.1528-1167.2012.03686.x. [DOI] [PubMed] [Google Scholar]
- 32.Perissinotti A, Setoain X, Aparicio J, Rubí S, Fuster BM, Donaire A, Carreño M, Bargalló N, Rumiá J, Garcia-Fructuoso G, Mayoral M, Sanmartí F, Pons F. Clinical Role of Subtraction Ictal SPECT Coregistered to MR Imaging and 18F-FDG PET in Pediatric Epilepsy. J Nucl Med. 2014;55:1099–1105. doi: 10.2967/jnumed.113.136432. [DOI] [PubMed] [Google Scholar]
- 33.Murphy MA, O’Brien TJ, Morris K, Cook MJ. Multimodality image-guided surgery for the treatment of medically refractory epilepsy. J Neurosurg. 2004;100:452–62. doi: 10.3171/jns.2004.100.3.0452. [DOI] [PubMed] [Google Scholar]
- 34.Lee KK, Salamon N. [18F] fluorodeoxyglucose-positron-emission tomography and MR imaging coregistration for presurgical evaluation of medically refractory epilepsy. AJNR Am J Neuroradiol. 2009;30:1811–6. doi: 10.3174/ajnr.A1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radtke RA, Hanson MW, Hoffman JM, Crain BJ, Walczak TS, Lewis DV, Beam C, Coleman RE, Friedman AH. Temporal lobe hypometabolism on PET: Predictor of seizure control after temporal lobectomy. Neurology. 1993;43:1088–1092. doi: 10.1212/wnl.43.6.1088. [DOI] [PubMed] [Google Scholar]
- 36.Theodore WH, Sato S, Kufta C, Balish MB, Bromfield EB, Leiderman DB. Temporal lobectomy for uncontrolled seizures: The role of positron emission tomography. Ann Neurol. 1992;32:789–794. doi: 10.1002/ana.410320613. [DOI] [PubMed] [Google Scholar]
- 37.Manno EM, Sperling MR, Ding X, Jaggi J, Alavi A, O’Connor MJ, Reivich M. Predictors of outcome after anterior temporal lobectomy: Positron emission tomography. Neurology. 1994;44:2331–2336. doi: 10.1212/wnl.44.12.2321. [DOI] [PubMed] [Google Scholar]
- 38.Swartz BE, Tomiyasu U, Delgado-Escueta AV, Mandelkern M, Khonsari A. Neuroimaging in temporal lobe epilepsy: test sensitivity and relationships to pathology and post-surgical outcome. Epilepsia. 1992;33:624–634. doi: 10.1111/j.1528-1157.1992.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 39.Blum DE, Ehsan T, Dungan D, Karis JP, Fisher RS. Bilateral temporal hypometabolism in epilepsy. Epilepsia. 1998;39:651–659. doi: 10.1111/j.1528-1157.1998.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 40.Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy A meta-analysis. Seizure. 2007;16:509–20. doi: 10.1016/j.seizure.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Siclari F, Prior JO, Rossetti AO. Ictal cerebral positron emission tomography (PET) in focal status epilepticus. Epilepsy Res. 2013;105:356–61. doi: 10.1016/j.eplepsyres.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Stayman A, Abou-Khalil B. FDG-PET in the diagnosis of complex partial status epilepticus originating from the frontal lobe. Epilepsy Behav. 2011;20:721–4. doi: 10.1016/j.yebeh.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Dong C, Sriram S, Delbeke D, Al-Kaylani M, Arain AM, Singh P, McLean MJ, Abou-Khalil B. Aphasic or amnesic status epilepticus detected on PET but not EEG. Epilepsia. 2009;50:251–5. doi: 10.1111/j.1528-1167.2008.01782.x. [DOI] [PubMed] [Google Scholar]
- 44.Mehta AD, Ettinger AB, Perrine K, Dhawan V, Patil A, Jain SK, Klein G, Schneider SJ, Eidelberg D. Seizure propagation in a patient with musicogenic epilepsy. Epilepsy Behav. 2009;14:421–4. doi: 10.1016/j.yebeh.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Fong CY, Delgado-Escueta AV. Ictal PET in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1999;67:409. doi: 10.1136/jnnp.67.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chugani HT, Shewmon DA, Khanna S, Phelps ME. Interictal and postictal focal hypermetabolism on positron emission tomography. Pediatr Neurol. 1993;9:10–5. doi: 10.1016/0887-8994(93)90003-u. [DOI] [PubMed] [Google Scholar]
- 47.Leiderman DB, Albert P, Balish M, Bromfield E, Theodore WH. The dynamics of metabolic change following seizures as measured by positron emission tomography with fludeoxyglucose F 18. Arch Neurol. 1994;51:932–6. doi: 10.1001/archneur.1994.00540210106019. [DOI] [PubMed] [Google Scholar]
- 48.Choi JY, Kim SJ, Hong SB, Seo DW, Hong SC, Kim BT, Kim SE. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging. 2003;30:581–7. doi: 10.1007/s00259-002-1079-8. [DOI] [PubMed] [Google Scholar]
- 49.Tepmongkol S, Srikijvilaikul T, Vasavid P. Factors affecting bilateral temporal lobe hypometabolism on 18F-FDG PET brain scan in unilateral medial temporal lobe epilepsy. Epilepsy Behav. 2013;29:386–9. doi: 10.1016/j.yebeh.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Meldrum BS. The role of glutamate in epilepsy and other CNS disorders. Neurology. 1994;44:S14–23. [PubMed] [Google Scholar]
- 51.Chapman AG. Glutamate and epilepsy. J Nutr. 2000;130:1043S–5S. doi: 10.1093/jn/130.4.1043S. [DOI] [PubMed] [Google Scholar]
- 52.Chagnac-Amitai Y, Connors BW. Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition. J Neurophysiol. 1989;61:747–758. doi: 10.1152/jn.1989.61.4.747. [DOI] [PubMed] [Google Scholar]
- 53.Tortella FC, Echevarria E, Robles L, Mosberg HI, Holaday JW. Anticonvulsant effects of mu (DAGO) and delta (DPDPE) enkephalins in rats. Peptides. 1988;9:1177–81. doi: 10.1016/0196-9781(88)90104-0. [DOI] [PubMed] [Google Scholar]
- 54.Clinckers R, Smolders I, Meurs A, Ebinger G, Michotte Y. Anticonvulsant action of hippocampal dopamine and serotonin is independently mediated by D and 5-HT receptors. J Neurochem. 2004;89:834–43. doi: 10.1111/j.1471-4159.2004.02355.x. [DOI] [PubMed] [Google Scholar]
- 55.Bozzi Y, Dunleavy M, Henshall DC. Cell signaling underlying epileptic behavior. Front Behav Neurosci. 2011;5:45. doi: 10.3389/fnbeh.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starr MS. The role of dopamine in epilepsy. Synapse. 1996;22:159–94. doi: 10.1002/(SICI)1098-2396(199602)22:2<159::AID-SYN8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 57.Masino SA, Kawamura M Jr, Ruskin DN. Adenosine receptors and epilepsy: current evidence and future potential. Int Rev Neurobiol. 2014;119:233–55. doi: 10.1016/B978-0-12-801022-8.00011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Savic I, Ingvar M, Stone-Elander S. Comparison of [11C] flumazenil and [18F] FDG as PET markers of epileptic foci. J Neurol Neurosurg Psychiatry. 1993;56:615–21. doi: 10.1136/jnnp.56.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savic I, Thorell JO, Roland P. [11C] flumazenil positron emission tomography visualizes frontal epileptogenic regions. Epilepsia. 1995;36:1225–32. doi: 10.1111/j.1528-1157.1995.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 60.Burdette DE, Sakurai SY, Henry TR, Ross DA, Pennell PB, Frey KA, Sackellares JC, Albin RL. Temporal lobe central benzodiazepine binding in unilateral mesial temporal lobe epilepsy. Neurology. 1995;45:934–41. doi: 10.1212/wnl.45.5.934. [DOI] [PubMed] [Google Scholar]
- 61.Szelies B, Weber-Luxenburger G, Pawlik G, Kessler J, Holthoff V, Mielke R, Herholz K, Bauer B, Wienhard K, Heiss WD. MRI-guided flumazenil- and FDG-PET in temporal lobe epilepsy. Neuroimage. 1996;3:109–18. doi: 10.1006/nimg.1996.0013. [DOI] [PubMed] [Google Scholar]
- 62.Vivash L, Gregoire MC, Lau EW, Ware RE, Binns D, Roselt P, Bouilleret V, Myers DE, Cook MJ, Hicks RJ, O’Brien TJ. 18F-flumazenil: a γ-aminobutyric acid A-specific PET radiotracer for the localization of drug-resistant temporal lobe epilepsy. J Nucl Med. 2013;54:1270–7. doi: 10.2967/jnumed.112.107359. [DOI] [PubMed] [Google Scholar]
- 63.Koepp MJ, Richardson MP, Labbé C, Brooks DJ, Cunningham VJ, Ashburner J, Van Paesschen W, Revesz T, Duncan JS. 11C-flumazenil PET, volumetric MRI, and quantitative pathology in mesial temporal lobe epilepsy. Neurology. 1997;49:764–73. doi: 10.1212/wnl.49.3.764. [DOI] [PubMed] [Google Scholar]
- 64.Muzik O, da Silva EA, Juhasz C, Chugani DC, Shah J, Nagy F, Canady A, von Stockhausen HM, Herholz K, Gates J, Frost M, Ritter F, Watson C, Chugani HT. Intracranial EEG versus flumazenil and glucose PET in children with extratemporal lobe epilepsy. Neurology. 2000;54:171–9. doi: 10.1212/wnl.54.1.171. [DOI] [PubMed] [Google Scholar]
- 65.Ryvlin P, Bouvard S, Le Bars D, De Lamérie G, Grégoire MC, Kahane P, Froment JC, Mauguière F. Clinical utility of flumazenil-PET versus [18F] fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. A prospective study in 100 patients. Brain. 1998;121:2067–81. doi: 10.1093/brain/121.11.2067. [DOI] [PubMed] [Google Scholar]
- 66.Yankam Njiwa J, Bouvard S, Catenoix H, Mauguiere F, Ryvlin P, Hammers A. Periventricular [(11)C] flumazenil binding for predicting postoperative outcome in individual patients with temporal lobe epilepsy and hippocampal sclerosis. Neuroimage Clin. 2013;3:242–8. doi: 10.1016/j.nicl.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hammers A, Koepp MJ, Brooks DJ, Duncan JS. Periventricular white matter flumazenil binding and postoperative outcome in hippocampal sclerosis. Epilepsia. 2005;46:944–8. doi: 10.1111/j.1528-1167.2005.30904.x. [DOI] [PubMed] [Google Scholar]
- 68.Savic I, Widen L, Thorell JO, Blomqvist G, Ericson K, Roland P. Cortical benzodiazepine receptor binding in patients with generalized and partial epilepsy. Epilepsia. 1990;31:724–30. doi: 10.1111/j.1528-1157.1990.tb05513.x. [DOI] [PubMed] [Google Scholar]
- 69.Frost JJ, Mayberg HS, Fisher RS, Douglass KH, Dannals RF, Links JM, Wilson AA, Ravert HT, Rosenbaum AE, Snyder SH, et al. Mu-opiate receptors measured by positron emission tomography are increased in temporal lobe epilepsy. Ann Neurol. 1988;23:231–7. doi: 10.1002/ana.410230304. [DOI] [PubMed] [Google Scholar]
- 70.Madar I, Lesser RP, Krauss G, Zubieta JK, Lever JR, Kinter CM, Ravert HT, Musachio JL, Mathews WB, Dannals RF, Frost JJ. Imaging of delta- and mu-opioid receptors in temporal lobe epilepsy by positron emission tomography. Ann Neurol. 1997;41:358–67. doi: 10.1002/ana.410410311. [DOI] [PubMed] [Google Scholar]
- 71.Mayberg HS, Sadzot B, Meltzer CC, Fisher RS, Lesser RP, Dannals RF, Lever JR, Wilson AA, Ravert HT, Wagner HN Jr. Quantification of mu and non-mu opiate receptors in temporal lobe epilepsy using positron emission tomography. Ann Neurol. 1991;30:3–11. doi: 10.1002/ana.410300103. [DOI] [PubMed] [Google Scholar]
- 72.Prevett MC, Cunningham VJ, Brooks DJ, Fish DR, Duncan JS. Opiate receptors in idiopathic generalised epilepsy measured with [11C] diprenorphine and positron emission tomography. Epilepsy Res. 1994;19:71–7. doi: 10.1016/0920-1211(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 73.Bartenstein PA, Prevett MC, Duncan JS, Hajek M, Wieser HG. Quantification of opiate receptors in two patients with mesiobasal temporal lobe epilepsy, before and after selective amygdalohippocampectomy, using positron emission tomography. Epilepsy Res. 1994;18:119–25. doi: 10.1016/0920-1211(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 74.Hammers A, Asselin MC, Hinz R, Kitchen I, Brooks DJ, Duncan JS, Koepp MJ. Upregulation of opioid receptor binding following spontaneous epileptic seizures. Brain. 2007;130:1009–16. doi: 10.1093/brain/awm012. [DOI] [PubMed] [Google Scholar]
- 75.McGinnity CJ, Shidahara M, Feldmann M, Keihaninejad S, Riaño Barros DA, Gousias IS, Duncan JS, Brooks DJ, Heckemann RA, Turkheimer FE, Hammers A, Koepp MJ. Quantification of opioid receptor availability following spontaneous epileptic seizures: correction of [11C] diprenorphine PET data for the partial-volume effect. Neuroimage. 2013;79:72–80. doi: 10.1016/j.neuroimage.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 76.Bartenstein PA, Duncan JS, Prevett MC, Cunningham VJ, Fish DR, Jones AK, Luthra SK, Sawle GV, Brooks DJ. Investigation of the opioid system in absence seizures with positron emission tomography. J Neurol Neurosurg Psychiatry. 1993;56:1295–302. doi: 10.1136/jnnp.56.12.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koepp MJ, Richardson MP, Brooks DJ, Duncan JS. Focal cortical release of endogenous opioids during reading-induced seizures. Lancet. 1998;352:952–5. doi: 10.1016/s0140-6736(97)09077-6. [DOI] [PubMed] [Google Scholar]
- 78.Toczek MT, Carson RE, Lang L, Ma Y, Spanaki MV, Der MG, Fazilat S, Kopylev L, Herscovitch P, Eckelman WC, Theodore WH. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–56. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- 79.Merlet I, Ostrowsky K, Costes N, Ryvlin P, Isnard J, Faillenot I, Lavenne F, Dufournel D, Le Bars D, Mauguière F. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F] MPPF-PET study. Brain. 2004;127:900–13. doi: 10.1093/brain/awh109. [DOI] [PubMed] [Google Scholar]
- 80.Savic I, Lindström P, Gulyás B, Halldin C, Andrée B, Farde L. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004;62:1343–51. doi: 10.1212/01.wnl.0000123696.98166.af. [DOI] [PubMed] [Google Scholar]
- 81.Merlet I, Ryvlin P, Costes N, Dufournel D, Isnard J, Faillenot I, Ostrowsky K, Lavenne F, Le Bars D, Mauguière F. Statistical parametric mapping of 5-HT1A receptor binding in temporal lobe epilepsy with hippocampal ictal onset on intracranial EEG. Neuroimage. 2004;22:886–96. doi: 10.1016/j.neuroimage.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 82.Giovacchini G, Toczek MT, Bonwetsch R, Bagic A, Lang L, Fraser C, Reeves-Tyer P, Herscovitch P, Eckelman WC, Carson RE, Theodore WH. 5-HT 1A receptors are reduced in temporal lobe epilepsy after partial-volume correction. J Nucl Med. 2005;46:1128–35. [PMC free article] [PubMed] [Google Scholar]
- 83.Ito S, Suhara T, Ito H, Yasuno F, Ichimiya T, Takano A, Maehara T, Matsuura M, Okubo Y. Changes in central 5-HT(1A) receptor binding in mesial temporal epilepsy measured by positron emission tomography with [(11)C] WAY100635. Epilepsy Res. 2007;73:111–8. doi: 10.1016/j.eplepsyres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 84.Didelot A, Ryvlin P, Lothe A, Merlet I, Hammers A, Mauguière F. PET imaging of brain 5-HT1A receptors in the preoperative evaluation of temporal lobe epilepsy. Brain. 2008;131:2751–64. doi: 10.1093/brain/awn220. [DOI] [PubMed] [Google Scholar]
- 85.Liew CJ, Lim YM, Bonwetsch R, Shamim S, Sato S, Reeves-Tyer P, Herscovitch P, Dustin I, Bagic A, Giovacchini G, Theodore WH. 18F-FCWAY and 18F-FDG PET in MRI-negative temporal lobe epilepsy. Epilepsia. 2009;50:234–9. doi: 10.1111/j.1528-1167.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Assem-Hilger E, Lanzenberger R, Savli M, Wadsak W, Mitterhauser M, Mien LK, Stögmann E, Baumgartner C, Kletter K, Asenbaum S. Central serotonin 1A receptor binding in temporal lobe epilepsy: a [carbonyl-(11)C] WAY-100635 PET study. Epilepsy Behav. 2010;19:467–73. doi: 10.1016/j.yebeh.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 87.Theodore WH, Hasler G, Giovacchini G, Kelley K, Reeves-Tyer P, Herscovitch P, Drevets W. Reduced hippocampal 5HT1A PET receptor binding and depression in temporal lobe epilepsy. Epilepsia. 2007;48:1526–30. doi: 10.1111/j.1528-1167.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- 88.Martinez A, Finegersh A, Cannon DM, Dustin I, Nugent A, Herscovitch P, Theodore WH. The 5-HT1A receptor and 5-HT transporter in temporal lobe epilepsy. Neurology. 2013;80:1465–71. doi: 10.1212/WNL.0b013e31828cf809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cuellar-Herrera M, Velasco AL, Velasco F, Trejo D, Alonso-Vanegas M, Nuche-Bricaire A, Vázquez-Barrón D, Guevara-Guzmán R, Rocha L. Alterations of 5-HT1A receptor-induced G-protein functional activation and relationship to memory deficits in patients with pharmacoresistant temporal lobe epilepsy. Epilepsy Res. 2014;108:1853–63. doi: 10.1016/j.eplepsyres.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 90.Werhahn KJ, Landvogt C, Klimpe S, Buchholz HG, Yakushev I, Siessmeier T, Müller-Forell W, Piel M, Rösch F, Glaser M, Schreckenberger M, Bartenstein P. Decreased dopamine D2/D3-receptor binding in temporal lobe epilepsy: an [18F] fallypride PET study. Epilepsia. 2006;47:1392–6. doi: 10.1111/j.1528-1167.2006.00561.x. [DOI] [PubMed] [Google Scholar]
- 91.Landvogt C, Buchholz HG, Bernedo V, Schreckenberger M, Werhahn KJ. Alteration of dopamine D2/D3 receptor binding in patients with juvenile myoclonic epilepsy. Epilepsia. 2010;51:1699–706. doi: 10.1111/j.1528-1167.2010.02569.x. [DOI] [PubMed] [Google Scholar]
- 92.Choi H, Kim YK, Oh SW, Im HJ, Hwang do W, Kang H, Lee B, Lee YS, Jeong JM, Kim EE, Chung JK, Lee DS. In vivo imaging of mGluR5 changes during epileptogenesis using [11C] ABP688 PET in pilocarpine-induced epilepsy rat model. PLoS One. 2014;9:e92765. doi: 10.1371/journal.pone.0092765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Picard F, Bruel D, Servent D, Saba W, Fruchart-Gaillard C, Schöllhorn-Peyronneau MA, Roumenov D, Brodtkorb E, Zuberi S, Gambardella A, Steinborn B, Hufnagel A, Valette H, Bottlaender M. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: a PET study. Brain. 2006;129:2047–60. doi: 10.1093/brain/awl156. [DOI] [PubMed] [Google Scholar]
- 94.Dupont S, Semah F, Loc’h C, Strijckmans V, Baulac M, Samson Y, Mazière B. In vivo imaging of muscarinic cholinergic receptors in temporal lobe epilepsy with a new PET tracer: [76Br] 4-bromodexetimide. J Nucl Med. 1999;40:935–41. [PubMed] [Google Scholar]
- 95.Kumlien E, Hartvig P, Valind S, Oye I, Tedroff J, Långström B. NMDA-receptor activity visualized with (S)-[N-methyl-11C] ketamine and positron emission tomography in patients with medial temporal lobe epilepsy. Epilepsia. 1999;40:30–7. doi: 10.1111/j.1528-1157.1999.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 96.Rubí S, Costes N, Heckemann RA, Bouvard S, Hammers A, Martí Fuster B, Ostrowsky K, Montavont A, Jung J, Setoain X, Catenoix H, Hino K, Liger F, Le Bars D, Ryvlin P. Positron emission tomography with α-[11C] methyl-Ltryptophan in tuberous sclerosis complex-related epilepsy. Epilepsia. 2013;54:2143–50. doi: 10.1111/epi.12412. [DOI] [PubMed] [Google Scholar]
- 97.Fedi M, Reutens DC, Andermann F, Okazawa H, Boling W, White C, Dubeau F, Nakai A, Gross DW, Andermann E, Diksic M. alpha-[11C] -Methyl-L-tryptophan PET identifies the epileptogenic tuber and correlates with interictal spike frequency. Epilepsy Res. 2003;52:203–13. doi: 10.1016/s0920-1211(02)00216-4. [DOI] [PubMed] [Google Scholar]
- 98.Natsume J, Kumakura Y, Bernasconi N, Soucy JP, Nakai A, Rosa P, Fedi M, Dubeau F, Andermann F, Lisbona R, Bernasconi A, Diksic M. Alpha-[11C] methyl-L-tryptophan and glucose metabolism in patients with temporal lobe epilepsy. Neurology. 2003;60:756–61. doi: 10.1212/01.wnl.0000052682.99812.f5. [DOI] [PubMed] [Google Scholar]
- 99.Wakamoto H, Chugani DC, Juhász C, Muzik O, Kupsky WJ, Chugani HT. Alpha-methyl-ltryptophan positron emission tomography in epilepsy with cortical developmental malformations. Pediatr Neurol. 2008;39:181–8. doi: 10.1016/j.pediatrneurol.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 100.Chugani DC, Chugani HT, Muzik O, Shah JR, Shah AK, Canady A, Mangner TJ, Chakraborty PK. Imaging epileptogenic tubers in children with tuberous sclerosis complex using alpha-[11C] methyl-L-tryptophan positron emission tomography. Ann Neurol. 1998;44:858–66. doi: 10.1002/ana.410440603. [DOI] [PubMed] [Google Scholar]
- 101.Chugani DC. α-methyl-L-tryptophan: mechanisms for tracer localization of epileptogenic brain regions. Biomark Med. 2011;5:567–75. doi: 10.2217/bmm.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Juhász C, Chugani DC, Muzik O, Shah A, Asano E, Mangner TJ, Chakraborty PK, Sood S, Chugani HT. Alpha-methyl-L-tryptophan PET detects epileptogenic cortex in children with intractable epilepsy. Neurology. 2003;60:960–8. doi: 10.1212/01.wnl.0000049468.05050.f2. [DOI] [PubMed] [Google Scholar]
- 103.Tatlidil R, Xiong J, Luther S. Presurgical lateralization of seizure focus and language dominant hemisphere with O-15 water PET imaging. Acta Neurol Scand. 2000;102:73–80. doi: 10.1034/j.1600-0404.2000.102002073.x. [DOI] [PubMed] [Google Scholar]
- 104.Tatlidil R, Luther S, West A, Jadvar H, Kingman T. Comparison of fluorine-18 deoxyglucose and O-15 water PET in temporal lobe epilepsy. Acta Neurol Belg. 2000;100:214–20. [PubMed] [Google Scholar]
- 105.Henry TR, Votaw JR, Pennell PB, Epstein CM, Bakay RA, Faber TL, Grafton ST, Hoffman JM. Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsy. Neurology. 1999;52:1166–73. doi: 10.1212/wnl.52.6.1166. [DOI] [PubMed] [Google Scholar]
- 106.Franck G, Sadzot B, Salmon E, Depresseux JC, Grisar T, Peters JM, Guillaume M, Quaglia L, Delfiore G, Lamotte D. Regional cerebral blood flow and metabolic rates in human focal epilepsy and status epilepticus. Adv Neurol. 1986;44:935–48. [PubMed] [Google Scholar]
- 107.Bernardi S, Trimble MR, Frackowiak RS, Wise RJ, Jones T. An interictal study of partial epilepsy using positron emission tomography and the oxygen - 15 inhalation technique. J Neurol Neurosurg Psychiatry. 1983;46:473–7. doi: 10.1136/jnnp.46.6.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sone D, Ito K, Taniguchi G, Murata Y, Nakata Y, Watanabe Y, Okazaki M, Sato N, Matsuda H, Watanabe M. Evaluation of amygdala pathology using (11)C-methionine positron emission tomography/computed tomography in patients with temporal lobe epilepsy and amygdala enlargement. Epilepsy Res. 2015;112:114–21. doi: 10.1016/j.eplepsyres.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 109.Sasaki M, Kuwabara Y, Yoshida T, Fukumura T, Morioka T, Nishio S, Fukui M, Masuda K. Carbon-11-methionine PET in focal cortical dysplasia: a comparison with fluorine-18-FDG PET and technetium-99m-ECD SPECT. J Nucl Med. 1998;39:974–7. [PubMed] [Google Scholar]


