Abstract
Positron emission tomography (PET) allows assessment of myocardial blood flow in absolute terms (ml/min/g). Quantification of myocardial blood flow (MBF) and myocardial flow reserve (MFR) extend the scope of conventional semi-quantitative myocardial perfusion imaging (MPI): e.g. in 1) identification of the extent of a multivessel coronary artery disease (CAD) burden, 2) patients with balanced 3-vessel CAD, 3) patients with subclinical CAD, and 4) patients with regional flow variance, despite of a high global MFR. A more accurate assessment of the ischemic burden in patients with intermediate pretest probability of CAD can support the clinical decision-making in treatment of CAD patients as a complementary tool to the invasive coronary angiography (CAG). Recently, several studies have proven Rubidium-82 (82Rb) PET’s long-term prognostic value by a significant association between compromised global MFR and major adverse cardiovascular events (MACE), and together with new diagnostic possibilities from measuring the longitudinal myocardial perfusion gradient, cardiac 82Rb PET faces a promising clinical future. This article reviews current evidence on quantitative 82Rb PET’s ability to diagnose and risk stratify CAD patients, while assessing the potential of the modality in clinical practice.
Keywords: PET 82Rb, quantification, myocardial blood flow, coronary artery disease
Introduction
Ischemic heart disease (IHD) tallied more than 7 million deaths worldwide in 2010 as the leading cause of death, and was together with stroke responsible for approximately a quarter of all deaths that year [1]. From 1990 to 2010, deaths caused by IHD have increased by 35% [1], disproportionately affecting developing countries [2]. Diagnostic imaging techniques are useful in optimization of patient treatment, including patients with known or suspected IHD. Techniques such as MPI, either by PET or single-photon emission computed tomography (SPECT), are useful for early diagnosis, risk stratification, and to shorten response time for appropriate treatment [3]. Decrease in cost of PET scanners and their wide implementation in oncology has led to a wider clinical adoption of cardiac PET, especially PET/CT hybrid scanners, which currently account for 80% of new PET units installed [3-5]. In contrast, limited health care resources and the invasive nature of CAG restrict its use in all IHD patients. Cardiac PET is therefore an attractive non-invasive alternative to CAG [6], and current published guidelines assign class IA recommendation to PET in patients with intermediate pre-test probability of CAD [7].
Several radiolabeled tracers are available for PET scanners. The most validated tracers for determination of cardiac perfusion are: 13N-ammonia, 15O-water, and 82RbCl [5]. 13N-ammonia and 15O-water require an on-site cyclotron, whereas 82RbCl requires only a generator with replacement every 4-5 weeks, thereby offering an alternative to departments without a cyclotron [8].
Although quantitative, 82Rb PET is often used primarily for semi-quantitative MPI. Perfusion in a myocardial segment, determined by regional tracer uptake, is assessed by reference to maximum uptake in the left ventricle (LV). However, semi-quantitative MPI can be associated with interpretation difficulties in patients with multivessel CAD because of the best supplied area being hypoperfused as well [4,9]. Quantitative MPI by 82Rb PET is an increasingly interesting supplement allowing quantification of flow in absolute terms (ml/min/g) [10] (Figure 1). From mainly being a research tool, the method is shifting to routine clinical practice [6,11], and different software solutions are available to secure fast and reproducible flow estimates [12-14]. The clinical feasibility of dynamic and quantitative 82Rb PET has already been confirmed in several studies [15-17], while the method is not yet fully implemented.
Figure 1.
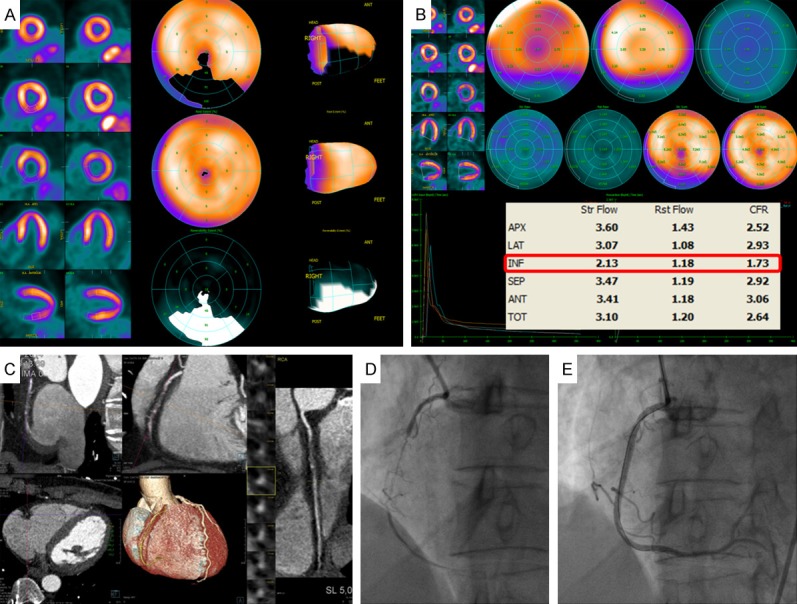
Integrated 82Rb PET/CTA study in a patient with chronic stable angina. Rest/stress 82Rb PET scan demonstrates a large and severe stress-induced perfusion defect throughout inferior LV wall (A). Results of quantitative assessment of MBF from 82Rb PET demonstrate impaired adenosine-stimulated flow in the inferior LV wall, resulting in a reduced MFR, compared to the other vascular territories in the LV (B). Cardiac CTA and coronary angiogram showing coronary stenosis in the right coronary artery (RCA) (C, D), and a normal post percutaneous coronary intervention (PCI) angiogram (E).
The aim of this review is to describe the current benefits of quantitative 82Rb PET in the diagnosis and risk stratification of IHD patients, and assess the potential in clinical practice.
Methods
This review is based on a search in PubMed, MEDLINE, and Cochrane. Entered search terms included: myocardial, flow, PET, CAD, and 82Rb, as well as the combinations. Furthermore, searching in free text and with MeSH terms ensured that the latest articles that have not yet been assigned MeSH terms were also searched for. The overall search yielded 154 hits, and together with bibliographies the 154 hits formed the basis for the subsequent inclusion of incorporated literature.
The oldest publication included was from year 1990.
Last update of the PubMed search has been completed at date June 17, 2015.
PET 82Rb and quantification of MBF
To calculate myocardial blood flow quantitatively, time activity curves of tracer input for the LV cavity and myocardium are obtained from volumes of interest drawn on the dynamic images, starting from the moment of tracer injection until uptake in myocardium is completed. The time activity curves are fitted to a physiological model to estimate MBF. Different models exist, but lately a majority of studies [8,12,13,18-21] use the Lortie et al. single-tissue compartment model [16], as it is the most commonly used model in 82Rb PET software today [13,14]. The tracers, 13N-ammonia and 15O-water, are already highly validated for calculating MBF in humans [6,22,23]. Quantitative 82Rb PET has been validated by comparison with these tracers with a high accuracy of MBF estimates in the clinically relevant range between 0.5 to 2.5 ml/min/g [6,15,17].
Generator produced 82RbCl is attractive as a tracer, since its distribution to cardiac PET centers without a cyclotron can potentially lead to wider availability and a larger global geographic distribution [8]. In addition, the short half-life of 76 seconds makes implementation of rest/stress paired studies possible under comparable conditions within a short study period of less than 30 minutes, while improving patient comfort and throughput [3]. Furthermore, patient and staff radiation exposure is significantly reduced compared to conventional technetium-99m (99mTc) SPECT because of the much shorter scan time and no waiting time to clear background radiation [24-26]. Several limitations with 82Rb are also evident. The extraction fraction of 82Rb in to the myocardium decreases significantly with an increased flow. This may underestimate the hyperemic flow during a stress test. In addition, positrons from 82Rb have higher energy with longer positron range, resulting in poorer resolution compared to 13N-ammonia and 15O-water [27]. Thus, using 82Rb it is necessary to apply a compartment model of the 82Rb kinetics, e.g. the single-tissue compartment model with a non-linear extraction function as proposed by Lortie and co-workers for cardiac PET [16]. The general compartment model includes the appropriate correction factors, but further technical details of these methods go beyond the scope of this literature study.
Hybrid scanners with both coronary anatomical and hemodynamic measurement capabilities are advantageous alternatives with a high diagnostic accuracy, compared to dedicated PET scanners alone [28,29]. CT-based attenuation correction and tissueboundaring are more effective than traditional radionuclide transmission sources used in dedicated PET. Furthermore, perfusion abnormalities can be based on the CT-derived anatomy without the use of frequently inaccurate standard assumptions about vascular distribution pattern [28].PET combined with magnetic resonance imaging (MRI) is also a possible hybrid scanner option offering promising capabilities for future clinical implementation [4,29,30].
Quantitative vs. semi-quantitative perfusion measurements
The quantitative perfusion analysis extends the scope and adds valuable information the traditional semi-quantitative MPI assessment; e.g. in 1) identification of the extent of a multivessel CAD burden, 2) patients with balanced 3-vessel CAD, 3) patients with subclinical CAD, and 4) patients with regional flow variance despite of a high global MFR [9,11,31].
Improved identification of CAD burden
The semi-quantitative assessment of a regional stress-induced myocardial perfusion defect with normalization of the data to the maximal tracer uptake can lead to a false negative conclusion of IHD [8]. In patients with multivessel CAD, myocardial perfusion is often reduced even in areas without significant stenosis, including the best supplied reference region, and only the poorest supplied areas are considered pathological [4,9]. Although sensitivity of semi-quantitative 82Rb PET is confirmed to be high (93%) by Sampson et al. [4] for detection of disease defined by CAG (≥70% stenosis), the correct diagnosis of the anatomical extent of multivessel disease was compromised (sensitivity of only 55%) [4]. Parkash et al. [10] illustrated sensitivity of semi-quantitative and quantitative 82Rb PET nearly on par (87% vs. 83%), but correct identification of disease in all three diseased vascular territories (≥70% stenosis in each territory defined by CAG) was significantly better with quantitative 82Rb PET (46% vs. 92%). In addition, the study of Yoshinaga et al. [19]showed that segments supplied by stenotic vessels had significantly lower regional stress MBF and MFR than segments without stenosis. Stenotic segments, but with normal perfusion rated by semi-quantitative 82Rb PET, still had reduced hyperemic MBF [19], and exemplifies an added diagnostic value of quantitative MPI compared to semi-quantitative MPI even in areas with significant stenosis.
Balanced 3-vessel CAD
The relative assessment of tracer uptake in the LV myocardium alone may also fail to recognize patients with balanced 3-vessel CAD. The uniform perfusion deficit in all three major vascular territories often leads to a false negative conclusion by semi-quantitative MPI. The study of Ziadi et al. [8] enrolled 120 patients with known or suspected CAD, and demonstrated that patients with 3-vessel CAD had a significantly lower MFR compared to patients without. Global MFR (<2) was concluded as an independent predictor of 3-vessel CAD, by results from a multivariable Cox analysis including summed stress score (SSS), MFR, and other significant risk factors. MFR had a diagnostic sensitivity of 88% for 3-vessel disease, whereas only 60% of these patients had other generally accepted risk factors, such as reduced ejection fraction, transient ischemic dilation, and ischemic ECG changes. The study is important in demonstrating that quantitative MFR has an advantage in the diagnosis of 3-vessel CAD, compared to semi-quantitative measurements. The incremental value of absolute flow quantification has recently been confirmed by another comprehensive diagnostic study [32]. While the interpretation of a balanced 3-vessel CAD by semi-quantitative analysis as being normal may be rare [11], nevertheless quantitative assessment of myocardial perfusion with 82Rb PET may be used in facilitating prediction of patients with balanced 3-vessel CAD over standard semi-quantitative MPI [8].
Subclinical CAD
A compromised global perfusion and no focal stress-induced perfusion abnormality can also be due to diffusely spread non-obstructive atherosclerosis and microvascular dysfunction. These burdens are different from luminal narrowing, and are often present together with atherosclerosis, confounding the relationship between percent stenosis and downstream coronary flow [32]. A previous study already documented a decreased MFR as a result of coronary microvascular dysfunction [33]. Without the presence of a flow limiting coronary artery stenosis, the quantitative values are thought to reflect the degree of microvascular dysfunction in patients with coronary risk factors [34]. This subgroup with normal epicardial vessels is particularly important. A reduced MFR and hyperemic MBF despite no detection of a semi-quantitative perfusion deficit are followed by a long-term higher outcome of MACE [18,20,34]. The WISE study [35] has shown that women with IHD symptoms, but without significant coronary artery stenosis were at higher risk of cardiovascular events (myocardial infarction, hospitalization for heart failure, stroke, or cardiac mortality) than women without any symptoms, probably because of microvascular disease. Because of absent regional perfusion abnormalities from a homogenously impaired hyperemic flow, semi-quantitative MPI will often overlook such abnormalities [9]. The quantitative possibility of identifying these patients is therefore essential, as intervention aiming at reducing traditional risk factors, as well as initiation of medical treatment may be undertaken.
Regional flow variance despite high global flow
Quantitative evaluation without normalization to maximum tracer uptake is desirable, as it may help to recognize patients with widespread flow reduction from a normal variance of tracer uptake [11,31]. In diseases such as Takotsubo cardiomyopathy, we recently demonstrated that what was initially interpreted, using semi-quantitative MPI, as hypoperfusion of the apical region with normal basal perfusion, turned out to be normal apical perfusion with hyperperfusion of the basal part when using quantitative 82Rb PET [36-38]. This also underscores how quantitative MPI can lead to new insight into pathophysiology mechanisms in cardiovascular disease.
CAD prognostication
Quantitative perfusion assessment with PET can provide information on both macro- and microvascular level, hence detection of early stages of CAD and more precise risk stratification of manifest CAD is possible [20]. Only few prospective studies exist regarding 82Rb PET’s long-term prognostic value. The study of Ziadi et al. [18] was a prospective cohort study with 704 consecutive patients assessed for ischemia. This study shows an association between patients with abnormal MFR (<2.0) and a significantly higher cardiac event rate after one year of follow-up. Using a multivariate Cox model pointed out MFR as the independent factor for prediction of MACE with the highest hazard ratio. The trend was supported and confirmed by two other prognostic studies with slightly smaller enrolled populations, n=275 and n=351, of patients referred for known or suspected CAD [20,34]. Same conclusions about quantitative MFR’s ability to predict long-term MACE were drawn, including hyperemic MBF as an equally good prognostic factor using 2.11 and 1.8 as cutoff for MFR. The semi-quantitative measurements had a significant prognostic value in all three studies as well. When patients were assessed as having normal MPI with a semi-quantitative evaluation (SSS <4 or summed difference score (SDS) ≤2), reduced MFR was still associated with a higher MACE prevalence in accordance with presence of 3-vessel and subclinical CAD [18,20,34]. Recently, a large study including 2,783 consecutive patients referred for known or suspected CAD, documented global MFR’s incremental value for prognostication over other recognized clinical risk factors, including LV systolic function and a semi-quantitative assessment of myocardial ischemia [39] (Figure 2). They were the first to demonstrate an association between decreased MFR and cardiac mortality with consistency across 29 subgroups, evaluated of different assumed cardiac risk factors. Moreover, patients in the lowest tertile of MFR (<1.5) had a significantly worse prognosis independent of other risk factors, and patients in the highest tertile of MFR (>2.0) had a good prognosis despite of other risk factors resulting in correct reclassification of risk in a large group of patients, including 35% of intermediate risk patients [39] (Figure 2).
Figure 2.

Illustration of risk reclassification after addition of global coronary flow reserve (CFR or MFR). The top horizontal bar represents risk categories of <1% (green), 1%-3% (blue), and ≥3% (red) annual rate of cardiac death, estimated by rest and stress LV ejection fractions (LVEFs), and the combination of myocardial ischemia and scar. The pie graphs show the percentages of patients in each pre-CFR risk category reclassified after addition of CFR (post-CFR risk). The bottom bar charts illustrate annualized rates of cardiac death in the three post-CFR risk categories. The benefit of CFR addition was greatest among intermediate pre-CFR risk patients (34.9% correctly reclassified). (Reproduced with permission of [39]).
MFR and specificity
Compromised MBF integrates epicardial stenosis dimensions, diffusely spread atherosclerosis, and microvascular dysfunction [32,40]. Thus, quantitative perfusion measurements are sensitive, but not necessarily specific for obstructive epicardial CAD. Microvascular disease together with epicardial stenosis coexist in many CAD patients with reduced global MFR, and the ability of separating these two when regional perfusion defects are missing, is compromised [32]. In patients without contraindications, the relative contribution can be revealed from a CT angiography (CTA) in a hybrid PET/CT session (Figure 1), with determination of the degree of epicardial coronary stenosis [32,41,42]. With exclusion of stenosis, functional information with decreased MFR will point to microvascular dysfunction and subclinical CAD. A high sensitivity for MFR has been confirmed in larger studies for detecting high-risk patients on angiography [8,32] (Figure 3). A preserved MFR above 2.0 excludes patients at high risk with a negative predictive value of nearly 1.0 independent of semi-quantitative perfusion results. While a stepwise reduction in MFR increases the likelihood of comprehensive obstructive CAD relative to other conditions, the positive predictive value and specificity will remain compromised because the method lacks ability to separate obstructive and non-obstructive CAD (Figure 3). According to several studies, the addition of MFR to other high-risk imaging findings increases the identification of flow-limiting stenosis, but at the expense of less specificity [8,9,32]. At the risk of an increased frequency of unnecessary catheterization, quantitative perfusion data should not be interpreted in isolation, but in clinical context with semi-quantitative perfusion data, cardiovascular risk factors, and coronary anatomy. More recently, increasing evidence has accumulated that the downstream hemodynamic consequences of an obstructive CAD burden may be better identified by measuring the longitudinal myocardial perfusion gradient from the base to the apex of the heart under hyperemic conditions together with MFR [43-46]. A longitudinal decrease in hyperemic MBF indicates a CAD related increase in epicardial resistance from an absent adequate flow-mediated vasodilation, which leads to a decline in coronary pressure and flow along the vessels [44,46]. These studies have so far only been performed with tracer 13N-ammonia [46]. Whether the method will assist MFR, improving the reduced specificity of CAD and be performed with easier accessible 82RbCl tracer, remains to be shown.
Figure 3.
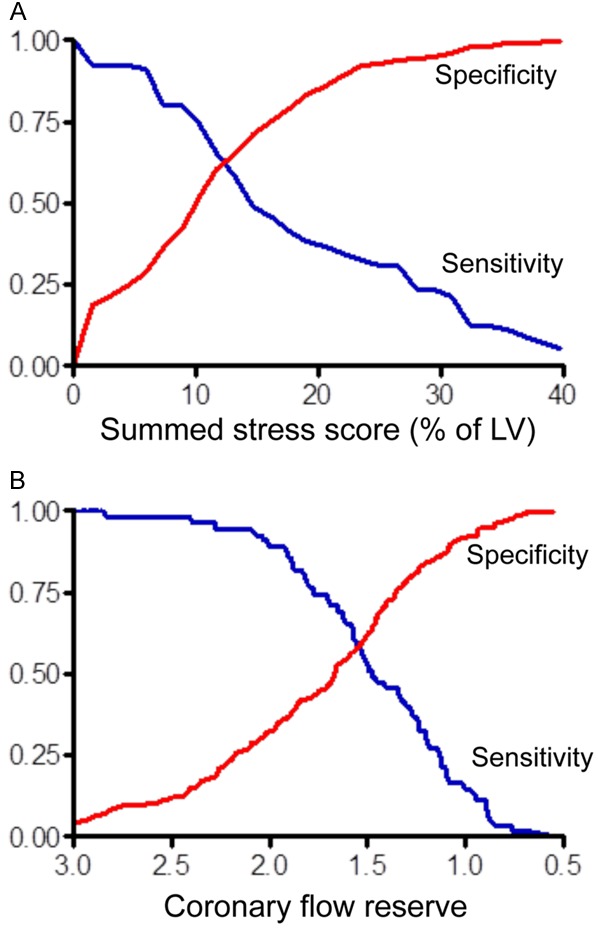
Univariate ROC curves for 82Rb PET semiquantitative MPI (A) and quantitative MPI (B). The ROC curves illustrate sensitivity (blue curves) and specificity (red curves) in pairs for identification of high risk CAD patients. The sensitivity/specificity trade-offs were obtained from different cutoff points of %SSS and global CFR, with CAG as a reference. Data demonstrate a general low sensitivity of semiquantitative MPI, and thereby a frequent risk of missing high risk patients. Furthermore, data is consistent with a high sensitivity of quantitative MPI for CFR above 2.0, but with a compromised positive predictive value because of the methods lack of specificity. Naya et al. [32] determined that a %SSS of 10.2% and a CFR of 1.93, resulted in the best trade-off between sensitivity and specificity. (This research was originally published in JNM, and reproduced with permission of [32]).
Concordance between anatomical and physiological assessment of CAD
Regional hyperemic MBF and MFR are thought to be inversely and non-linearly correlated with percent diameter coronary stenosis [19,47-49].Quantitative coronary angiography (QCA) commonly represents the primary tool for investigation of stenosis severity [9]. The relationship between stenosis severity and hyperemic MBF is, however, more complex with relatively high flow variability with coronary stenosis of intermediate severity [47,48]. Several factors, including physiological adaptive vasodilation to balance the increase in resistance and induction of collaterals to compensate hypoxic conditions, can result in the discordance. Furthermore, a tendency for the operator to overestimate stenosis diameter exists, especially when assessed by CAG rated visually [9,42]. A relatively preserved MFR because of local compensatory vasodilation may in fact be seen in some individuals with intermediate epicardial CAD lesions, thereby preventing stress-induced ischemia [9,42]. Quantitative 82Rb PET has an improved ability to differentiate stenosis of intermediate severity based on their hemodynamic significance, and can facilitate the diagnostic testing of suspected CAD [7,19,49]. Whether addition of quantitative 82Rb PET will play a role in future revascularization planning, and aid for a more objective determination of mechanical versus medical treatment of CAD, requires more clinical validation and trials.
For most of the included clinical studies, the extent of ischemia, determined with 82Rb PET, was compared to an anatomical test carried out with CAG or QCA. A comparison of physiological and anatomical metrics is not ideal, as an anatomical stenosis does not necessarily result in ischemia [46], and therefore it may be challenged why anatomy is considered the reference. However, consideration about whether the quantitative perfusion measurements with PET would have a greater diagnostic precision compared to a physiological test such as the invasively measured fractional flow reserve by intracoronary pressure measurement, is therefore relevant.
PET MPI parameters
Semi-quantitative MPI provides high sensitivity for detecting isolated flow-limiting defects [4,10]. Quantitative absolute MPI shows the real ischemic burden in the LV myocardium [9]. Thus, the combined assessment allows true evaluation of global disease severity together with high sensitivity of a potential focal lesion [9,50]. Absence of a focal stress-induced perfusion abnormality with a low absolute perfusion indicates microvascular disease, and usually no benefit of revascularization. In this case though, isolated lesions can be overlooked when the regional perfusion deficits are balancing each other. With both diffusely reduced perfusion and a focal defect, possible benefits of revascularization can be judged independently from regional quantitative MPI and the degree of ischemia.
Hyperemic MBF and MFR, accounting for rest MBF, are both important for covering the wide spectrum of conditions seen clinically. In a small but significant subgroup of patients, a mildly reduced hyperemic MBF may lead to two opposite diagnoses because of an abnormal flow at rest [40,51]. When treated with beta blockade, a low rest MBF may lead to a preserved MFR and capacity for increased flow to meet increased demand, in spite of the reduced hyperemic MBF, hence, no ischemia [40,51]. A high rest MBF however, due to uncontrolled blood pressure or anxiety, may lead to a definite low MFR, hence, inadequate flow capacity under high workload and possible ischemia [40,51,52]. Furthermore, in patients with suspected myocardial infarct, assessment of rest MBF is necessary to differentiate scar tissue from reversible ischemia in a stress perfusion test [6]. Selection of each patient should always be considered in a clinical perspective including symptoms, comorbidities, response to medication, and general accepted CAD risk factor.
A clinical implementation of quantitative 82Rb PET - how far are we?
Several studies have investigated the efficacy of revascularization in addition to optimal medical therapy in initial treatment of patients with CAD [53-55], and they found no significant differences in long-term outcome regarding rates of death or MACE. It seems that only when the invasive strategy is combined with an assessment of significant ischemia from functional imaging, can it lead to a better outcome for CAD patients than with optimal medical therapy [56].
The new European Society of Cardiology (ESC) guidelines on myocardial revascularization [7] and management of stable CAD [57] from 2014 and 2013 respectively stress the role of non-invasive testing including cardiac PET in patients with suspected CAD. After initial pre-testing of CAD likelihood, patients with an intermediate risk of significant CAD (15-85%) are advised to undergo functional testing or CTA [7,57], with the purpose of distinguishing between obstructive and non-obstructive CAD. PET is one of the imaging modalities recommended for this purpose. However, availability still restricts the use of PET perfusion and hybrid imaging compared to SPECT, even though they are confirmed as being superior [7,57]. Thus, SPECT and echocardiography are still the widely used functional imaging techniques today [7], probably due to their availability.
The complex PET technology, including the mathematical data modeling necessary to quantitative blood flow, is prone to artifacts and measurement uncertainties. Because of the rapid technical development of cardiac PET, and so far primary use as a research tool, the quantitative 82Rb PET procedure is still missing standardization [40]. A standardized protocol and treatment algorithm across diagnostic centers is desired, and the knowledge from experienced centers should be globally disseminated. Furthermore, the heterogeneity between the majority of studies, including different scanners, software algorithms, and clinical practice, limits the comparability and interpretation.
Routine list mode acquisition provides an easy way to combine quantitative and semi-quantitative perfusion analysis facilitating the integration of the benefits of quantitative 82Rb PET into clinical practice [11] (Figure 1). For a further implementation of quantitative 82Rb PET, an optimized consistency of output data is necessary. Various studies have confirmed a high reproducibility with a low intra-, and interoperator variability when using the same software packages and hardware [12,58-60]. The inter-operator variability can be minimized when using robust and fully automated software packages already available [12]. Recently, several studies have shown a very good consistency of quantitative perfusion data from 82Rb PET when using the single-tissue compartment model of Lortie et al. [12-14]. Software which implement the suggested kinetic model [16] produced results close enough to be used interchangeably, and the independence of a particular software package provides an opportunity for patient follow-up across different nuclear cardiology centers [13,14]. Moreover, the RUBY-10 study revealed that different tracer kinetic models produce different results from the same 82Rb PET data, with possible variances in global MBF up to 90%, and MBF patient data presented without information on the implemented mathematical model can therefore not be compared directly [13]. MBF quantification reproducibility and accuracy have also been evaluated versus the previously validated tracer 13N-ammonia [15]. With excellent same-day repeatability within 60 minutes in healthy patients [21] it is possible for serial quantitative 82Rb PET studies to be used for monitoring the effect of clinical interventions. Beyond an increased credibility, the approved reproducibility and commutability supports larger multicenter trials, and can aid a further implementation of quantitative 82Rb PET in clinical practice.
Conclusion
Due to the high temporal resolution and attenuation correction of PET/CT scanners, quantification of perfusion in ml/g/min from dynamic images is feasible. This allows for more accurate assessment of the CAD burden in individuals with cardiovascular risk than with semiquantitative PET MPI. The correct diagnosis of severity and improved identification of patients with multivessel CAD and subclinical CAD are interesting advantages of assessing quantitative global MFR and hyperemic MBF with 82Rb PET. Quantification of MFR may reveal the relationship between cardiovascular risk factors and coronary artery circulation. It may clarify early signs of a CAD burden before its clinical breakthrough, together with an improved risk stratification of manifest CAD. The additional information obtained may help guide decisions of life style changes and medical therapy.
Severely reduced global perfusion is usually caused by obstructive multivessel CAD, diffuse non-obstructive atherosclerosis, microvascular dysfunction, or combinations thereof [32,40]. The ability to separate coexisting ischemic conditions can be quite challenging, and may be supported by the addition of CTA to the PET/CT session. Furthermore, decision of potential revascularization of patients with an intermediate risk of CAD may be further supported by quantitative 82Rb PET.
The easy combination of quantitative perfusion analysis with traditional semi-quantitative PET MPI facilitates implementation in clinical routine. In spite of technical challenges, including requirement of a high reproducibility and a standardized protocol and software algorithm between nuclear cardiology centers, it appears from current evidence that quantitative MPI with 82Rb PET has the power to become a reliable non-invasive imaging tool assisting in diagnosis, treatment, and risk stratification of IHD patients.
Disclosure of conflict of interest
None.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. doi: 10.1161/01.cir.97.6.596. [DOI] [PubMed] [Google Scholar]
- 3.Yoshinaga K, Klein R, Tamaki N. Generatorproduced rubidium-82 positron emission tomography myocardial perfusion imaging-From basic aspects to clinical applications. J Cardiol. 2010;55:163–173. doi: 10.1016/j.jjcc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49:1052–1058. doi: 10.1016/j.jacc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Ghotbi AA, Kjaer A, Hasbak P. Review: comparison of PET rubidium-82 with conventional SPECT myocardial perfusion imaging. Clin Physiol Funct Imaging. 2014;34:163–170. doi: 10.1111/cpf.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saraste A, Kajander S, Han C, Nesterov SV, Knuuti J. PET: Is myocardial flow quantification a clinical reality? J Nucl Cardiol. 2012;19:1044–1059. doi: 10.1007/s12350-012-9588-8. [DOI] [PubMed] [Google Scholar]
- 7.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 2015;10:1024–1094. doi: 10.4244/EIJY14M09_01. [DOI] [PubMed] [Google Scholar]
- 8.Ziadi MC, Dekemp RA, Williams K, Guo A, Renaud JM, Chow BJ, Klein R, Ruddy TD, Aung M, Garrard L, Beanlands RS. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol. 2012;19:670–680. doi: 10.1007/s12350-011-9506-5. [DOI] [PubMed] [Google Scholar]
- 9.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–640. doi: 10.1016/j.jcmg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Parkash R, deKemp RA, Ruddy TD, Kitsikis A, Hart R, Beauchesne L, Williams K, Davies RA, Labinaz M, Beanlands RS. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol. 2004;11:440–449. doi: 10.1016/j.nuclcard.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Knuuti J, Saraste A. Advances in clinical application of quantitative myocardial perfusion imaging. J Nucl Cardiol. 2012;19:643–646. doi: 10.1007/s12350-012-9530-0. [DOI] [PubMed] [Google Scholar]
- 12.Tahari AK, Lee A, Rajaram M, Fukushima K, Lodge MA, Lee BC, Ficaro EP, Nekolla S, Klein R, Dekemp RA, Wahl RL, Bengel FM, Bravo PE. Absolute myocardial flow quantification with Rb PET/CT: comparison of different software packages and methods. Eur J Nucl Med Mol Imaging. 2014;41:126–35. doi: 10.1007/s00259-013-2537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nesterov SV, Deshayes E, Sciagra R, Settimo L, Declerck JM, Pan XB, Yoshinaga K, Katoh C, Slomka PJ, Germano G, Han C, Aalto V, Alessio AM, Ficaro EP, Lee BC, Nekolla SG, Gwet KL, deKemp RA, Klein R, Dickson J, Case JA, Bateman T, Prior JO, Knuuti JM. Quantification of Myocardial Blood Flow in Absolute Terms Using Rb PET Imaging: Results of RUBY-10 Study. JACC Cardiovasc Imaging. 2014;7:1119–27. doi: 10.1016/j.jcmg.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dekemp RA, Declerck J, Klein R, Pan XB, Nakazato R, Tonge C, Arumugam P, Berman DS, Germano G, Beanlands RS, Slomka PJ. Multisoftware reproducibility study of stress and rest myocardial blood flow assessed with 3D dynamic PET/CT and a 1-tissue-compartment model of 82Rb kinetics. J Nucl Med. 2013;54:571–577. doi: 10.2967/jnumed.112.112219. [DOI] [PubMed] [Google Scholar]
- 15.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med. 2009;50:1062–1071. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lortie M, Beanlands RS, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging. 2007;34:1765–1774. doi: 10.1007/s00259-007-0478-2. [DOI] [PubMed] [Google Scholar]
- 17.Prior JO, Allenbach G, Valenta I, Kosinski M, Burger C, Verdun FR, Bischof Delaloye A, Kaufmann PA. Quantification of myocardial blood flow with 82Rb positron emission tomography: clinical validation with 15O-water. Eur J Nucl Med Mol Imaging. 2012;39:1037–1047. doi: 10.1007/s00259-012-2082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RS. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 19.Yoshinaga K, Katoh C, Manabe O, Klein R, Naya M, Sakakibara M, Yamada S, Dekemp RA, Tsutsui H, Tamaki N. Incremental diagnostic value of regional myocardial blood flow quantification over relative perfusion imaging with generator-produced rubidium-82 PET. Circ J. 2011;75:2628–2634. doi: 10.1253/circj.cj-11-0502. [DOI] [PubMed] [Google Scholar]
- 20.Farhad H, Dunet V, Bachelard K, Allenbach G, Kaufmann PA, Prior JO. Added prognostic value of myocardial blood flow quantitation in rubidium-82 positron emission tomography imaging. Eur Heart J Cardiovasc Imaging. 2013;14:1203–10. doi: 10.1093/ehjci/jet068. [DOI] [PubMed] [Google Scholar]
- 21.Manabe O, Yoshinaga K, Katoh C, Naya M, deKemp RA, Tamaki N. Repeatability of rest and hyperemic myocardial blood flow measurements with 82Rb dynamic PET. J Nucl Med. 2009;50:68–71. doi: 10.2967/jnumed.108.055673. [DOI] [PubMed] [Google Scholar]
- 22.Nitzsche EU, Choi Y, Czernin J, Hoh CK, Huang SC, Schelbert HR. Noninvasive quantification of myocardial blood flow in humans. A direct comparison of the [13N] ammonia and the [15O] water techniques. Circulation. 1996;93:2000–2006. doi: 10.1161/01.cir.93.11.2000. [DOI] [PubMed] [Google Scholar]
- 23.Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032–1042. doi: 10.1016/0735-1097(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 24.Senthamizhchelvan S, Bravo PE, Lodge MA, Merrill J, Bengel FM, Sgouros G. Radiation dosimetry of 82Rb in humans under pharmacologic stress. J Nucl Med. 2011;52:485–491. doi: 10.2967/jnumed.110.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merhige ME, Breen WJ, Shelton V, Houston T, D’Arcy BJ, Perna AF. Impact of myocardial perfusion imaging with PET and (82)Rb on downstream invasive procedure utilization, costs, and outcomes in coronary disease management. J Nucl Med. 2007;48:1069–1076. doi: 10.2967/jnumed.106.038323. [DOI] [PubMed] [Google Scholar]
- 26.Tout D, Davidson G, Hurley C, Bartley M, Arumugam P, Bradley A. Comparison of occupational radiation exposure from myocardial perfusion imaging with Rb-82 PET and Tc-99m SPECT. Nucl Med Commun. 2014;35:1032–1037. doi: 10.1097/MNM.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 27.Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. J Nucl Med. 2009;50:1076–1087. doi: 10.2967/jnumed.108.054478. [DOI] [PubMed] [Google Scholar]
- 28.Javadi MS, Lautamaki R, Merrill J, Voicu C, Epley W, McBride G, Bengel FM. Definition of vascular territories on myocardial perfusion images by integration with true coronary anatomy: a hybrid PET/CT analysis. J Nucl Med. 2010;51:198–203. doi: 10.2967/jnumed.109.067488. [DOI] [PubMed] [Google Scholar]
- 29.Gaemperli O, Saraste A, Knuuti J. Cardiac hybrid imaging. Eur Heart J Cardiovasc Imaging. 2012;13:51–60. doi: 10.1093/ejechocard/jer240. [DOI] [PubMed] [Google Scholar]
- 30.Anagnostopoulos C, Georgakopoulos A, Pianou N, Nekolla SG. Assessment of myocardial perfusion and viability by Positron Emission Tomography. Int J Cardiol. 2013;167:1737–1749. doi: 10.1016/j.ijcard.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Bengel FM. Leaving relativity behind: quantitative clinical perfusion imaging. J Am Coll Cardiol. 2011;58:749–751. doi: 10.1016/j.jacc.2011.02.068. [DOI] [PubMed] [Google Scholar]
- 32.Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, Dorbala S, Hainer J, Blankstein R, Resnic F, Di Carli MF. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med. 2014;55:248–255. doi: 10.2967/jnumed.113.121442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 34.Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG, Bengel FM. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med. 2011;52:726–732. doi: 10.2967/jnumed.110.081828. [DOI] [PubMed] [Google Scholar]
- 35.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen TE, Bang LE, Holmvang L, Ghotbi AA, Lassen ML, Andersen F, Ihlemann N, Andersson H, Grande P, Kjaer A, Hasbak P. Cardiac (9)(9)mTc sestamibi SPECT and (1)(8) F FDG PET as viability markers in Takotsubo cardiomyopathy. Int J Cardiovasc Imaging. 2014;30:1407–1416. doi: 10.1007/s10554-014-0453-5. [DOI] [PubMed] [Google Scholar]
- 37.Christensen TE, Ahtarovski KA, Andersson H, Vejlstrup N, Ihlemann N, Kjaer A, Holmvang L, Bang L, Grande P, Hasbak P. Takotsubocardiomyopathy: a case of extremely fast recovery described by multimodality cardiac imaging. J Nucl Cardiol. 2012;19:1240–1242. doi: 10.1007/s12350-012-9620-z. [DOI] [PubMed] [Google Scholar]
- 38.Hasbak P, Kjaer A, Skovgaard D, Bang LE, Grande P, Holmvang L. Preserved myocardial blood flow in the apical region involved in takotsubo cardiomyopathy by quantitative cardiac PET assessment. J Nucl Cardiol. 2012;19:169–171. doi: 10.1007/s12350-011-9451-3. [DOI] [PubMed] [Google Scholar]
- 39.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJ, Di Carli MF, Dorbala S, Gewirtz H, Gropler RJ, Kaufmann PA, Knaapen P, Knuuti J, Merhige ME, Rentrop KP, Ruddy TD, Schelbert HR, Schindler TH, Schwaiger M, Sdringola S, Vitarello J, Williams KA Sr, Gordon D, Dilsizian V, Narula J. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–1653. doi: 10.1016/j.jacc.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 41.Chow BJ, Dennie C, Hoffmann U, So D, de Kemp RA, Ruddy TD, Beanlands RS. Comparison of computed tomographic angiography versus rubidium-82 positron emission tomography for the detection of patients with anatomical coronary artery disease. Can J Cardiol. 2007;23:801–807. doi: 10.1016/s0828-282x(07)70831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valenta I, Dilsizian V, Quercioli A, Ruddy TD, Schindler TH. Quantitative PET/CT measures of myocardial flow reserve and atherosclerosis for cardiac risk assessment and predicting adverse patient outcomes. Curr Cardiol Rep. 2013;15:344. doi: 10.1007/s11886-012-0344-0. [DOI] [PubMed] [Google Scholar]
- 43.Valenta I, Quercioli A, Schindler TH. Diagnostic Value of PET-Measured Longitudinal Flow Gradient for the Identification of Coronary Artery Disease. JACC Cardiovasc Imaging. 2014;7:387–396. doi: 10.1016/j.jcmg.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Gould KL, Nakagawa Y, Nakagawa K, Sdringola S, Hess MJ, Haynie M, Parker N, Mullani N, Kirkeeide R. Frequency and clinical implications of fluid dynamically significant diffuse coronary artery disease manifest as graded, longitudinal, base-to-apex myocardial perfusion abnormalities by noninvasive positron emission tomography. Circulation. 2000;101:1931–1939. doi: 10.1161/01.cir.101.16.1931. [DOI] [PubMed] [Google Scholar]
- 45.Schindler TH, Quercioli A, Valenta I, Ambrosio G, Wahl RL, Dilsizian V. Quantitative assessment of myocardial blood flow--clinical and research applications. Semin Nucl Med. 2014;44:274–293. doi: 10.1053/j.semnuclmed.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Valenta I, Wahl RL, Schindler TH. Longitudinal Myocardial Blood Flow Gradient and CAD Detection. Curr Cardiol Rep. 2015;17:550. doi: 10.1007/s11886-014-0550-z. [DOI] [PubMed] [Google Scholar]
- 47.Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330:1782–1788. doi: 10.1056/NEJM199406233302503. [DOI] [PubMed] [Google Scholar]
- 48.Di Carli M, Czernin J, Hoh CK, Gerbaudo VH, Brunken RC, Huang SC, Phelps ME, Schelbert HR. Relation among stenosis severity, myocardial blood flow, and flow reserve in patients with coronary artery disease. Circulation. 1995;91:1944–1951. doi: 10.1161/01.cir.91.7.1944. [DOI] [PubMed] [Google Scholar]
- 49.Anagnostopoulos C, Almonacid A, El Fakhri G, Curillova Z, Sitek A, Roughton M, Dorbala S, Popma JJ, Di Carli MF. Quantitative relationship between coronary vasodilator reserve assessed by 82Rb PET imaging and coronary artery stenosis severity. Eur J Nucl Med Mol Imaging. 2008;35:1593–1601. doi: 10.1007/s00259-008-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging. 2009;2:1009–1023. doi: 10.1016/j.jcmg.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012;5:430–440. doi: 10.1016/j.jcmg.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Kjaer A, Meyer C, Wachtell K, Olsen MH, Ibsen H, Opie L, Holm S, Hesse B. Positron emission tomographic evaluation of regulation of myocardial perfusion in physiological (elite athletes) and pathological (systemic hypertension) left ventricular hypertrophy. Am J Cardiol. 2005;96:1692–1698. doi: 10.1016/j.amjcard.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 53.BARI 2D Study Group. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 55.Stergiopoulos K, Brown DL. Initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:312–319. doi: 10.1001/archinternmed.2011.1484. [DOI] [PubMed] [Google Scholar]
- 56.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 57.Task Force Members; Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ ESC Committee for Practice Guidelines; Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S Document Reviewers. Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 58.Knesaurek K, Machac J, Zhang Z. Repeatability of regional myocardial blood flow calculation in 82Rb PET imaging. BMC Med Phys. 2009;9:2. doi: 10.1186/1756-6649-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klein R, Renaud JM, Ziadi MC, Thorn SL, Adler A, Beanlands RS, deKemp RA. Intra- and inter-operator repeatability of myocardial blood flow and myocardial flow reserve measurements using rubidium-82 pet and a highly automated analysis program. J Nucl Cardiol. 2010;17:600–616. doi: 10.1007/s12350-010-9225-3. [DOI] [PubMed] [Google Scholar]
- 60.Efseaff M, Klein R, Ziadi MC, Beanlands RS, deKemp RA. Short-term repeatability of resting myocardial blood flow measurements using rubidium-82 PET imaging. J Nucl Cardiol. 2012;19:997–1006. doi: 10.1007/s12350-012-9600-3. [DOI] [PubMed] [Google Scholar]


