Abstract
Bavituximab is a chimeric monoclonal antibody with immune modulating and tumor-associated vascular disrupting properties demonstrated in models of non-small cell lung cancer (NSCLC). The molecular target of Bavituximab, phosphatidylserine (PS), is exposed on the outer leaflet of the membrane bi-layer of malignant vascular endothelial cells and tumor cells to a greater extent than on normal tissues. We evaluated the tumor-targeting properties of Bavituximab for imaging of NSCLC xenografts when radiolabeled with 111In through conjugation with a bifunctional chelating agent, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). In vitro binding of 111In-DOTA-Bavituximab to PS was determined by enzyme-linked immunosorbent assay (ELISA). Biodistribution of 111In-DOTA-Bavituximab was conducted in normal rats, which provided data for dosimetry calculation. Single-photon emission computed tomography/computed tomography (SPECT/CT) imaging was performed in athymic nude rats bearing A549 NSCLC xenografts. At the molar conjugation ratio of 0.54 DOTA per Bavituximab, the PS binding affinity of 111In-DOTA-Bavituximab was comparable to that of unmodified Bavituximab. Based on the quantitative SPECT/CT imaging data analysis, 111In-DOTA-Bavituximab demonstrated tumor-specific uptake as measured by the tumor-tomuscle ratio, which peaked at 5.2 at 72 hr post-injection. In contrast, the control antibody only presented a contrast of 1.2 at the same time point.These findings may underlie the diagnostic efficacy and relative low rates of systemic vascular and immune-related toxicities of this immunoconjugate. Future applications of 111In-DOTA-bavituximab may include prediction of efficacy, indication of tumor immunologic status, or characterization of radiographic findings.
Keywords: Bavituximab, phosphatidylserine, lung cancer, immunotherapy, vascular targeting, 111In
Introduction
Under normal cellular homeostasis, phosphatidylserine (PS) is an anionic membrane phospholipid preferentially located on the inner leaflet of the plasma membrane. This asymmetry is maintained by ATP-dependent aminophospholipid translocases that catalyze the transfer of aminophospholipids from the external to the internal plasma membrane [1,2]. In certain physiologic states, such as apoptosis and cell activation, PS asymmetry is disrupted, resulting in exposure of PS on the external membrane leaflet [3-6]. This process occurs due to inactivation of aminophospholipid translocases or the activation of scramblases and ABC floppases [7]. Stresses within tumors, such as hypoxia and acidity, also trigger these events through the generation of reactive oxygen species [6]. Exposed PS in the cellular microenvironment restricts the production of pro-inflammatory cytokines and increases the production of anti-inflammatory cytokines. This role is consistent with PS exposure during apoptosis, a class of cell death that does not induce inflammatory responses [8]. On apoptotic cells, PS exposure results in down-regulated macrophage secretion of TNF-α and IL-1β, and upregulated production of TGF-β and IL-10 [8]. In addition, PS exposure suppresses immune responses by reducing dendritic cell maturation and antigen presentation [9]. Therefore, PS can be applied for targeted cancer immunotherapy. The molecular distribution of PS appears to result in favorable pharmacodynamic properties. In normal cells, including vascular endothelium, PS is segregated to the inner membrane leaflet and is therefore not available for antibody binding. In contrast, the exposure of PS on cancer cells and vascular endothelial cells in the tumor microenvironment permits this antibody to target the tumor microenvironment.
Bavituximab is a human-mouse chimeric IgG1 monoclonal antibody that targets PS by recognizing the PS-binding protein β2 glycoprotein-1 (β2GP1). Specifically, by cross-linking two β2GP1 molecules at surface of cells with exposed PS, Bavituximab localizes to tumors [10]. Once bound, Bavituximab activates host effector (immune) functions, such as antibody dependent cellular cytotoxicity (ADCC), resulting in tumor vessel destruction. Additionally, Bavituximab conveys anticancer effects by reversing the immune and inflammatory suppression normally exerted by tumors. Specifically, PS-targeting antibodies such as Bavituximab have been shown to result in promotion of pro-inflammatory pathways, recruitment of tumoricidal M1 macrophages, dendritic cell maturation and antigen presentation leading to adaptive T lymphocyte responses [11,12].
The PS-targeting antibody, Bavituximab, has completed phase 1 [13] and phase 2 testing in lung cancer [14] and is currently under investigation in combination with chemotherapy and other agents for a number of malignancies, including lung, breast, rectal, liver, and prostate cancer. A phase 3 trial in previously treated advanced nonsquamous NSCLC is underway [15]. Clinical experience with Bavituximab to date shows relatively low rates of systemic vascular and immune-related toxicities. These observations also suggest tumor-specific targeting by Bavituximab. In this study, we presented a proof-of-concept method for the imaging evaluation of an 111In-labeled Bavituximab immunoconjugate by single-photon emission computed tomography (SPECT) with the goal to understand the low rates of systemic vascular and immune-related toxicities of Bavituximab seen clinically along with its anti-tumor effects.
Materials and methods
All chemicals were of reagent grade and used as received. All chemicals, solvents, and reagents were purchased from Sigma-Aldrich and Fisher Chemical unless otherwise noted. The 111InCl3 solution in 0.1 M HCl was purchased from Perkin-Elmer. The 64CuCl2 solution was purchased from Washington University, St. Louis. Rituximab (human IgG1 chimeric mAb) was obtained from the University of Texas Southwestern pharmacy. Radio-TLC analysis was performed on a Rita Star Radioisotope TLC Analyzer (Straubenhardt, Germany) to monitor the radiolabeling reaction. Instant Thin Layer Chromatography Medium (ITLC) acted as the supporter, and 1 × PBS buffer was the developing eluent.
Preparation of DOTA-Bavituximab conjugate
The DOTA-Bavituximab conjugate formation was via the coupling reaction between the activated 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) bifunctional chelating agent and free lysine amino groups in the antibody. The conjugation was undertaken in aqueous conditions. Briefly, a solution of 9.4 mg (19.9 μmol) of DOTA·4H2O in 300 μL of H2O was adjusted to pH 5-5.5 with 1 M NaOH and set in an ice-water bath. To the DOTA solution was added 1.9 mg (9.94 μmol) of 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) in 76 μL of H2O. Then, 4.3 mg (19.9 μmol) of N-Hydroxysulfosuccinimide (s-NHS) in 86 μL of H2O freshly prepared was added, and the reaction mixture was stirred in the ice-water bath for 30 min and then used without separation for the protein conjugation. An aliquot of 5.8 mg of Bavituximab (provided by Peregrine Pharmaceuticals, Tustin, CA) in 0.6 mL of PBS, pH 7.4, was pre-conditioned by 4 times of washing with 0.1 M Na2HPO4, pH 7.5, using an Amicon® Ultra 4 mL filter (50,000 Da) at 4°C. Subsequently, the solution of N-Hydroxysulfosuccinimidyl DOTA (DOTA-OSSu) (0.66 μ mol) was added to the preconditioned Bavituximab solution. After the pH was adjusted to 8.0, the reaction mixture was incubated at 4°C overnight with continuous mixing. The DOTA-Bavituximab conjugate was separated and purified by centrifuging the reaction mixture 5 times in an Amicon® Ultra 4 mL filter (50,000 Da) at 4°C using 0.1 M NaOAc, pH 5.0 as the washing solution. The same procedure was also applied to the control antibody (Rituximab).
Determination of average number of chelates per antibody molecule
The average number of DOTA chelator per Bavituximab molecule was determined by the isotope dilution method. Briefly, a series of standardized CuCl2 dilutions was prepared to react with the DOTA-Bavituximab conjugates. For each Cu(II) concentration, 3 μL of DOTA-Bavituximab containing approximately 150 pmol of the conjugate was added to 56 μL of 0.1 M NH4OAc, pH 5.0, followed by adding 40 μL of the standardized CuCl2 solution and 1 μL of 64CuCl2. The reaction mixture was incubated at 37°C for 1 h, after which 10 μL of 5 mM diethylenetriamine pentaacetic acid (DTPA) was added to remove non-specifically bound 64Cu(II)/Cu(II). Radio-TLC was performed to analyze the labeling reaction yields. The number of chelator per antibody molecule was calculated from the ratio of counts remaining at the origin to the total number of counts, using the method of Meares et al. [16].
Indium-111 (111In) radiolabeling of DOTA-Bavituximab
The isotope of 111In was selected for this work to match the biological half-life of Bavituximab. To label DOTA-Bavituximab with 111In, 230 MBq of 111InCl3 in 10 μL of 0.1 M HCl was added to 1.6 mg of DOTA-Bavituximabin 300 μL of 0.1 M sodium acetate, pH 5.0. The reaction mixture was incubated at 37°C for 0.5 h, and then the reaction mixture was mixed with 1 μL of 5 mM DTPA solution. The reaction mixture was allowed to stand at room temperature for 5 min, and the radiolabeling yield was ~90% as accessed by radio-ITLC. The 111In-DOTA-Bavituximabwas purified by a Bio-Spin P6 columnto reach > 95% of radiochemical purity.The same procedure was also applied to the control antibody (Rituximab).
Characterization of 111In-DOTA-Bavituximab target binding
For phospholipid ELISAs, 50 µl of PS in n-hexane (10 µg/mL) was added to wells of Immulon 1B 96-well plates. The solvent was evaporated at room temperature and the plates were blocked for 1 h with 1% BSA in PBS and then washed with PBS. Unlabeled Bavituximab, Rituximab, and 111In-DOTA-Bavituximab were pre-diluted in 10% fetal bovine serum to provide β2GP1 and further diluted in blocking buffer at an initial concentration of 200 µg/mL and 2-fold dilutions were performed in a separate 96-well plates (100 µl per well). The dilutions (100 µl/well) were then transferred from the dilution plates to PS-coated plates. The plates were incubated for 1 h at room temperature. The plates were washed and bound antibody was detected with goat anti-human IgG conjugated to HRP (1:2000 in blocking buffer) and developed with the chromogenic substrate ODP. The plates were read at 490 nm with a microplate reader (BioTek Instruments, Winooski, VT).
Generation of A549 xenograft model
All animal studies were approved by the UT Southwestern Institutional Animal Care and Use Committee (IACUC). A549 human lung carcinoma cellswere maintained at 37°C in a mixture of 5% CO2 and 95% air in Gibco® DMEM supplemented with 10% fetal bovine serum (Sigma Aldrich). The cells were allowed to grow until 90% confluent. Cells were washed with PBS and mixed with Basement Membrane Matrix BD MatrigelTM (BD Biosciences) in a 1:1 ratio such that there would be 5 million cells/300 µl of suspension. The cell/Matrigel suspension was stored on ice until implantation into animals. Athymic nude rats were chosen because the use of the xenograft model calls for the use of immune-suppressed animals and because Bavituximab interacts with rat, but not mouse, β2GP1. Animals were housed in a pathogen-free facility. Fifteen 5-week old female athymic nude rats were purchased from the National Cancer Institute. 5 million cells were injected subcutaneously into the right flank of each rat. Tumor growth was monitored by caliper measurement three times per week. Tumors were allowed to grow until reaching a diameter of 1.5 cm for SPECT/CT imaging.
Dosimetry studies
Distributions of radioactivity were determined in normal rats with tail vein injection of 111In-DOTA-Bavituximab. The organs of interest [blood, heart, lung, liver, spleen, stomach, pancreas, large intestine, small intestine, uterus, kidney, muscle, fat, bone, brain, and thyroid] were excised after whole-body perfusion at 4, 8, 24, 48, and 72 hr post-injection (p.i.) (n = 4), and then the radioactivity was quantitated by automatic gamma counter. The concentration in each sample was expressed as percentage injected dose per gram (%ID/g) [17].
Radiation dose estimates for human organs were calculated based on mass-based extrapolation of animal data. Specifically, the concentration in the animal organs was converted to a concentration in human organs by multiplying the animal concentration by a ratio of the total body weight of the animals and humans [18]. Then the percentage in the human organs was derived using organ masses taken from a standard model of the human body for either adult males [19]. Data were fit using the SAAM II software [20]. Time integrals of activity [21] were entered into the OLINDA/EXM software [22] using the adult male model. Time-activity data in the bladder was fit with a single, in-growing exponential, and no bladder voiding was assumed. From the observed activity in the intestines, 5% of the administered activity was assumed to enter the gastrointestinal tract (at the small intestine) and follow normal transit kinetics thereafter.
SPECT/CT imaging
SPECT/CT Imaging was performed using a NanoSPECT/CT PlusSystem (Bioscan, Washington, DC, USA) on A549 tumor bearing rats. After the intravenous injection of around 14-16 MBq of 111In-DOTA-Bavituximab or control antibody (111In-DOTA-Rituximab) (n = 1), SPECT images were acquired at 48, 72, and 96 hr p.i. The SPECT data were collected with 4 detector arrays collimated with multi-pinhole apertures giving a post-reconstruction resolution of 1.23 mm. Tumor focused images were acquired with 20 projections per 360° rotation, with a movement of 48 mm per 360°. The count rate of the tumors was used to determine the optimum exposure time, achieving at least 20,000 counts per projection. The CT imaging was performed using 360 projections per rotation with 45 kVp, 1500 ms exposure, and the binning factor of 1:4. The SPECT image reconstruction was carried out using HiSPECT NG (Sciviswissenschaftliche Bildverarbeitung GmbH, Germany) with 35% smoothing, 100% resolution, and 3 × 3 iterations (Standard mode). The quantification of the organ activity was performed using Bioscan’s In Vivo Scope 1.43 software package (Bioscan, Wash-ington, DC). After co-registration of the CT and SPECT images, a cylindrical region of interest (ROI) was drawn, encompassing the organs in all planes containing the organs. The total activity in the tumor was quantified as percentage injected dose per gram (%ID/g) by normalizing the signal imaged at a given location for the amount of tracer injected into the subject.
Results
Antibody modification and radiochemistry
The conjugation of DOTA to Bavituximab (Figure 1) was performed by combining the antibody with 15 molequiv of DOTA-OSSu intermediate at pH 8 and the reaction was allowed to proceed overnight at 4°C. The molar conjugation ratio of DOTA to Bavituximab was determined by the 64Cu/natCu isotope dilution method (Figure 2). Under the reaction conditions, the desired low chelator per antibody ratio (0.54) was achieved. The radiolabeling yield with 111In varied from 50% to over 95% depending on the relative amounts between 111In and the DOTA-Bavituximab conjugate. After purification, the radiochemical purity of 111In-DOTA-Bavituximab was determined to be > 95% by radio-TLC analysis. The average specific activity of 111In-DOTA-Bavituximab in this study was about 140 MBq/mg. Rituximab without PS-specific binding [10,23] was chosen as the negative control. Its DOTA conjugation and radiochemistry followed the same procedure as that for Bavituximab.
Figure 1.
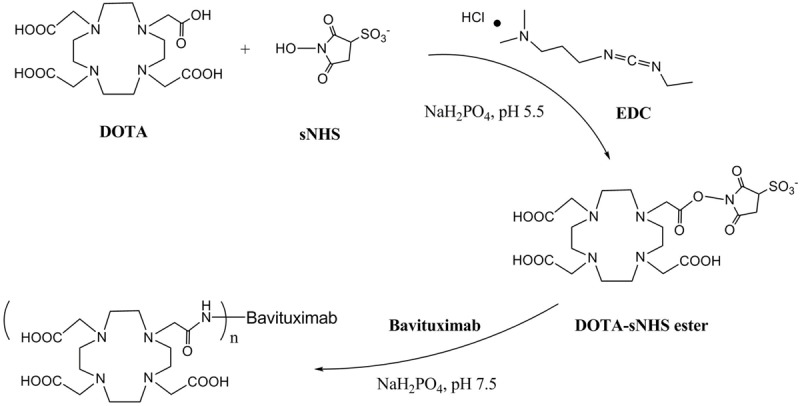
Schematic conjugation procedure of Bavituximab with a bifunctional chelator, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). EDC: 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide; s-NHS: N-Hydroxysulfosuccinimide.
Figure 2.
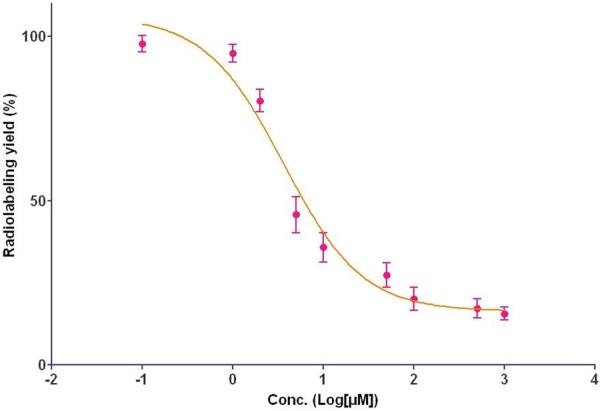
Determination of the average number of DOTA per Bavituximab using an isotopic dilution method.
111In-DOTA-Bavituximab target binding
Immunoreactivity of 111In-DOTA-Bavituximab was determined by ELISA. As shown in Figure 3, the PS binding curve of 111In-DOTA-Bavituximab was almost identical to that of the intact Bavituximab, which correlated with their calculated EC50 values (111In-DOTA-Bavituximab: 0.23 μg/mL; Bavituximab: 0.18 μg/mL). The radiolabeled control antibody Rituximab did not demonstrate significant PS binding at sub-µg/mL concentrations. These data indicated that the immunoreactivity of Bavituximab was not significantly altered by DOTA conjugation or radiolabeling, showing the plausibility of using 111In-DOTA-Bavituximab for in vivo assessment of the PS status.
Figure 3.
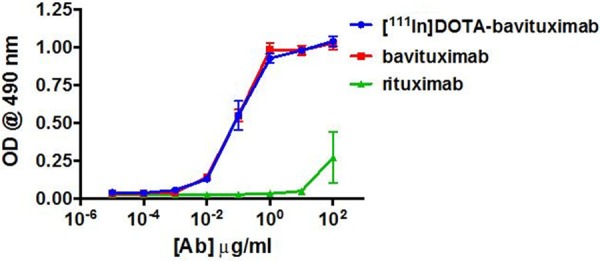
Phosphatidylserine (PS) binding by 111In-DOTA-Bavituximab, native Bavituximab, and control antibody (Rituximab).
Dosimetry
In order to investigate the dosimetry of 111In-DOTA-Bavituximab for potential clinical application, biodistribution was performed in normal/healthy (non-tumor-bearing) rats at multiple time points (4, 8, 24, 48, 72 hrp.i.) (Figure 4, Table 1). As expected, liver and spleen demonstrated the most activity. Human radiation dose estimates based on an adult male model revealed thatthe skin received the least radiation exposure (7.32 × 10-2 mSv/MBq or 2.71 × 10-1 rem/mCi), while most organs received around 0.08-0.15 mSv/MBq. Not surprisingly, the bone and liver received the highest doses, which were around 0.31 and 0.28 mSv/MBq, respectively. The effective dose was approximately 0.15 mSv/MBq.
Figure 4.
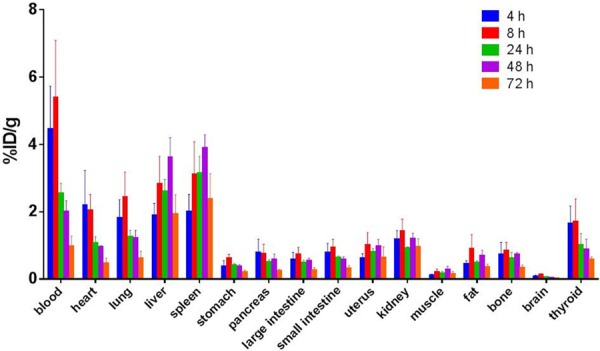
Biodistribution of 111In-DOTA-Bavituximab in normal rats (4, 8, 24, 48, 72 hr; n = 4) as percent injected dose per gram of tissue (%ID/g).
Table 1.
Estimates of the human radiation dosimetry (adult male model) obtained from the biodistribution data summarized in Figure 4. Results were obtained by employing a mass-based extrapolation of the biodistribution data to estimate the human organ concentrations used as input for the OLINDA/EXM dosimetry software
| Estimated Dose | ||
|---|---|---|
|
|
||
| Target Organ | mSv/MBq | rem/mCi |
| Adrenals | 1.59E-01 | 5.89E-01 |
| Brain | 9.44E-02 | 3.49E-01 |
| Breasts | 8.46E-02 | 3.13E-01 |
| Gallbladder Wall | 1.89E-01 | 6.98E-01 |
| LLI Wall | 2.26E-01 | 8.36E-01 |
| Small Intestine | 1.59E-01 | 5.87E-01 |
| Stomach Wall | 1.41E-01 | 5.21E-01 |
| ULI Wall | 1.81E-01 | 6.71E-01 |
| Heart Wall | 1.46E-01 | 5.42E-01 |
| Kidneys | 1.61E-01 | 5.95E-01 |
| Liver | 2.79E-01 | 1.03E+00 |
| Lungs | 1.34E-01 | 4.95E-01 |
| Muscle | 1.10E-01 | 4.08E-01 |
| Ovaries | 1.58E-01 | 5.84E-01 |
| Pancreas | 1.70E-01 | 6.27E-01 |
| Red Marrow | 1.16E-01 | 4.31E-01 |
| Osteogenic Cells | 3.14E-01 | 1.16E+00 |
| Skin | 7.32E-02 | 2.71E-01 |
| Spleen | 2.29E-01 | 8.49E-01 |
| Testes | 1.06E-01 | 3.93E-01 |
| Thymus | 1.20E-01 | 4.42E-01 |
| Thyroid | 1.21E-01 | 4.46E-01 |
| Urinary Bladder Wall | 1.70E-01 | 6.28E-01 |
| Uterus | 1.54E-01 | 5.69E-01 |
| Total Body | 1.20E-01 | 4.46E-01 |
| Effective Dose Equivalent | 1.61E-01 | 5.97E-01 |
| Effective Dose | 1.50E-01 | 5.54E-01 |
SPECT/CT imaging
Small animal SPECT/CT imaging studies were conducted in athymic nude rats bearing A549 NSCLC xenografts. Representative coronal SPECT/CT images are presented in Figure 5. A549 tumors were clearly visualized by 111In-DOTA-Bavituximab at 48 hrp.i. when the images provided the greatest tumor contrast. At 72 and 96 hrp.i., A549 tumors could still be identified by 111In-DOTA-Bavituximab. Besides A549 tumors, other surrounding tissues were observed with minimal uptake within the SPECT imaging window. In contrast, 111In-DOTA-Rituximab could not show significant contrast of tumor to the surrounding tissues, indicating only non-specific distributionwas associated with this control antibody.
Figure 5.
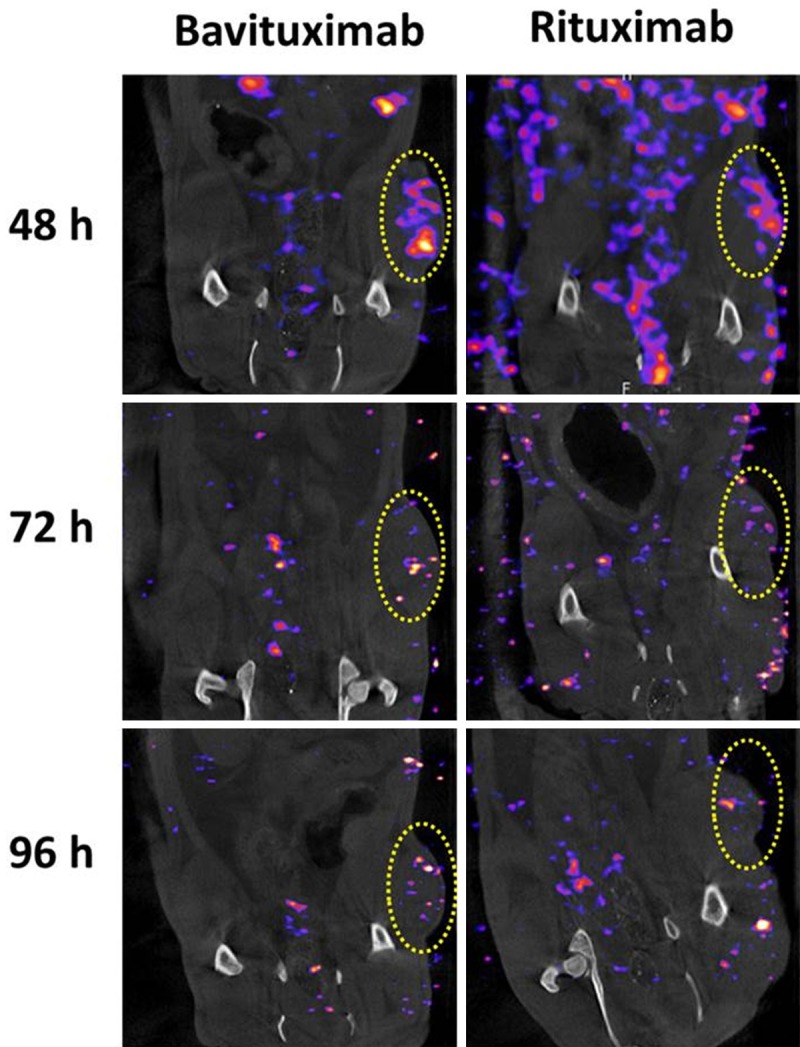
Representative SPECT/CT images of 111In-DOTA-Bavituximab and radiolabeled control antibody (111In-DOTA-Rituximab) in rats bearing A549 NSCLC tumors (coronal view) at 48, 72, and 96 hr p.i. Yellow circles denote uptake in tumors.
The quantitative imaging analysis was performed using the manufacturer’s software. Tumor-to-muscle ratio, the metric applicable in clinical practice, was calculated based on the imaging quantification data (Figure 6). At all time-points, 111In-DOTA-Bavituximab demonstrated a substantially increased tumor-to-muscle ratio (3.7, 5.2, and 3.8 at 48, 72, and 96 hrp.i., respectively) compared to 111In-DOTA-Rituximab (2.4, 1.2, and 0.2 at 48, 72, and 96 hrp.i., respectively).
Figure 6.
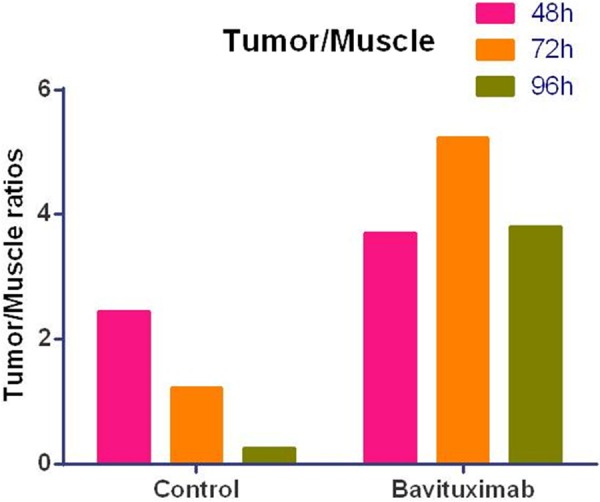
Tumor-to-muscle uptake ratios in A549 NSCLC tumor-bearing rats treated with 111In-DOTA-Bavituximab and radiolabeled control antibody (111In-DOTA-Rituximab) derived from SPECT/CT imaging quantitative analysis.
Discussion
Phosphatidylserine (PS) is a membrane phospholipid normally segregated to the inner membrane leaflet. In apoptotic cells or activated cells such as tumor vascular endothelial cells, PS is exposed on the outer membrane leaflet. In addition, PS in the tumor microenvironment generates an immunosuppressive signal allowing the tumor to escape immune surveillance.PS-targeting antibodies, such as Bavituximab, reawaken antitumor immunity, exert a vascular targeting effect, and have demonstrated anti-tumor effects in a number of cancer models [11,24-27]. In clinical studies, Bavituximab has been investigated in numerous malignancies. In lung cancer, encouraging initial efficacy and safety results have led to phase 3 development. In this study, the 111In-labeled Bavituximab was prepared to explainits low rates of systemic vascular and immune-related toxicities seen clinically and help understand its anti-tumor effects. The tumor-specific activity shown from the in vivo imaging of 111In-DOTA-Bavituximab can help distinguish Bavituximab from other immune modulating and vascular targeting agents.
The isotope of 111In (t1/2: 67 hr) was selected for labeling to best match the biological half-life of Bavituximab (approximately 48 hr) [13]. Our DOTA modification and radiolabeling did not appear to alter immunoreactivity of Bavituximab to PS, as 111In-DOTA-Bavituximab exhibited almost identical PS binding in the ELISA assay as unconjugated Bavituximab antibody did. The biodistribution data showed minimal uptake across the whole rat body within a 72-hr period. Meanwhile, the SPECT/CT imaging data revealed PS-specific tumor targeting from 111In-DOTA-Bavituximab, not the control antibody of Rituximab. These results correlate with the low rates of systemic vascular and immune-related toxicities of Bavituximab seen clinically, due to the unique feature of PS between normal and cancerous environments.
Dosimetric analysis suggested that 111In-DOTA-Bavituximab could be administered at radiographically useful doses to human patients without imposing significant amount of radiation doses. As with any dose estimates based on data obtained from an animal model, uncertainties always exist in the actual human dose estimates, particularly in the extrapolation models used to produce the human dose calculations and the assumptions applied about excretion of activity. Nevertheless, the acquired dosimetry data still provides a reasonable basis for predicting the approximate human dosimetry.
In this work, we achieved a ratio of 0.54 DOTA per Bavituximab molecule by controlling the conjugation conditions. We intentionally aimed for such a low ratio as it would not alter antibody immunoreactivity after DOTA modification and 111In-labeling. Another consideration was that the resulted 111In-DOTA-Bavituximab might have a significant amount of non-specific binding during the circulation, which would result in a high level of systemic radiation exposure, so high specific activity was not pursued. Although the radiolabeled antibody appeared to have good tumor targeting, absolute tumor uptake mayhave been compromised by this low ratio due to the resulted low specific activity. The low specific activity might also lead to the lower tumor-to-muscle ratios than those observed with the previously reported 74As-Bavituximab [23], in addition to other determinants of in vivo tumor targeting. Further studies on 111In-DOTA-Bavituximab at higher specific activity are warranted if this radioimmunoconjugateis intended for PS-targeted imaging.
Although the A549 NSCLC tumors could be visualized on SPECT/CT images at 48 hrp.i., we noticed the relatively weak tumor accumulation shown at 72 and 96 hr p.i. Low specific activity of 111In-DOTA-Bavituximab aside, the suboptimal sensitivity of SPECT imaging with 111In might play a role as the NanoSPECT/CT system is optimized for the detection of 99mTc’s gamma emission at 141 keV. Therefore, positron emission tomography (PET) will offer advantages to the development of Bavituximab-based immuno-diagnostic techniques for noninvasive assessment of PS status before and after therapeutic interventions of cancer. Actually, immunoPET with Bavituximab would be straightforward when labeled with 89Zr or 124I [28,29]. Arsenic-74 is another choice but perhaps not ideal due to limited availability and a not-so-well-established radiochemistry procedure [30].
In addition to this proof of principle study, there are numerous potential clinical applications for PS-targeting radiolabeled Bavituximab. Such a radiolabeled antibody could yield insights into Bavituximabtherapeutic efficacy, indicate a tumor’s immunologic status, or distinguish between malignant and benign molecularimaging findings. Because standard tissue immunohistochemistry cannot determine whether PS is internalized or exposed on the outer membrane leaflet, only with an in vivo diagnostic can tumors be characterized as PS-positive for the purpose of predicting response to PS-directed therapy. Furthermore, because PS is segregated to the inner cell membrane leaflet in most normal tissues, a PS-targeting imaging agent might assist with the determination of whether radiographic abnormalities represent malignancy. Preclinical studies have shown that cancer treatments such as cytotoxic chemotherapy, ionizing radiation, and certain kinase inhibitors enhance PS flipping [25]. The extent to which such effects occur in patients, with which agents they occur most, and whether these effects predict outcomes might be evaluated with a radiolabeled PS-targeting antibody. Separately, in vivo characterization of tumor PS exposure might provide insight into a tumor’s immunomodulating properties and the potential role for immunotherapies such as vaccines and checkpoint inhibitors. Other possibilities include conjugation of Bavituximab to a therapeutic radioisotope, toxin, or drug to capitalize on the antibody’s apparent tumor specificity.
In conclusion, we demonstrated that 111In-DOTA-Bavituximab preserved the in vivo PS targeting of Bavituximab, an acceptable dosimetry profile, and specific accumulation in NSCLC xenografts. These findings may underlie the efficacy and low rates of systemic vascular and immune-related toxicities of Bavituximab seen clinically. In the future, potential clinical applications of 111In-DOTA-Bavituximab may include prediction of Bavituximab efficacy, indication of tumor immunologic status, or distinguishing between malignant and benign radiographic findings. In the near term, modifications of the current radiolabeled compound may improve its future performance, such as altering the DOTA: Bavituximab ratio and employing PET radioisotopes.
Acknowledgements
This work was supported by an American Society of Clinical Oncology (ASCO) Career Development Award (to D.E.G.) and by a research grant from Peregrine Pharmaceuticals (to D.E.G.). SPECT/CT imaging was performed on a NanoSPECT/CT Plus System purchased with funds provided in part by an NIH NCRR grant (1S10RR029674-01 to O.K.O.). We thank Michael Stabin, PhD, from Vanderbilt University for assistance with dosimetry analyses. We also thank Dru Gray from UT Southwestern for assistance with manuscript preparation.
Disclosure of conflict of interest
Dr. Gerber reports grants from the American Society of Clinical Oncology, grants from Peregrine Pharmaceuticals, during the conduct of the study. Dr. Hao has nothing to disclose. Dr. Watkins has nothing to disclose. Dr. Barbero has nothing to disclose. Dr. Stafford has nothing to disclose. Dr. Anderson has nothing to disclose. Dr. Holbein has nothing to disclose. Dr. Oz reports grants from NIH NCRR grant (1S10RR029674-01) during the conduct of the study. Dr. Mathews has nothing to disclose. Dr. Thorpe reports grants from Peregrine Pharmaceuticals during the conduct of the study; grants from Peregrine Pharmaceuticals outside the submitted work. Dr. Brekken reports grants from Peregrine Pharmaceuticals during the conduct of the study; grants from Peregrine Pharmaceuticals outside the submitted work. Dr. Sun has nothing to disclose.
References
- 1.Bitbol M, Fellmann P, Zachowski A, Devaux PF. Ion regulation of phosphatidylserine and phosphatidylethanolamine outside-inside translocation in human erythrocytes. Biochim Biophys Acta. 1987;904:268–282. doi: 10.1016/0005-2736(87)90376-2. [DOI] [PubMed] [Google Scholar]
- 2.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 3.Dillon SR, Mancini M, Rosen A, Schlissel MS. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol. 2000;164:1322–1332. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- 4.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132–6140. [PubMed] [Google Scholar]
- 6.Ran S, Thorpe PE. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int J Radiat Oncol Biol Phys. 2002;54:1479–1484. doi: 10.1016/s0360-3016(02)03928-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Zhou Q, Wiedmer T, Sims PJ. Level of expression of phospholipid scramblase regulates induced movement of phosphatidylserine to the cell surface. J Biol Chem. 1998;273:6603–6606. doi: 10.1074/jbc.273.12.6603. [DOI] [PubMed] [Google Scholar]
- 8.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Doffek K, Sugg SL, Shilyansky J. Phosphatidylserine regulates the maturation of human dendritic cells. J Immunol. 2004;173:2985–2994. doi: 10.4049/jimmunol.173.5.2985. [DOI] [PubMed] [Google Scholar]
- 10.Luster TA, He J, Huang X, Maiti SN, Schroit AJ, de Groot PG, Thorpe PE. Plasma protein beta-2-glycoprotein 1 mediates interaction between the anti-tumor monoclonal antibody 3G4 and anionic phospholipids on endothelial cells. J Biol Chem. 2006;281:29863–29871. doi: 10.1074/jbc.M605252200. [DOI] [PubMed] [Google Scholar]
- 11.He J, Yin Y, Luster TA, Watkins L, Thorpe PE. Antiphosphatidylserine antibody combined with irradiation damages tumor blood vessels and induces tumor immunity in a rat model of glioblastoma. Clin Cancer Res. 2009;15:6871–6880. doi: 10.1158/1078-0432.CCR-09-1499. [DOI] [PubMed] [Google Scholar]
- 12.Yin Y, Huang X, Lynn KD, Thorpe PE. Phosphatidylserine-targeting antibody induces M1 macrophage polarization and promotes myeloid-derived suppressor cell differentiation. Cancer Immunol Res. 2013;1:256–268. doi: 10.1158/2326-6066.CIR-13-0073. [DOI] [PubMed] [Google Scholar]
- 13.Gerber DE, Stopeck AT, Wong L, Rosen LS, Thorpe PE, Shan JS, Ibrahim NK. Phase I safety and pharmacokinetic study of bavituximab, a chimeric phosphatidylserine-targeting monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17:6888–6896. doi: 10.1158/1078-0432.CCR-11-1074. [DOI] [PubMed] [Google Scholar]
- 14.Shtivelband M, Spigel DR, Gerber DE, Jain MM, Ponomarova OV, Giorgadze D, Shan J, Menander KB. Randomized, blinded, placebocontrolled phase II trial of docetaxel and bavituximab as second-line therapy in locally advanced or metastatic non-squamous nonsmall cell lung cancer. J Clin Oncol. 2013:31. [Google Scholar]
- 15.Gerber DE, Shan J, Keilholz U, Spigel DR, Horn L, Heist RS, Sanborn RE, Mainwaring PN, Belani CP, Edelman MJ. Stimulating an immune response through bavituximab in a phase III lung cancer study. J Clin Oncol. 2014;32(Suppl) abstract TPS8129. [Google Scholar]
- 16.Meares CF, McCall MJ, Reardan DT, Goodwin DA, Diamanti CI, McTigue M. Conjugation of antibodies with bifunctional chelating agents: isothiocyanate and bromoacetamide reagents, methods of analysis, and subsequent addition of metal ions. Anal Biochem. 1984;142:68–78. doi: 10.1016/0003-2697(84)90517-7. [DOI] [PubMed] [Google Scholar]
- 17.Singh AN, Liu W, Hao G, Kumar A, Gupta A, Oz OK, Hsieh JT, Sun X. Multivalent bifunctional chelator scaffolds for gallium-68 based positron emission tomography imaging probe design: signal amplification via multivalency. Bioconjug Chem. 2011;22:1650–1662. doi: 10.1021/bc200227d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirschner A, Ice R, Beierwaltes W. Radiation dosimetry of 131I-19-iodocholesterol: the pitfalls of using tissue concentration data, the author’s reply. J Nucl Med. 1975;16:248–249. [Google Scholar]
- 19.Cristy M, Eckerman K. Specific absorbed fractions of energy at various ages from internal photon sources. Oak Ridge, TN: Oak Ridge National Laboratory; 1987. [Google Scholar]
- 20.Education ORIfSa. Developing and testing integrated multicompartment models to describe a single-input multiple-output study using the SAAM II software system. In: Foster D, Barrett P, editors. Proceedings of the Sixth International Radiopharmaceutical Dosimetry Symposium; 1998. [DOI] [PubMed] [Google Scholar]
- 21.Stabin MG, Siegel JA. Physical models and dose factors for use in internal dose assessment. Health Phys. 2003;85:294–310. doi: 10.1097/00004032-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 23.Jennewein M, Lewis MA, Zhao D, Tsyganov E, Slavine N, He J, Watkins L, Kodibagkar VD, O'Kelly S, Kulkarni P, Antich PP, Hermanne A, Rosch F, Mason RP, Thorpe PE. Vascular imaging of solid tumors in rats with a radioactive arsenic-labeled antibody that binds exposed phosphatidylserine. Clin Cancer Res. 2008;14:1377–1385. doi: 10.1158/1078-0432.CCR-07-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorpe PE. Targeting anionic phospholipids on tumor blood vessels and tumor cells. Thromb Res. 2010;125(Suppl 2):S134–137. doi: 10.1016/S0049-3848(10)70031-1. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, Bennett M, Thorpe PE. A monoclonal antibody that binds anionic phospholipids on tumor blood vessels enhances the antitumor effect of docetaxel on human breast tumors in mice. Cancer Res. 2005;65:4408–4416. doi: 10.1158/0008-5472.CAN-05-0031. [DOI] [PubMed] [Google Scholar]
- 26.Beck AW, Luster TA, Miller AF, Holloway SE, Conner CR, Barnett CC, Thorpe PE, Fleming JB, Brekken RA. Combination of a monoclonal anti-phosphatidylserine antibody with gemcitabine strongly inhibits the growth and metastasis of orthotopic pancreatic tumors in mice. Int J Cancer. 2006;118:2639–2643. doi: 10.1002/ijc.21684. [DOI] [PubMed] [Google Scholar]
- 27.He J, Luster TA, Thorpe PE. Radiationenhanced vascular targeting of human lung cancers in mice with a monoclonal antibody that binds anionic phospholipids. Clin Cancer Res. 2007;13:5211–5218. doi: 10.1158/1078-0432.CCR-07-0793. [DOI] [PubMed] [Google Scholar]
- 28.Stafford JH, Hao GY, Best AM, Sun XK, Thorpe PE. Highly Specific PET Imaging of Prostate Tumors in Mice with an Iodine-124-Labeled Antibody Fragment That Targets Phosphatidylserine. PLoS One. 2013;8:e84864. doi: 10.1371/journal.pone.0084864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogasawara A, Tinianow JN, Vanderbilt AN, Gill HS, Yee S, Flores JE, Williams SP, Ashkenazi A, Marik J. ImmunoPET imaging of phosphatidylserine in pro-apoptotic therapy treated tumor models. Nucl Med Biol. 2013;40:15–22. doi: 10.1016/j.nucmedbio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Hao GY, Singh AN, Liu W, Sun XK. PET with Non-Standard Nuclides. Curr Top Med Chem. 2010;10:1096–1112. doi: 10.2174/156802610791384289. [DOI] [PubMed] [Google Scholar]


