Abstract
Pigment epithelium-derived factor (PEDF) is a glycoprotein that belongs to the family of non-inhibitory serpins. The broad spectrum of PEDF biological activity is evident when considering its effects in promoting cell survival and proliferation, as well as its antiangiogenic, antitumor, and anti-metastatic properties. Although the structural domains of the PEDF gene that mediate such diverse effects and their mechanisms of action have not been completely elucidated, there is a large body of evidence describing their diverse range of activities; this evidence combined with the regulation of PEDF expression by sex steroids and their receptors have led to the idea that PEDF is not only a diagnostic and prognostic marker for certain diseases such as cancer, but is also a potential therapeutic target. In this manner, this paper aims to generally review the regulation of PEDF expression and PEDF interactions, as well as the findings that relate PEDF to the role of estrogens and estrogen receptors. In addition, this manuscript will review major advances toward potential therapeutic applications of PEDF.
Keywords: Anti-tumorigenesis, Antiangiogenesis, Estrogens, Estrogen receptors, Hormonal regulation, PEDF, Synthetic peptides
Introduction
Pigment epithelium-derived factor (PEDF) was initially described as a neurotrophic and antiangiogenic factor secreted by the human fetal retinal pigment epithelium (1). Expression of PEDF has been reported in a variety of organs and tissues, such as the brain (2), spinal cord (3), eyes (1), lung, heart (4), liver (5), uterus (6–8), ovary (9), prostate, pancreas (10), bone (11), and plasma (12). The multiple biological actions of PEDF have been identified not only in healthy conditions but also in several diseases, such as cancer (12–15), endometriosis (16–19), polycystic ovarian syndrome (20, 21), insulin resistance (22, 23), metabolic syndrome, type II diabetes (24, 25), diabetic retinopathy (26, 27), and cardiovascular disease (28, 29), among others.
The available evidence indicates that the multifunctionality of PEDF is due to the large number of signaling pathways it activates and to the various microenvironments in which PEDF can interact with multiple molecules and undergo various post-translational modifications (30).
The high levels of PEDF expression in normal tissues, in contrast to the low levels found in cancer tissues, have been attributed to tumor suppressive activity (31, 32). In addition, PEDF has become a central focus of research in the past decade due to the discovery that in normal cells such as neurons, PEDF induces cell survival and differentiation (1, 33), whereas in animal and human models of malignant tumors, PEDF has potent antiangiogenic and anti-metastatic activity (7, 34–37).
PEDF: the gene and protein
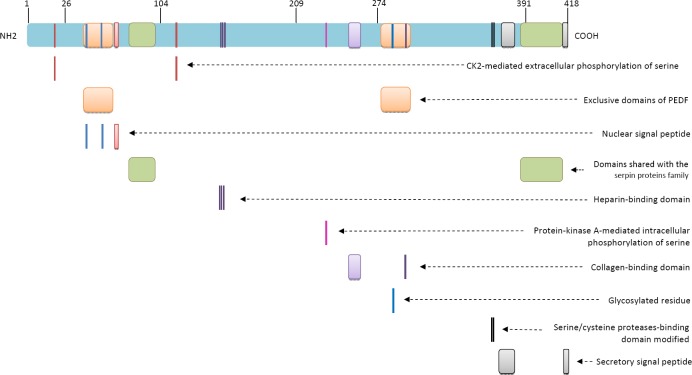
The gene encoding PEDF, also known as SERPINF1, is located on chromosome 17p13.3 and contains eight exons and seven introns and spans approximately 16 kb (30) (Figure 1) while the encoded glycoprotein consists of 418 amino acids and has a molecular weight of 50 kDa. Similarly to other serpins, the tertiary structure of PEDF is composed of three β sheets and ten α-helices plus a typical reactive center loop (RCL) near the C-terminus. However, compared with the serpins that inhibit serine/cysteine proteases, the RCL region of PEDF contains substitutions such as the replacement of four consecutive alanines (aa 360-364) with one phenylalanine and one glutamic acid in the last two positions, which explains why PEDF is unable to bind to the target serine/cysteine proteases (38-40).
Figure 1.
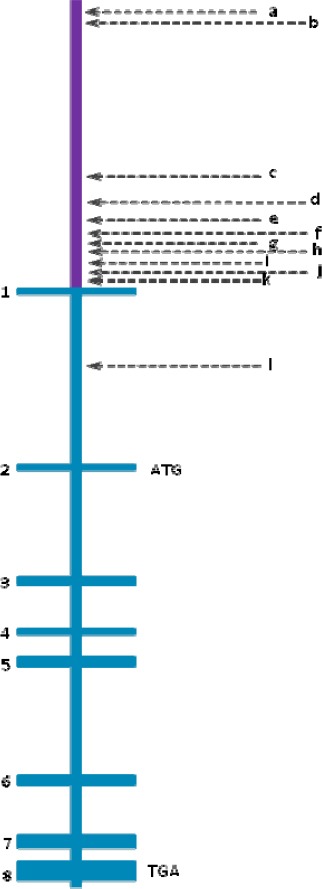
Pigment epithelium-derived factor (PEDF) gene structure containing its promoter region and its eight exons and seven introns
-
▪Promoter is represented in purplemmmm
-
▪a, b and c represent three different putative response elements to p63 y p73, which are located at -6362, -5996, and -1366
-
▪d, f and h are binding sites for transcription factors, located at ALU sequences (-682, -480, and -150). The most striking and unique feature of this region is the dense cluster of Alu elements which comprises 70% of the most proximal 1 kb upstream region. A sequence identical to Alu subclass (consensus sequence GGTCA(n)3 TGGTC(n)9 TGACC), which can function as an estrogen receptor-dependent transcriptional enhancer, is present in the PEDF upstream Alu repeat and a sequence differing by only one nucleotide is present in the proximal Alu repeat, i.e., within 200 bps of the translational start site141.
-
▪e is a sequence that is recognized by the C/EBP (CAAT-enhancer binding protein) family of transcription factors
-
▪g represents a sequence contained within TREp (palindromic thyroid hormone-responsive element). It is similar to the developmentally-regulated RAR (retinoic acid receptor) (-204).
-
▪i is the sequence for PEA3 (polyomavirus enhancer activator-3). It is present in tandem at -122,-129 and again at -141
-
▪j is a binding element for transcription factors such as the Oct (octamer-binding factor) family (-113)
-
▪k represents binding sites for CAAT-enhancer-binding proteins, C/EBPs or CHOP (-40), HNF4 (hepatocyte nuclear factor 4) (-60) and USF (upstream stimulatory factor)
-
▪l represents the MITF binding site (intron 1)
- ▪
In positions 63-70, PEDF contains an unconventional nuclear localization signal (NLS) peptide (Figure 2); arginines 67 and 69 in this sequence interact with lysines 48 and 53, from which they are separated by 12 residues, so that a bipartite NLS signal is generated from its own YxxYRVRS sequence and a sequence similar to the consensus NLS sequence KKRK (41, 42). This signal mediates the transport of PEDF by TRN-SR2 (transportin 3) to the nucleus, where it has specific functions such as participating in cell cycle regulation (43). In addition to the nuclear localization signal, PEDF contains other evolutionarily conserved regions, such as one signal peptide for the secretion of the protein, one C-terminal glycosylation site, and four peptide regions: two near the N-terminus (aa 40–67 and aa 78–95) and two near the C-terminus (aa 277–301 and aa 384–415) (44) (Figure 2). The high degree of homology between the N-terminal amino acids 78–95 and the C-terminal residues 384–415 with all other serpins has led researchers to suggest that these regions are responsible for the shared functions of PEDF and other serpins. Additionally, the finding that the N-terminal residues 40–67 and the C-terminal amino acids 277–301 are unique to PEDF, suggests that they are related to unique functions of the protein (39).
Figure 2.
Pigment epithelium-derived factor (PEDF) protein has binding domains for heparin (residues 145, 146 and 148), collagen (regions 255–257, 299) and a modified binding domain for serine/cysteine proteases. It further contains a bipartite nuclear localization peptide (NLS) (regions 48, 53 and 67–69), and a secretory peptide (regions 373–380 and 415–418). It has exclusive domains (regions 40–67 and 277–301) and also domains shared with other serpins (regions 78–95 and 384–415). The protein is target of various post-translational modifications, such as extracellular phosphorylation at serines 24 and 114 by CK2 as well as intracellular phosphorylation of serine 277 by PKA and glycosylation at residue 285
The presence of a secretion signal peptide (Figure 2) located in the RCL C-terminus indicates that PEDF can also be transported outside of the cell, where it acts on target cells by binding to various receptor types.
Cellular localization and functions
PEDF may localize to either the nucleus or the cytoplasm (44–46) or may be secreted, demonstrating that the various functions of the protein can be performed both inside and outside of the cell that produces the protein.
Little is known about the specific activity of PEDF in the nuclear and cytoplasmic compartments, although other members of the same family, such as the avian serpin MENT, have been associated with an inhibition of gene expression by epigenetic mechanisms and the nuclear inhibition of cysteine proteases similar to papain thus promoting the accumulation of the target proteins of papain, for example, Rb (47) and the expression of certain genes by epigenetic mechanisms. These findings coupled with the presence of the nuclear localization peptide and the strong immunolocalization of PEDF in the nucleus of many mammalian cell types indicate the relevance of the PEDF protein in the nuclear compartment.
Cell cycle regulation
In the nucleus, PEDF interacts with transcription factors that regulate the cell cycle. It has been established that the promoter region involved in this process is located at least 1759 bp upstream of the transcriptional start site (48). In the 5 kb adjacent to the 5’ end of the gene, there is an accumulation of Alu repeats that are involved in the regulation of gene expression (49) through their binding motifs for transcription factors involved in cell cycle regulation, such as ERα, YY1 and Sp1 (48).
There is also evidence that the PEDF gene promoter contains response elements for the nuclear proteins p63 and p73 (-1366 to -1330), which belong to the p53 family (Figure 1). The amino acid sequence and molecular structure of both proteins have a significant level of homology with p53, which suggests that p63 and p73 share similar functions with p53. In this regard, Sasaki et al 2005 (50) noted that although p63 and p73 can induce PEDF expression and promote cell differentiation, the physiological role of these proteins as potential inducers of PEDF expression has not yet been clarified.
Induction of p53
The indirect effect by which PEDF induces p53 expression (51) by activation of the PEDF receptor (PEDFR) is particularly important. Certain fatty acids such as arachidonic acid metabolites that are released upon activation of this receptor and oxysterol derivatives of the cholesterol biosynthetic pathway in turn activate PPARγ (peroxisome proliferator-activated receptor gamma) transcription factors. The same effect can also be observed when cPLA(2)-α (cytosolic calcium-dependent phospholipase A(2)-alpha) and p38 MAPK (mitogen-activated protein kinase) are sequentially activated (52). It is worth mentioning that PPARγ also can trigger anti-neoplastic processes, including the inhibition of cell proliferation and the induction of apoptosis, due to the affinity of these proteins for the p53 promoter region (52–54). Evidence indicates that PEDF inhibits the cell cycle by inducing p53 expression, suggesting that this effect does not correspond to an immediate cell response but rather to a long-term response.
Extracellular localization and interactions
Outside of the cell, PEDF is found in body fluids, in which it reaches significant concentrations, and in the extracellular matrix, where it establishes interactions with collagen and glycosaminoglycans such as hyaluronan, heparan sulfate, chondroitin sulfate, chondroitin sulfate-C, dermatan sulfate, and dextran sulfate (4, 55); each of these glycoproteins interacts with a different binding domain of PEDF. Thus, whereas the heparan sulfate-binding domain includes a positively charged region, the collagen-binding region requires Asp(255), Asp(257), and Asp(299), and the heparin-binding domain contains three acidic residues: Arg(145), Lys(146), and Arg(148) (56).
The PEDF protein has affinity for various receptor types, such as PEDFR, the laminin receptor (LR), F1-ATPase synthase, LRP6 (low-density lipoprotein receptor-related protein 6), ATGL (adipose triglyceride lipase), LRP5 (low-density lipoprotein receptor-related protein 5), and PLA2 (phospholipase A2), and specific receptor interactions determine the broad pleiotropy of PEDF biological activity (39, 55, 57–59). For example, anti-apoptotic activity of PEDF has been demonstrated in retinal cells as a result of binding between PEDF and the PEDFR transmembrane receptor (60). In this case, PEDF-PEDFR binding triggers the activity of phospholipase A2 (61) and, therefore, the release of membrane fatty acids or bioactive lipids capable of inducing apoptosis (62). In cerebellar granule neurons, the PEDF-PEDFR complex activates NFκB (nuclear factor kappa beta) target genes with anti-apoptotic and neuroprotective functions against glutamate cytotoxicity. NFκB signaling is released via IP3 and RAS downstream of PEDF-PEDFR complex formation (63). In contrast, PEDF binding to the LR induces pro-apoptotic signaling in endothelial cells (64–66), whereas PLA binding triggers a similar signal in neurons and cardiomyocytes (67). In cultured cells and in animal models, the activation of the LR by PEDF has antiangiogenic, anti-thrombogenic, and anti-inflammatory effects (59, 66). It has further been reported that PEDF binding to the ATGL receptor triggers antiangiogenic and proinflammatory activity (68, 69).
In neurons, PEDF induces the expression of genes that promote survival, such as c-IAP1, c-IAP2, FLIPs, A1/Bfl-1, and Mn-SOD. In murine models, PEDF secretion in the nervous system has been demonstrated in certain regions of neurogenesis, such as in the hippocampus and subventricular zone; here, PEDF may specifically regulate the induction of self-renewal in neural stem cells (70).
In addition to the above mentioned cell types, other cells such as neonatal pericytes and astrocytes are also PEDF targets. PEDF stimulates the synthesis of PDGF-B (platelet-derived growth factor-B), a factor necessary for survival and microvascular homeostasis (71) in pericytes, whereas neonatal astrocytes, in turn, begin to express early and proinflammatory genes as a result of NFκB or CREB (cyclin AMP-responsive element binding protein) activation (72). In recent studies, it has been reported that adipocytes secrete PEDF, which has been associated with insulin resistance and inflammatory processes in adipocytes and muscle cells (42).
The PEDF is the most potent inhibitor of angiogenesis
Although PEDF lacks the serine/cysteine protease inhibitory activity characteristic of many serpins (39), PEDF does share antiangiogenic and antitumor properties with some serpin family members, such as antithrombin, angiotensinogen, and maspin (73), presumably due to the presence of a common structural determinant. However, among all naturalangiogenesis inhibitors known thus far, PEDF is the most effective, with greater potency than angiostatin, endostatin, and thrombospondin 1 (74). This antiangiogenic activity is the result of various PEDF functions, including the induction of endothelial cell apoptosis, the inhibition of cell migration and new vessel formation, and the inhibition of the response to VEGF (vascular endothelial cell growth factor), the most potent proangiogenic factor, as well as the response to bFGF (basic fibroblast growth factor) (74).
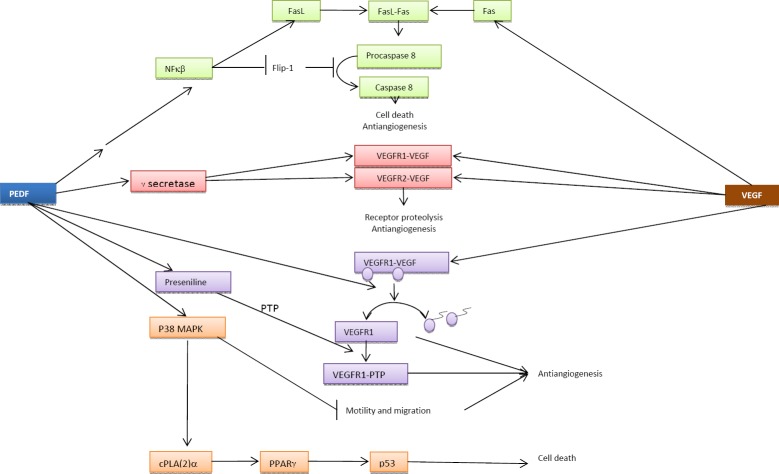
PEDF has been shown to inhibit the neovasculari-zation promoted by VEGF through different pathways (Figure 3):
Figure 3.
Signaling transduction pathways involved in the antiangiogenic activity of Pigment epithelium-derived factor(PEDF). The antiangiogenic response depends on the balance between PEDF and vascular endothelial cell growth factor (VEGF) and on the regulatory factors induced by them
-
-
The PEDF-PEDFR complex can act as an upstream inducer of NFκB, a factor that, in turn, downregulates the anti-apoptotic factor FLIP 1 (FLICE inhibitory protein) which means that PEDF-PEDFR complex interferes with caspase 8 activation and increases cell resistance to the pro-apoptotic effects of FasL (75).
-
-
PEDF can promote γ-secretase-dependent proteolysis of the transmembrane portion of VEGFR1 and VEGFR2 (76, 77) and also inhibit VEGF-induced VEGFR1 phosphorylation, or promote the dephosphorylation of this receptor, by inducing the upregulation of presenilin-1 and the subsequent aggregation of vascular endothelial protein-tyrosine phosphatase and VEGFR1 (78).
-
-
PEDF inhibits motility and thus migratory behavior both in endothelial and cancer cells, and this effect is mediated by the PEDF-LR complex and subsequent activation of the p38 MAPK pathway (79).
-
-
PEDF directly induces apoptosis in endothelial cells (74) by upregulating the expression of the pro-apoptotic gene p53 in human umbilical vein and mouse cornea endothelial cells in vitro through the sequential activation of p38 MAPK, cPLA(2)-α, and PPARγ (80). In a more recent study, it was shown that PEDF induces the expression of TRAIL (TNF-related apoptosis inducing ligand) also through cPLA(2)-α and PPARγ to generate a pro-apoptotic effect mediated by macrophages in tumor cells in vitro (52) and also suggested that macrophages may be involved in the antiangiogenic mechanism of PEDF.
There is evidence that the inhibition and induction of angiogenesis depend on the differential secretion of regulatory factors by the fibroblasts present in the tissues. Thus, when fibroblasts are quiescent, they secrete PEDF (43), whereas cells in the proliferative state secrete VEGF to arrest or induce mitosis in the endothelial cells of adjacent blood vessels depending on the situation (81). Accordingly, it has been proposed that the regulation of angiogenesis depends on changes in the balance between PEDF and VEGF. In this vein, both the increase in PEDF expression and the decrease in VEGF have a negative effect on angiogenesis, whereas the increase in VEGF promotes blood vessel formation (82). Similarly, studies conducted in mice (83) showed that bone marrow neovascularization depends on the ratio of VEGF expression and PEDF produced by stromal cells. In any case, the mechanisms underlying the antiangiogenic property of PEDF are not fully elucidated, underscoring the need for further studies.
The role of the PEDF in senescence and aging
PEDF expression declines not only during tumor progression but also during senescence and aging. Senescence is defined as the state of irreversible cell cycle arrest reached by cells that have exhausted their capacity for proliferation, either as part of the natural aging process or as a result of mechanisms such as the activation of certain oncogenes, the inactivation of tumor suppressors, oxidative stress, and genotoxic damage, among others (84, 85). Aging is the result of a gradual decrease in the ability to repair and regenerate tissues in complex organisms (86).
Similarly to mitotic cells, PEDF is not expressed in senescent cells (43). However, when cells have recently become senescent or, in the case of fibroblasts, when cells are cultured under serum deprivation, PEDF levels are elevated, in contrast to the low levels found in long-term senescent cells (43). In cells that have recently become senescent, these elevated levels of PEDF begin to decline until they become undetectable, which has led to the suggestion that PEDF may induce senescence in mitotically active cells, after which senescence would be maintained by other genes (43, 87). However, it has been reported that in certain cells, such as senescent melanocytes, PEDF expression is upregulated by MITF (microphthalmia-associated transcription factor), which activates gene transcription by directly binding to the first intron (88). Thus, the elevated PEDF expression is likely associated with a recent acquisition of the senescent state in these cells, which is in agreement with the findings reported by Pignolo et al 1993 (87).
In cultured human mesenchymal cells, Cao et al 2013 (89) established that PEDF reduces oxidative stress and thus indirectly delays the onset of senescence and preserves the proliferative and differentiation potentials of the cells. Considering that oxidative stress precedes p53 activation (90), it is reasonable that when stress is reduced, the expression of genes associated with senescence, such as p53 and p16, also decreases, as determined by Cao et al 2008 (89).
In contrast to this finding, Steinle et al 2008 (91) showed that during normal aging in rats, the retinal pigment choroid-epithelium complex shows a decrease in PEDF and VEGF expression levels. The decrease in PEDF may be due to its antiangiogenic properties. In this vein, the lack of PEDF may be the result of a compensatory effect to offset the decline in microvessel density, a common event during this stage of life. Furthermore, the loss of PEDF may generate an imbalance in angiogenic modulators, contributing to the onset of diseases characterized by abnormal vasculogenesis (92). The decreased PEDF expression associated with aging could also be ascribed to the activity of transcription factors whose activity decreases with age, such as NFκB and AP-1, which can potentially bind to specific binding motifs located at multiple Alu repeats present in the PEDF promoter (48).
Antitumor activity of the PEDF
Several studies have shown that PEDF exerts strong antitumor activity and is downregulated in solid tumors and cancer cell lines. Assays aimed at restoring gene expression have allowed researchers to assess the impact of PEDF loss on tumor progression. The restoration of PEDF expression in prostate, osteosarcoma, ovary, pancreas, malignant glioma, and neuroblastoma cells reduces cell growth rate and microvessel density of the tumor mass (32, 35, 93–96), indicating that low PEDF levels are significantly associated with metastatic potential, tumor grade, and poor prognosis (51).
Because the expression pattern of PEDF is strongly linked to angiogenesis, disease progression is dependent on the balance between PEDF and VEGF. Increased PEDF or decreased VEGF expression inhibits angiogenesis, whereas increased VEGF promotes angiogenesis (82). In invasive ductal breast tumors, decreased PEDF expression is correlated with an increase in microvessel density; for this reason, PEDF is considered to predict poor prognosis (26). Moreover, a decrease in PEDF in this type of tumor and in others, such as pancreatic ductal adenocarcinoma and melanoma, has been associated not only with increased tumor aggressiveness but also with increased metastatic potential and shorter survival time (97). Thus, PEDF may serve as a therapeutic target for certain cancers, including breast, liver, lung, colorectal, and Wilms tumor (97–100) and may also provide prognostic value where high PEDF expression is associated with better total and disease-free survival.
Although the PEDF mechanism of action in tumors has not yet been revealed, it has been established in malignant melanoma that PEDF expression is upregulated by MITF, which activates gene transcription in a manner similar to what occurs in senescent melanocytes (88).
The PEDF in normal and pathological estrogen-dependent tissues
Estrogens are steroid hormones that act on a number of tissues to promote growth and differentiation. Estrogens possess a wide range of influence, which allows them to participate in a myriad of physiological processes. For this reason, it is not surprising that estrogens are considered to be important players in the onset and progression of certain cancers (breast, ovarian, prostate, endometrial, and colorectal) and other diseases such as diabetic retinopathy, osteoporosis, endometriosis and obesity as well as various types of neuropathies, systemic lupus erythematosus, and cardiovascular disease (101–109).
To date, three estrogen receptor (ER) subtypes have been described: ERα and ERβ, which are present in the cytoplasm, nucleus and mitochondria, and GPER (G protein-coupled estrogen receptor), which is localized in the plasma membrane.
ERα and ERβ
Despite differences in tissue distribution and downstream signaling, ERα and ERβ share structural similarities. Their DNA-binding domains share approximately 95% homology, whereas the ligand binding domains are approximately 55% identical at the amino acid level and the N-terminal domains share only 15% homology (110).
At the genomic level, ERα and ERβ act as modulators of estrogen physiological function because they regulate gene expression as transcription factors (107, 111, 112). These ERs are primarily located in the cytoplasm, where they form complexes with the heat shock proteins (HSP) 50, 70, and 90. Once activated by ligand binding, ERs dissociate from HSPs so that the DNA-binding domain is released. ERs form αα or ββ homodimers or αβ heterodimers. In the dimerized form, the receptors can interact with specific DNA sequences in the promoter region of target genes, known as E2 response elements (EREs). When not bound to DNA, the ERs interact with other factors such as SP1, NFκB, and AP1 (activator protein 1), thus affecting the transcription of target genes of these factors (113, 114). After binding to DNA, ERs recruit coregulatory molecules of the P160 family such as SRCs (steroid receptor coactivators) (115). The finding that ERα preferentially binds to EREs whereas ERβ binds to AP1 recognition sites established that DNA-binding domains are less relevant for the induction of ERβ transcriptional activity than for that of ERα (102).
A wide variety of rapid non-genomic effects have also been described resulting from the activation of ERα and ERβ. In the presence of estrogens, these ERs interact with various molecules associated with membrane receptors, as tyrosine kinase, striatin, G proteins and non-receptor tyrosine kinases and with caveolin-1, allowing them to form complexes that activate different signaling pathways, as MAPK/ERK and PI3K/AKT (phosphoinositide 3-kinase) pathways (103, 116); they also increase Ca2+ and nitric oxide levels (117), among others.
Both the ERα and ERβ receptors are expressed in a wide range of cells and tissues and are involved in regulating the functions of the reproductive, immune, skeletal, cardiovascular, and central nervous systems, on which they exert distinct and non-redundant effects (118, 119) despite their structural similarities.
Due to its effects on cell proliferation, ERα has been implicated not only in the development of the reproductive tract and the mammary gland but also in estrogen-dependent carcinogenesis (120). The antiproliferative effect of ERβ, meanwhile, is evident due to its antitumor (121) and anti-inflammatory activities (122). However, when both ERs are present at the promoter region of genes involved in proliferation, they exert opposing actions, so that the result of E2 stimulation corresponds to the balance of ERα and ERβ signaling (123).
GPER
The expression of GPER has been described in various organ systems, such as the reproductive, urinary, endocrine, nervous, immune, musculo-skeletal, and cardiovascular systems (119, 124). In these tissues, GPER participates in rapid signal transduction events triggered by estrogens and, to a lesser extent, in genomic signaling (125). It has been shown that in the cardiovascular and immune systems, estrogen-mediated rapid signaling responses occur as a result of GPER activation of multiple signaling pathways, an effect that occurs independently of the signal generated by steroid nuclear receptors (119, 126). This indicates that GPER increases the complexity of the physiological responses to estrogens (125).
PEDF is an estrogen target gene
The PEDF gene promoter contains estrogen response elements, which may explain why the protein levels change in tissues, both normal and pathological, whose growth is subject to hormonal regulation. The finding of a putative response element to 17β-estradiol (E2) in the region near the 5’ end of the gene (127) and the fact that E2 modulates PEDF expression indicate that estrogens are involved in the transcriptional regulation of PEDF and that the ERs can influence its biological functions.
Estrogen-ER-PEDF signaling Endothelium
Using ginsenoside-Rb1, a steroidal saponin with a structure similar to those of the estrogens, Leung et al 2007 (128) demonstrated that more so than ERα activation, ERβ activation caused an increase in the expression and secretion of PEDF in human umbilical vein endothelial cells in vitro, resulting in the inhibition of endothelial tube network formation. Consequently, these authors related the antiangiogenic role of ERβ to the findings of Garvin et al 2006 (129), who found that E2 decreases VEGF expression in breast cells transfected with ERβ, and those of Li et al 2006 (130), who reported an increased expression of PEDF mRNA in retinal Müller cells, which contain ERβ.
Ovary
Many studies have reported differential expression patterns of ERα and ERβ in the different cell types present in normal ovaries. Thus ERβ is confined to follicle granulosa cells, whereas ERα is only expressed in theca or ovary stromal cells (131, 132). This localization was described by Emmen et al 2005 (133), who noted that ERβ plays a key role in the differentiation and function of granulosa cells and that ERα is responsible for regulating the negative feedback of pituitary gonadotropins. However, Lenie and Smitz 2008 (134) showed that the two receptor types were expressed in vitro in the granulosa cells of mouse follicles, although with a different subcellular localization, and that ERα was expressed in theca cells. The results of this study indicated that ERβ is exclusively present in the nucleus during follicular development, whereas ERα is initially expressed in the cytoplasm and subsequently undergoes translocation to the nucleus, an event that also occurs in theca cells, showing that expression of the two receptors is subject to the hormonal environment.
In the human ovarian surface epithelium (OSE), ER activation is an important regulatory event upstream of PEDF expression as Cheung et al 2006 (32) demonstrated, In normal OSE cells, E2 directly repressed the PEDF promoter, which resulted in a rapid decrease in PEDF mRNA and protein levels in a dose-dependent and time-dependent manner that was independent of de novo synthesis of an intermediate protein or a change in mRNA stability. It is worth noting that in this tissue, ERα is the main estrogen receptor (135), which may be related to the fact that approximately 90% of ovarian tumors arise from this tissue. It also explains how E2 may exert a dual effect, suppressing or inducing PEDF according to the cell type and the activated receptor. These authors also reported that PEDF not only induces apoptosis but is also a potent inhibitor of estrogen-induced proliferation in ovarian epithelial cells, an effect that was shared by cancer cell lines of the same estrogen-responsive tissue. According to these researchers, the elevated PEDF levels that occur prior to ovulation induce apoptosis of ovarian epithelial cells adjacent to the preovulatory follicle, favoring its subsequent rupture and the release of the secondary oocyte. During ovulation, the reduction observed in the amount of PEDF is consistent with the preovulatory estrogen secretion peak, which promotes the survival and proliferation of cells around the rupture site (32).
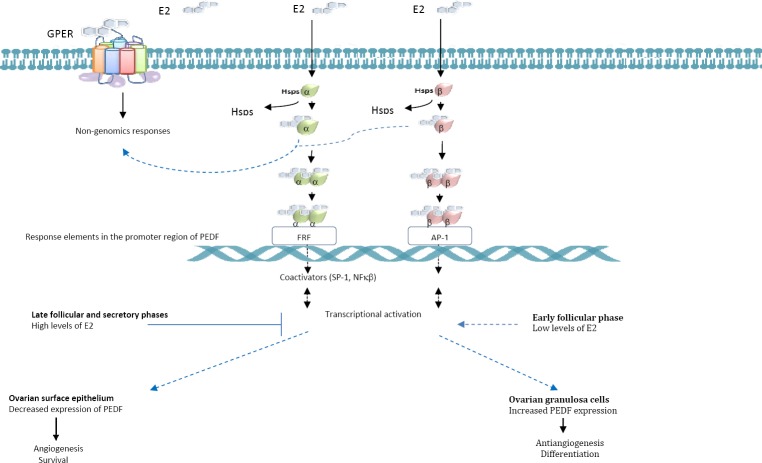
The presence of PEDF has also been established in the ovarian follicular fluid of patients undergoing in vitro fertilization procedures (136, 137). The circulation, the oocyte, and the surrounding granulosa cells appear to be the source of the factor (9), which decreases follicular angiogenesis, preventing vascularization of the granular compartment of the follicles before ovulation and luteinization occur (138). Based on the finding that high doses of E2 and hCG (human chorionic gonadotropin) induced a gradual decrease in PEDF expression in human granulosa cells and rodent follicles, Chuderland et al 2013b (9) postulated that the regulation of PEDF was dependent on the hormonal environment predominant in each phase of the ovarian cycle, with expression increased during the early proliferative phase when E2 levels are low and decreased as the ovulation cycle progresses towards the secretory phase, when levels of E2 and progesterone increase (Figure 4). Consistent with this hypothesis, the fact that ERβ, which is the predominant estrogen receptor in the granulosa cells, maintains its nuclear localization during follicular development suggests that this receptor induces not only the differentiation of these cells but also PEDF expression. Strikingly, ERα translocation to the nucleus occurs only during the late follicular phase in a manner that appears to parallel the decrease in the secretion of PEDF and E2 that occurs a few hours before ovulation and the decline in the levels of pituitary gonadotropins, hormones whose downregulation is controlled by ERα (133).
Figure 4.
Effects of estrogen signaling on Pigment epithelium-derived factor (PEDF) expression and of the PEDF transcriptional activation in ovarian granulosa cells and in epithelial cells along the ovarian cycle. The response to estrogen shows differences according to the phase of the cycle and the target ovarian cell
According to Kampfer et al 2014 (138), in the avascular granulosa cell compartment, PEDF is responsible for generating reactive oxygen species and hence oxidative stress, which in turn leads to apoptosis of the granulosa cells. This may be involved in the genesis of fertility disorders, such as polycystic ovary syndrome (PCOS). Despite its antiangiogenic effect, PEDF has also been found in the corpus luteum, a structure characterized by its large blood supply, as well as in cultured granulosa-lutein cells.
In addition to PCOS, other fertility disorders such as OHSS (ovarian hyper stimulation syndrome) have been associated with alterations in ovarian angiogenesis in a manner that involves VEGF and PEDF. PCOS appears to be common in women of reproductive age with risk factors such as insulin resistance, hyperinsulinemia, and genetic predisposition. After the appearance of seemingly contradictory reports on the finding of serum levels of PEDF—both decreases and increases have been reported—in patients with PCOS (20, 139, 140), it was recently demonstrated that in the ovaries of PCOS-induced mice, low levels of PEDF and elevated levels of VEGF coexist (141). OHSS has been described as a complication from the use of gonadotropins during in vitro fertilization treatments and is more common in women with PCOS (142). PEDF and VEGF also participate in the pathogenesis of OHSS according to reports that describe an increase in vascular permeability due to the action of VEGF (143) and a decrease in PEDF levels in the ovaries of OHSS-induced mice (144) as well.
Because ovarian carcinoma is one of the most lethal malignancies, the presence of low PEDF expression levels in various ovarian tumor types is of particular interest (145). One of the major risk factors for ovarian cancer is the presence of elevated estrogen serum levels; thus, it is understandable that under these circumstances, PEDF levels tend to decrease, which is in line with the results of Cheung et al 2006 (32). Because it is generally thought that ERβ expression in ovarian tumors decreases and that of ERα increases, the reduction in PEDF expression may also be related to the low levels of ERβ expression reported in ovarian tumors (146–148). Similarly, the low expression of ERβ suggests that the pro-proliferative effect of ERα is not counterbalanced. Chan et al 2008 (147) demons-trated that ERβ expression is significantly higher in normal tissue compared with malignant ovarian tissues and that the expression level is inversely correlated with disease stage. However, Halon et al 2011 (148) determined that although ERβ expression is decreased in ovarian tumors, ERβ expression did not significantly correlate with histopathological parameters (histological type and grade) or tumor stage. This study further established that increased ERβ expression is associated with increased both disease-free survival and overall survival. Furthermore, Fujimoto et al 2000 (149) demonstrated that when the ERα/ERβ ratio is close to one, prognosis improves compared with very high or very low ratios, which are associated with poor prognosis.
However, based on the observation that gene expression was only lower in malignant ovarian tissues where there was also low VEGF expression, Tsuchiya et al 2009 (145) proposed that the malignancy of ovarian tumors with low VEGF expression was strongly dependent on PEDF expression. Thus, decreased PEDF expression could be not only an informative diagnostic marker but also a good prognostic factor for patients with ovarian cancer.
Endometrium
The endometrial tissue is subject to differential hormonal regulation by estrogen and progesterone. Morphological and functional changes in the human endometrium during the menstrual cycle are closely linked to PEDF expression, as demonstrated by Chuderland et al 2014a (8), who stated that PEDF levels varied according to a dynamic pattern, with low levels during the proliferative and early secretory cycle phase-when estrogen levels are high-and higher levels in the late secretory phase-when estrogen levels decrease. This finding suggested the existence of an association between PEDF expression and the variation of steroid hormone dominance during each phase of the cycle. E2 pro-angiogenic activity appears to be mediated by ERα (150), whereas the effect of progesterone is less well understood. Although the mechanisms involved are not fully elucidated, these authors suggested that PEDF counterbalances the pro-angiogenic factor VEGF in a manner similar to what occurs in other tissues, with elevated levels of VEGF during the proliferative and early secretory cycle phases and decreased levels during the late secretory phase, indicating that endometrial angiogenesis is controlled by the balance between VEGF and PEDF. They also argued that the regulation exerted by PEDF in the endometrium is autocrine-paracrine taking into account that endometrial glandular cells not only express and secrete PEDF but also respond to it via the PEDFR (8). The VEGF-dependent antiangiogenic response mediated by PEDFR combines with the VEGF-independent antiangiogenic response mediated by the LR in endothelial cells, which was previously described by Bernard et al 2009 (66).
Angiogenesis defects have been associated with the development of certain endometrial diseases such as endometriosis (151), endometrial cancer (152), and repetitive implantation failure (153). In endometriosis, the formation of ectopic foci of functional endometrium is necessarily preceded by a process of neovascularization. This led Chen et al 2011 and 2012 (16, 17) to hypothesize about the possible role of PEDF in the pathogenesis of this disease. Indeed, these authors demonstrated that PEDF expression levels are reduced in serum, peritoneal fluid, and endometriotic lesions of women with endometriosis and established that the expression level is correlated with disease severity.
Mammary gland
ERβ and ERα are expressed in the normal adult breast tissue of humans and rodents. ERα is expressed in approximately 10% and ERβ in 70%–80% of epithelial cells. ERβ is also expressed in stromal and immune cells present in glandular tissue (154, 155). ERα is necessary for growth and branching of the mammary ducts, whereas ERβ promotes the terminal differentiation of ductal epithelial cells and maintains their differentiated status (156). In contrast to normal breast tissue, the concentration of ERβ declines and that of ERα increases in tumor tissue (157).
As tumor mass increases, hypoxic regions appear, which, together with other factors, induce angiogenesis. Hypoxic regions are associated with decreased PEDF expression and thus with a poor prognosis and tumor cell metastasis. Therefore, the presence of PEDF in breast tumors has been regarded as an important biomarker because the level of PEDF is regulated by the degree of hypoxia and by VEGF, as demonstrated by Zhou et al 2010 (26). In breast cancer metastatic foci in the brain, it has been observed that PEDF expression is inversely related to patient survival (158). Assays restoring PEDF expression in mice with brain metastasis have shown a reduction in the size of the tumor mass without affecting microvessel development, a finding that is the first evidence that PEDF protects neurons from damage induced by cancer (158).
Clinical implications and perspectives
Because the role of PEDF in modifying the tumor microenvironment has been amply demonstrated, research on the use of this molecule for therapeutic purposes against cancer is highly important (51, 159). The design of PEDF-derived synthetic peptides forecasts a promising future therapy for cancer. Due to their high specificity for and easy penetration of target tissues and the lack of genotoxic or genotype-specific effects, PEDF synthetic peptides are currently a spearhead in the therapy to restore PEDF expression in cancer tissues (159, 160).
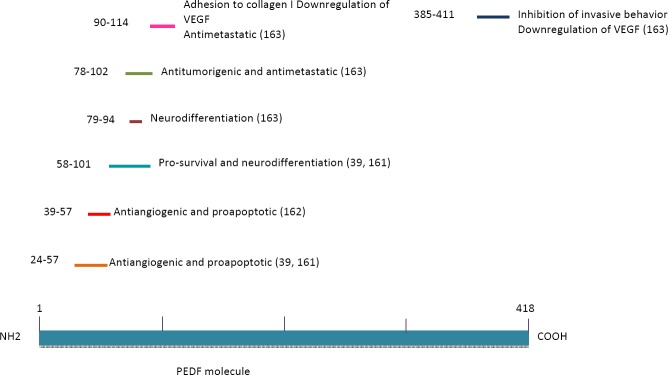
Some studies using peptides synthesized from functional epitopes located in the PEDF N-terminal region have ascribed properties to the peptides similar to those of the whole protein (Figure 5). For example, a 34-mer peptide (residues 24–57) with antiangiogenic and pro-apoptotic activities and a 44-mer peptide (residues 58–101) with neurotrophic activity (161) showed significant antitumor activity when introduced into prostate cancer cells (39). Within the 44-mer peptide sequence, a shorter ERT fragment (residues 79–94) was identified that causes neuroendocrine differentiation in prostate cancer cells (39). From the 34-mer peptide, Mirochnik et al 2009 (162) obtained a shorter 18-mer peptide (residues 39–57) that retains the ability to inhibit angiogenesis and tumor growth in prostate tumors in mice. Based on known PEDF functional epitopes, Ek et al 2007b (163) designed four synthetic peptides and used them against cells of the human osteosarcoma cell line SaOS2. In this study, the StVOrth-2 peptide (residues 78 to 102) exhibited an inhibitory effect on tumor cell proliferation, whereas StVOrth-3 (residues 90–114) promoted adhesion to collagen I. StVOrth-4 (residues 387–411) was the most potent inhibitor of invasion, and StVOrth-1 (residues 40–64), -2, and -3 induced osteoblast differentiation. Although none of the peptides inhibited the formation of tubular networks in the angiogenesis assay conducted in this study, StVOrth-3 and -4 downregulated VEGF expression. In addition, the StVOrth-2 and StVOrth-3 peptides inhibited in vivo primary tumor growth and the development of pulmonary metastases in an orthotopic disease model (35). In another orthotopic murine model of tibial osteosarcoma with spontaneous metastasis, Broadhead et al 2012 (159) evaluated treatment with the StVOrth-2 and StVOrth-3 peptides, which were supplied continuously and systemically by micro-osmotic pumps. Their results showed that the peptides had little effect on the extent of local tumor invasion or on the induction of apoptosis or necrosis when administered together. However, when administered individually, StVOrth-2 reduced the growth of primary tumors, whereas StVOrth-3 limited the development of pulmonary metastases. Furthermore, there was no evidence of immunogenic effects or treatment-related toxicity.
Figure 5.
Some synthetic peptides derived from Pigment epithelium-derived factor (PEDF) as summarized here, have shown remarkable effects in various experimental models, especially as it relates to counteract the malignant progression
Given that the size of PEDF can be a limiting factor for its use, it is important to use smaller peptides derived from the protein that can easily access systems such as the central nervous system, where features such as the permeability of the blood-brain barrier restrict the passage of a large number of pharmacological agents, proteins, and peptides. In the nervous tissue, the use of PEDF-derived peptides that retain their neuroprotective activity may be feasible to protect neurons from damage caused by metastatic processes (164).
Using a somewhat different approach to investigate the effect of recombinant PEDF (rPEDF) on neovascularization and the growth of cervical carcinoma, Yang et al 2010 (165) injected the protein intraperitoneally into xenografted mice. The results indicated that PEDF significantly reduced tumor growth, possibly due to its antiangiogenic effect, and microvessel density, due to its pro-apoptotic and antiproliferative activities in endothelial cells. In combination with hyperthermia, Wu et al 2014 (166) evaluated the therapeutic effect of an adeno-associated viral vector expressing PEDF (rAVV-PEDF) in solid tumors both in vitro and in vivo. Thus, in Meth-A cells, it was possible to identify PEDF expression and bioactivity, whereas in a mouse model of subcutaneous fibrosarcoma, therapy suppressed tumor growth and increased survival. This same type of vector and low doses of the chemotherapeutic agent cisplatin were used by He et al 2014 (167) in a mouse model of Lewis lung cancer. Therapy was effective in suppressing tumor angiogenesis and inducing the death of malignant cells, which resulted in inhibition of tumor growth and increased survival.
rPEDF has been proposed as a potential therapeutic strategy against OHSS based on the finding that it restored VEGF expression in a mouse model to physiological levels without affecting normal functions such as ovulation, implantation, or pregnancy (141). In addition, the possible exogenous application of rPEDF for the treatment of PCOS is also feasible, given that in theory, any change in the physiological balance of PEDF-VEGF can be reversed by rPEDF (141).
Regarding the treatment of endometriosis, Sun et al 2012 (18) determined that in vivo -with prior use of gene therapy- PEDF induces regression and atrophy of human endometriotic cells transplanted in a mouse model by inducing apoptosis and antiangiogenesis. These authors also described similar effects of PEDF on ovarian endometriotic stromal cells in vitro. In addition, Chuderland et al 2013d (168) were able to eradicate endometrial lesions and cause a significant decrease in VEGF levels using rPEDF.
PEDF has also been used in combination with radiotherapy: In a heterogenic human xenograft model of nasopharyngeal carcinoma (NPC) in nude mice, the effectiveness of PEDF treatment on NPC was evaluated. The results showed that the combination therapy is much more effective than the single administration of PEDF or radiotherapy in reducing VEGF levels and microvessel density (169).
Given that one of the major difficulties for therapies against deep tumors, such as osteosarcoma, is inefficient drug delivery, Dass et al 2007 (170) reported that the application of chitosan biopolymer microparticles containing plasmid-expressed PEDF (pPEDF) decreased invasiveness and promoted adhesion in osteosarcoma (SaOS2) cells, whereas in an orthotopic model of the same disease, pPEDF decreased primary tumor growth and also decreased bone resorption and pulmonary metastasis. In an in situ assay, Ta et al 2009 (171) combined pPEDF treatment with chemotherapy and found greater suppression of tumor growth with no side effects.
Conclusion
The multimodality of PEDF potential under normal and pathological conditions has made PEDF a strong candidate for a therapeutic agent to successfully replace the combined cancer therapies in use today. However, before proceeding to clinical trials in humans, it is necessary to elucidate completely the clues that determine its activity, i.e. gene expression, post-translational modifications and half-lives of PEDF mRNA and protein as well as the signal transduction associated with each of the activities of the protein in both normal and pathological tissues. It is also desirable to identify functional epitopes that play a key role in tumor suppression and cell protection and possess the ability to recognize receptors on cells and tissues of interest.
Integrating the findings of the genomics, proteomics, transcriptomics, and epigenomics of PEDF will result in the development of therapies against diseases caused by defects in its expression and function.
References
- 1.Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 2.Bilak MM, Corse AM, Bilak SR, Lehar M, Tombran-Tink J, Kuncl RW. Pigment epithelium-derived factor (PEDF) protects motor neurons from chronic glutamate-mediated neurodegeneration. J Neuropathol Exp Neurol. 1999;58:719–728. doi: 10.1097/00005072-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kuncl RW, Bilak MM, Bilak SR, Corse AM, Royal W, Becerra SP. Pigment epithelium-derived factor is elevated in CSF of patients with amyotrophic lateral sclerosis. J Neurochem. 2002;81:178–184. doi: 10.1046/j.1471-4159.2002.00813.x. [DOI] [PubMed] [Google Scholar]
- 4.Alberdi E, Hyde CC, Becerra SP. Pigment epithelium-derived factor (PEDF) binds to glycosaminoglycans: analysis of the binding site. Biochemistry. 1998;37:10643–10652. doi: 10.1021/bi9802317. [DOI] [PubMed] [Google Scholar]
- 5.Sawant S, Aparicio S, Tink AR, Lara N, Barnstable CJ, Tombran-Tink J. Regulation of factors controlling angiogenesis in liver development: a role for PEDF in the formation and maintenance of normal vasculature. Biochem Biophys Res Commun. 2004;325:408–413. doi: 10.1016/j.bbrc.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 6.Palmieri D, Watson JM, Rinehart CA. Age-related expression of PEDF/EPC-1 in human endometrial stromal fibroblasts: implications for interactive senescence. Exp Cell Res. 1999;247:142–147. doi: 10.1006/excr.1998.4341. [DOI] [PubMed] [Google Scholar]
- 7.Tombran-Tink J, Barnstable CJ. Therapeutic prospects for PEDF: more than a promising angiogenesis inhibitor. Trends Mol Med. 2003;9:244–250. doi: 10.1016/s1471-4914(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 8.Chuderland D, Ben-Ami I, Friedler S, Hasky N, Ninio-Many L, Goldberg K, et al. Hormonal regulation of pigment epithelium-derived factor (PEDF) expression in the endometrium. Mol Cell Endocrinol. 2014a;390:85–92. doi: 10.1016/j.mce.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Chuderland D, Ben-Ami I, Kaplan-Kraicer R, Grossman H, Komsky A, Satchi-Fainaro R, et al. Hormonal regulation of pigment epithelium-derived factor (PEDF) in granulosa cells. Mol Hum Reprod. 2013a;19:72–81. doi: 10.1093/molehr/gas046. [DOI] [PubMed] [Google Scholar]
- 10.Doll JA, Stellmach VM, Bouck NP, Bergh AR, Lee C, Abramson LP, et al. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med. 2003;9:774–780. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]
- 11.Quan GM, Ojaimi J, Li Y, Kartsogiannis V, Zhou H, Choong PF. Localization of pigment epithelium-derived factor in growing mouse bone. Calcif Tissue Int. 2005;76:146–153. doi: 10.1007/s00223-004-0068-2. [DOI] [PubMed] [Google Scholar]
- 12.Maik-Rachline G, Shaltiel S, Seger R. Extracellular phosphorylation converts pigment epithelium-derived factor from a neurotrophic to an antiangiogenic factor. Blood. 2005;105:670–678. doi: 10.1182/blood-2004-04-1569. [DOI] [PubMed] [Google Scholar]
- 13.Tsuru M, Arima N, Toyozumi Y, Kato S. Pigment epithelium-derived factor as a new diagnostic marker for melanocytic tumors. Kurume Med J. 2005;52:81–87. doi: 10.2739/kurumemedj.52.81. [DOI] [PubMed] [Google Scholar]
- 14.Hoshina D, Abe R, Yamagishi SI, Shimizu H. The role of PEDF in tumor growth and metastasis. Curr Mol Med. 2010;10:292–295. doi: 10.2174/156652410791065327. [DOI] [PubMed] [Google Scholar]
- 15.Jan R, Huang M, Lewis-Wambi J. Loss of pigment epithelium-derived factor: a novel mechanism for the development of endocrine resistance in breast cancer. Breast Cancer Res. 2012;14:R146. doi: 10.1186/bcr3356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Chen L, Fan R, Huang X, Xu H, Zhang X. Decreased concentrations of pigment epithelium-derived factor in peritoneal fluid of patients with endometriosis. Fertil Steril. 2011;95:1798–1800. doi: 10.1016/j.fertnstert.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Fan R, Huang X, Xu H, Zhang X. Reduced levels of serum pigment epithelium-derived factor in women with endometriosis. Reprod Sci. 2012;19:64–69. doi: 10.1177/1933719111413300. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Che X, Zhu L, Zhao M, Fu G, Huang X, et al. Pigment epithelium derived factor inhibits the growth of human endometrial implants in nude mice and of ovarian endometriotic stromal cells in vitro. PLoS One. 2012;7:e45223. doi: 10.1371/journal.pone.0045223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu G, Che X, Sun Y, Huang X, Xu H, Zhou C, et al. Pigment epithelial-derived factor expression in endometriotic lesions in a rat model of endometriosis. Acta Histochem. 2013;115:301–307. doi: 10.1016/j.acthis.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Li Q, Zhong L, Song Y, Tian B, Cheng Q, et al. Serum pigment epithelium-derived factor is elevated in women with polycystic ovary syndrome and correlates with insulin resistance. J Clin Endocrinol Metab. 2011;96:831–836. doi: 10.1210/jc.2010-2140. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Q, Xia W, Yang S, Ye P, Mei M, Song Y, et al. Association of serum pigment epithelium-derived factor with high-sensitivity C-reactive protein in women with polycystic ovary syndrome. J Endocrinol Invest. 2013a;36:632–635. doi: 10.1007/BF03346755. [DOI] [PubMed] [Google Scholar]
- 22.Gattu AK, Birkenfeld AL, Jornayvaz F, Dziura J, Li F, Crawford SE, et al. Insulin resistance is associated with elevated serum pigment epithelium-derived factor (PEDF) levels in morbidly obese patients. Acta Diabetol. 2012;49:S161–169. doi: 10.1007/s00592-012-0397-y. [DOI] [PubMed] [Google Scholar]
- 23.Moreno-Navarrete JM, Touskova V, Sabater M, Mraz M, Drapalova J, Ortega F, et al. Liver, but not adipose tissue PEDF gene expression is associated with insulin resistance. Int J Obes (Lond) 2013;37:1230–1237. doi: 10.1038/ijo.2012.223. [DOI] [PubMed] [Google Scholar]
- 24.Choi KM, Hwang SY, Hong HC, Yang SJ, Choi HY, Yoo HJ, et al. C1q/TNF-related protein-3 (CTRP-3) and pigment epithelium-derived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes. 2012;61:2932–2936. doi: 10.2337/db12-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins AJ, Fu D, Azar M, Stoner JA, Kaufman DG, Zhang S, et al. Clinical correlates of serum pigment epithelium-derived factor in type 2 diabetes patients. J Diabetes Complications. 2014;28:353–359. doi: 10.1016/j.jdiacomp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou D, Cheng SQ, Ji HF, Wang JS, Xu HT, Zhang GQ, et al. Evaluation of protein pigment epithelium-derived factor (PEDF) and microvessel density (MVD) as prognostic indicators in breast cancer. J Cancer Res Clin Oncol. 2010;136(11):1719–1727. doi: 10.1007/s00432-010-0830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Feng L, Hu JW, Xie CL, Wang F. Characterization of the vitreous proteome in proliferative diabetic retinopathy. Proteome Sci. 2012;10:15. doi: 10.1186/1477-5956-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Ma X, Zhou M, Pan X, Ni J, Gao M, et al. Serum pigment epithelium-derived factor levels are independently correlated with the presence of coronary artery disease. Cardiovasc Diabetol. 2013;12:56. doi: 10.1186/1475-2840-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamagishi S, Matsui T. Pigment epithelium-derived factor (PEDF) and cardiometabolic disorders. Curr Pharm Des. 2014;20:2377–2386. doi: 10.2174/13816128113199990473. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Zhang SS, Barnstable CJ, Tombran-Tink J. Molecular phylogeny of the antiangiogenic and neurotrophic serpin, pigment epithelium derived factor in vertebrates. BMC Genomics. 2006;7:248. doi: 10.1186/1471-2164-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips NJ, Ziegler MR, Radford DM, Fair KL, Steinbrueck T, Xynos FP, et al. Allelic deletion on chromosome 17p13.3 in early ovarian cancer. Cancer Res. 1996;56:606–611. [PubMed] [Google Scholar]
- 32.Cheung LW, Au SC, Cheung AN, Ngan HY, Tombran-Tink J, Auersperg N, et al. Pigment epithelium-derived factor is estrogen sensitive and inhibits the growth of human ovarian cancer and ovarian surface epithelial cells. Endocrinology. 2006;147:4179–4191. doi: 10.1210/en.2006-0168. [DOI] [PubMed] [Google Scholar]
- 33.Houenou LJ, D’Costa AP, Li L, Turgeon VL, Enyadike C, Alberdi E, et al. Pigment epithelium-derived factor promotes the survival and differentiation of developing spinal motor neurons. J Comp Neurol. 1999;412:506–514. [PubMed] [Google Scholar]
- 34.Murray AR, Ma JX. PEDF as a treatment for cervical cancer. Cancer Biol Ther. 2010;9:975–977. doi: 10.4161/cbt.9.12.11985. [DOI] [PubMed] [Google Scholar]
- 35.Ek ET, Dass CR, Contreras KG, Choong PF. Inhibition of orthotopic osteosarcoma growth and metastasis by multitargeted antitumour activities of pigment epithelium-derived factor. Clin Exp Metastasis. 2007a;24:93–106. doi: 10.1007/s10585-007-9062-1. [DOI] [PubMed] [Google Scholar]
- 36.Dass CR, Ek ET, Choong PF. PEDF as an emerging therapeutic candidate for osteosarcoma. Curr Cancer Drug Targets. 2008;8:683–690. doi: 10.2174/156800908786733487. [DOI] [PubMed] [Google Scholar]
- 37.Lattier JM, Yang H, Crawford S, Grossniklaus HE. Host pigment epithelium-derived factor (PEDF) prevents progression of liver metastasis in a mouse model of uveal melanoma. Clin Exp Metastasis. 2013;30:969–976. doi: 10.1007/s10585-013-9596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simonovic M, Gettins PG, Volz K. Crystal structure of human PEDF, a potent anti-angiogenic and neurite growth-promoting factor. Proc Natl Acad Sci USA. 2001;98:11131–11135. doi: 10.1073/pnas.211268598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi-functional serpin family protein. J Cell Biochem. 2009;106:769–775. doi: 10.1002/jcb.22072. [DOI] [PubMed] [Google Scholar]
- 40.Broadhead ML, Becerra SP, Choong PF, Dass CR. The applied biochemistry of PEDF and implications for tissue homeostasis. Growth Factors. 2010;28:280–285. doi: 10.3109/08977191003604513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaguchi T, Yamagishi SI, Sata Ms. Structure-Function Relationships of PEDF. Curr Mol Med. 2010;10:302–311. doi: 10.2174/156652410791065255. [DOI] [PubMed] [Google Scholar]
- 42.Anguissola S, McCormack WJ, Morrin MA, Higgins WJ, Fox DM, Worrall DM. Pigment epithelium-derived factor (PEDF) interacts with transportin SR2, and active nuclear import is facilitated by a novel nuclear localization motif. PLoS One. 2011;6:e26234. doi: 10.1371/journal.pone.0026234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pignolo RJ, Francis MK, Rotenberg MO, Cristofalo VJ. Putative role for EPC-1/PEDF in the G0 growth arrest of human diploid fibroblasts. J Cell Physiol. 2003;195:12–20. doi: 10.1002/jcp.10212. [DOI] [PubMed] [Google Scholar]
- 44.Tombran-Tink J, Aparicio S, Xu X, Tink AR, Lara N, Sawant S, et al. PEDF and the serpins: phylogeny, sequence conservation, and functional domains. J Struct Biol. 2005;151:130–150. doi: 10.1016/j.jsb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Tombran-Tink J, Shivaram SM, Chader GJ, Johnson LV, Bok D. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci. 1995;15:4992–5003. doi: 10.1523/JNEUROSCI.15-07-04992.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tombran-Tink J, Lara N, Aparicio SE, Potluri P, Gee S, Ma JX, et al. Retinoic acid and dexamethasone regulate the expression of PEDF in retinal and endothelial cells. Exp Eye Res. 2004;78:945–955. doi: 10.1016/j.exer.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Irving JA, Shushanov SS, Pike RN, Popova EY, Bromme D, Coetzer TH, et al. Inhibitory activity of a heterochromatin-associated serpin (MENT) against papain-like cysteine proteinases affects chromatin structure and blocks cell proliferation. J Biol Chem. 2002;277:13192–13201. doi: 10.1074/jbc.M108460200. [DOI] [PubMed] [Google Scholar]
- 48.Kojima T, Nakahama K, Yamamoto K, Uematsu H, Morita I. Age- and cell cycle-dependent changes in EPC-1/PEDF promoter activity in human diploid fibroblast-like (HDF) cells. Mol Cell Biochem. 2006;293:63–69. doi: 10.1007/s11010-006-2680-0. [DOI] [PubMed] [Google Scholar]
- 49.Oei SL, Babich VS, Kazakov VI, Usmanova NM, Kropov AV, Tomilin NV. Clusters of regulatory signals for RNA polymerase 2 transcription associated with Alu family repeats and CpG islands in human promoters. Genomics. 2004;83:873–882. doi: 10.1016/j.ygeno.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki Y, Naishiro Y, Oshima Y, Imai K, Nakamura Y, Tokino T. Identification of pigment epithelium-derived factor as a direct target of the p53 family member genes. Oncogene. 2005;24:5131513–5131516. doi: 10.1038/sj.onc.1208695. [DOI] [PubMed] [Google Scholar]
- 51.Broadhead ML, Dass CR, Choong PF. Cancer cell apoptotic pathways mediated by PEDF: prospects for therapy. Trends Mol Med. 2009;15:461–467. doi: 10.1016/j.molmed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Ho TC, Chen SL, Shih SC, Chang SJ, Yang SL, Hsieh JW, et al. Pigment epithelium-derived factor (PEDF) promotes tumor cell death by inducing macrophage membrane tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Biol Chem. 2011;286:35943–35954. doi: 10.1074/jbc.M111.266064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonofiglio D, Aquila S, Catalano S, Gabriele S, Belmonte M, Middea E, et al. Peroxisome proliferator-activated receptor-gamma activates p53 gene promoter binding to the nuclear factor-kappaB sequence in human MCF7 breast cancer cells. Mol Endocrinol. 2006;20:3083–3092. doi: 10.1210/me.2006-0192. [DOI] [PubMed] [Google Scholar]
- 54.Gaetano C, Colussi C, Capogrossi MC. PEDF, PPAR-gamma, p53: deadly circuits arise when worlds collide. Cardiovasc Res. 2007;76:195–196. doi: 10.1016/j.cardiores.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat Rev Cancer. 2013;13:258–271. doi: 10.1038/nrc3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yasui N, Mori T, Morito D, Matsushita O, Kourai H, Nagata K, et al. Dual-site recognition of different extracellular matrix components by anti-angiogenic/neurotrophic serpin, PEDF. Biochemistry. 2003;42:3160–3167. doi: 10.1021/bi0206558. [DOI] [PubMed] [Google Scholar]
- 57.Notari L, Arakaki N, Mueller D, Meier S, Amaral J, Becerra SP. Pigment epithelium-derived factor binds to cell-surface F(1)-ATP synthase. FEBS J. 2010;277:2192–2205. doi: 10.1111/j.1742-4658.2010.07641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anne SL, Govek EE, Ayrault O, Kim JH, Zhu X, Murphy DA, et al. WNT3 inhibits cerebellar granule neuron progenitor proliferation and medulloblastoma formation via MAPK activation. PLoS One. 2013;8:e81769. doi: 10.1371/journal.pone.0081769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsui T, Higashimoto Y, Yamagishi S. Laminin receptor mediates anti-inflammatory and anti-thrombogenic effects of pigment epithelium-derived factor in myeloma cells. Biochem Biophys Res Commun. 2014;443:847–851. doi: 10.1016/j.bbrc.2013.12.060. [DOI] [PubMed] [Google Scholar]
- 60.Subramanian P, Locatelli-Hoops S, Kenealey J, DesJardin J, Notari L, Becerra SP. Pigment epithelium-derived factor (PEDF) prevents retinal cell death via PEDF Receptor (PEDF-R): identification of a functional ligand binding site. J Biol Chem. 2013;288:23928–23942. doi: 10.1074/jbc.M113.487884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Notari L, Baladron V, Aroca-Aguilar JD, Balko N, Heredia R, Meyer C, et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- 62.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signalling. Proc Natl Acad Sci USA. 2007;104:13152–13157. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taniwaki T, Hirashima N, Becerra SP, Chader GJ, Etcheberrigaray R, Schwartz JP. Pigment epithelium-derived factor protects cultured cerebellar granule cells against glutamate-induced neurotoxicity. J Neurochem. 1997;68:26–32. doi: 10.1046/j.1471-4159.1997.68010026.x. [DOI] [PubMed] [Google Scholar]
- 64.Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, et al. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 65.Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends Mol Med. 2002;8:330–334. doi: 10.1016/s1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- 66.Bernard A, Gao-Li J, Franco CA, Bouceba T, Huet A, Li Z. Laminin receptor involvement in the anti-angiogenic activity of pigment epithelium-derived factor. J Biol Chem. 2009;284:10480–10490. doi: 10.1074/jbc.M809259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li JK, Liang HL, Li Z, Gu CH, Yi DH, Pei JM. Pigment epithelium-derived factor promotes Fas-induced cardiomyocyte apoptosis via its receptor phospholipase A2. Life Sci. 2014;99:18–23. doi: 10.1016/j.lfs.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 68.Chung C, Doll JA, Gattu AK, Shugrue C, Cornwell M, Fitchev P, et al. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J Hepatol. 2008;48:471–478. doi: 10.1016/j.jhep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 69.Chavan SS, Hudson LK, Li JH, Ochani M, Harris Y, Patel NB, et al. Identification of pigment epithelium-derived factor as an adipocyte-derived inflammatory factor. Mol Med. 2012;18:1161–1168. doi: 10.2119/molmed.2012.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramírez-Castillejo C, Sánchez-Sánchez F, Andreu-Agulló C, Ferrón SR, Aroca-Aguilar JD, Sánchez P, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 71.Yamagishi S, Nakamura K, Takenaka K, Matsui T, Jinnouchi Y, Imaizumi T. Pigment epithelium-derived factor (PEDF) promotes growth of pericytes through autocrine production of platelet-derived growth factor-B. Microvasc Res. 2005;69:128–1234. doi: 10.1016/j.mvr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Yabe T, Kanemitsu K, Sanagi T, Schwartz JP, Yamada H. Pigment epithelium-derived factor induces pro-survival genes through cyclic AMP-responsive element binding protein and nuclear factor kappa B activation in rat cultured cerebellar granule cells: Implication for its neuroprotective effect. Neuroscience. 2005;133:691–700. doi: 10.1016/j.neuroscience.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Becerra SP, Sagasti A, Spinella P, Notario V. Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem. 1995;270:25992–25999. doi: 10.1074/jbc.270.43.25992. [DOI] [PubMed] [Google Scholar]
- 74.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 75.Yabe T, Wilson D, Schwartz JP. NFkappaB activation is required for the neuroprotective effects of pigment epithelium-derived factor (PEDF) on cerebellar granule neurons. J Biol Chem. 2001;276:43313–43319. doi: 10.1074/jbc.M107831200. [DOI] [PubMed] [Google Scholar]
- 76.Cai J, Jiang WG, Grant MB, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem. 2006;281:3604–3613. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 77.Ablonczy Z, Prakasam A, Fant J, Fauq A, Crosson C, Sambamurti K. Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through gamma-secretase. J Biol Chem. 2009;284:30177–30186. doi: 10.1074/jbc.M109.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cai J, Chen Z, Ruan Q, Han S, Liu L, Qi X, et al. γ-Secretase and presenilin mediate cleavage and phosphorylation of vascular endothelial growth factor receptor-1. J Biol Chem. 2011;286:42514–42523. doi: 10.1074/jbc.M111.296590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konson A, Pradeep S, D’Acunto CW, Seger R. Pigment epithelium-derived factor and its phosphomimetic mutant induce JNK-dependent apoptosis and p38-mediated migration arrest. J Biol Chem. 2011;286:3540–3551. doi: 10.1074/jbc.M110.151548. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Ho TC, Chen SL, Yang YC, Lo TH, Hsieh JW, Cheng HC, et al. Cytosolic phospholipase A2-{alpha} is an early apoptotic activator in PEDF-induced endothelial cell apoptosis. Am J Physiol Cell Physiol. 2009;296:C273, 284. doi: 10.1152/ajpcell.00432.2008. [DOI] [PubMed] [Google Scholar]
- 81.Pollina EA, Legesse-Miller A, Haley EM, Goodpaster T, Randolph-Habecker J, Coller HA. Regulating the angiogenic balance in tissues. Cell Cycle. 2008;7:2056–2070. doi: 10.4161/cc.7.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J, Ye L, Zhang L, Jian WG. The molecular impact of pigment epithelium-derived factor, PEDF, on lung cancer cells and the clinical significance. Int J Oncol. 2009;35:159–166. doi: 10.3892/ijo_00000324. [DOI] [PubMed] [Google Scholar]
- 83.Fan W, Crawford R, Xiao Y. The ratio of VEGF/PEDF expression in bone marrow mesenchymal stem cells regulates neovascularization. Differentiation. 2011;81:181–191. doi: 10.1016/j.diff.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Patil CK, Mian S, Campisi J. The thorny path linking cellular senescence to organismal aging. Mech Ageing Dev. 2005;126:1040–1045. doi: 10.1016/j.mad.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Chuaire-Noack L, Sánchez-Corredor MC, Ramírez-Clavijo SR. The dual role of senescence in tumorigenesis. Int J Morphol. 2010a;28:37–50. [Google Scholar]
- 86.Chuaire-Noack L, Rondón-Lagos S, Sánchez-Corredor M, Ibáñez-Pinilla M, Ramírez-Clavijo S. Beta-galactosidase activity as a marker of senescence in primary cultures of the ovarian surface epithelium. Invest Clin. 2010b;51:351–367. [PubMed] [Google Scholar]
- 87.Pignolo RJ, Cristofalo VJ, Rotenberg MO. Senescent WI-38 cells fail to express EPC-1, a gene induced in young cells upon entry into the G0 state. J Biol Chem. 1993;268:8949–8957. [PubMed] [Google Scholar]
- 88.Fernández-Barral A, Orgaz JL, Baquero P, Ali Z, Moreno A, Tiana M, et al. Regulatory and functional connection of microphthalmia-associated transcription factor and anti-metastatic pigment epithelium derived factor in melanoma. Neoplasia. 2014;16:529–542. doi: 10.1016/j.neo.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cao Y, Yang T, Gu C, Yi D. Pigment epithelium-derived factor delays cellular senescence of human mesenchymal stem cells in vitro by reducing oxidative stress. Cell Biol Int. 2013;34:305–313. doi: 10.1002/cbin.10041. [DOI] [PubMed] [Google Scholar]
- 90.Chuaire-Noack L, Sánchez-Corredor MC, Ramírez-Clavijo S. P53 and its role in the ovarian surface epithelium. Invest Clin. 2008;49:561–593. [PubMed] [Google Scholar]
- 91.Steinle JJ, Sharma S, Chin VC. Normal aging involves altered expression of growth factors in the rat choroid. J Gerontol A Biol Sci Med Sci. 2008;63A:135–140. doi: 10.1093/gerona/63.2.135. [DOI] [PubMed] [Google Scholar]
- 92.Francis MK, Appel S, Meyer C, Balin SJ, Balin AK, Cristofalo VJ. Loss of EPC-1/PEDF expression during skin aging In vivo. J Invest Dermatol. 2004;122:1096–1105. doi: 10.1111/j.0022-202X.2004.22510.x. [DOI] [PubMed] [Google Scholar]
- 93.Guan M, Pang CP, Yam HF, Cheung KF, Liu WW, Lu Y. Inhibition of glioma invasion by overexpression of pigment epithelium-derived factor. Cancer Gene Ther. 2004;11:325–332. doi: 10.1038/sj.cgt.7700675. [DOI] [PubMed] [Google Scholar]
- 94.Hase R, Miyamoto M, Uehara H, Kadoya M, Ebihara Y, Murakami Y, et al. Pigment epithelium-derived factor gene therapy inhibits human pancreatic cancer in mice. Clin Cancer Res. 2005;11:8737–8344. doi: 10.1158/1078-0432.CCR-05-1323. [DOI] [PubMed] [Google Scholar]
- 95.Streck CJ, Zhang Y, Zhou J, Ng C, Nathwani AC, Davidoff AM. Adeno-associated virus vector-mediated delivery of pigment epithelium-derived factor restricts neuroblastoma angiogenesis and growth. J Pediatr Surg. 2005;40:236–243. doi: 10.1016/j.jpedsurg.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 96.Guan M, Jiang H, Xu C, Xu R, Chen Z, Lu Y. Adenovirus-mediated PEDF expression inhibits prostate cancer cell growth and results in augmented expression of PAI-2. Cancer Biol Ther. 2007;6:419–425. doi: 10.4161/cbt.6.3.3757. [DOI] [PubMed] [Google Scholar]
- 97.Uehara H, Miyamoto M, Kato K, Ebihara Y, Kaneko H, Hashimoto H, et al. Expression of pigment epithelium-derived factor decreases liver metastasis and correlates with favorable prognosis for patients with ductal pancreatic adenocarcinoma. Cancer Res. 2004;64:3533–3537. doi: 10.1158/0008-5472.CAN-03-3725. [DOI] [PubMed] [Google Scholar]
- 98.Abramson LP, Browne M, Stellmach V, Doll J, Cornwell M, Reynolds M, et al. Pigment epithelium-derived factor targets endothelial and epithelial cells in Wilms’ tumour. J Pediatr Surg. 2006;41:1351–1356. doi: 10.1016/j.jpedsurg.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 99.Ji D, Li M, Zhan T, Yao Y, Shen J, Tian H, et al. Prognostic role of serum AZGP1, PEDF and PRDX2 in colorectal cancer patients. Carcinogenesis. 2013;34:1265–1272. doi: 10.1093/carcin/bgt056. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L, Chen J, Ke Y, Mansel RE, Jiang WG. Expression of pigment epithelial derived factor is reduced in non-small cell lung cancer and is linked to clinical outcome. Int J Mol Med. 2006;17:937–944. [PubMed] [Google Scholar]
- 101.Caldon CE. Estrogen signaling and the DNA damage response in hormone dependent breast cancers. Front Oncol. 2014;4:106. doi: 10.3389/fonc.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dey P, Barros RP, Warner M, Ström A, Gustafsson JÅ. Insight into the mechanisms of action of estrogen receptor β in the breast, prostate, colon, and CNS. J Mol Endocrinol. 2013;51:T61–74. doi: 10.1530/JME-13-0150. [DOI] [PubMed] [Google Scholar]
- 103.Parvathaneni K, Grigsby JG, Betts BS, Tsin AT. Estrogen-induced retinal endothelial cell proliferation: Possible involvement of Pigment Epithelium-Derived Factor and Phosphoinositide 3-Kinase/Mitogen-Activated Protein Kinase pathways. J Ocul Pharmacol Ther. 2013;29:27–32. doi: 10.1089/jop.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9:699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shao R, Cao S, Wang X, Feng Y, Billig H. The elusive and controversial roles of estrogen and progesterone receptors in human endometriosis. Am J Transl Res. 2014;6:104–113. [PMC free article] [PubMed] [Google Scholar]
- 106.Grantham JP, Henneberg M. The estrogen hypothesis of obesity. PLoS One. 2014;9:e99776. doi: 10.1371/journal.pone.0099776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids. 2014;90:13–29. doi: 10.1016/j.steroids.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Young NA, Friedman AK, Kaffenberger B, Rajaram MV, Birmingham DJ, Rovin BH, et al. Novel estrogen target gene ZAS3 is overexpressed in systemic lupus erythematosus. Mol Immunol. 2013;54:23–31. doi: 10.1016/j.molimm.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 110.Kumar R, Zakharov MN, Khan SH, Miki R, Jang H, Toraldo G, et al. The dynamic structure of the estrogen receptor. J Amino Acids. 2011;2011:812540. doi: 10.4061/2011/812540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miller JH, Gates RE, Jr, Ong DE, King LE., Jr A miniature molecular-sieving column assay for cytoplasmic vitamin A-binding proteins. Anal Biochem. 1984;139:104–114. doi: 10.1016/0003-2697(84)90395-6. [DOI] [PubMed] [Google Scholar]
- 112.Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P. Functional domains of the human estrogen receptor. Cell. 1987;51:941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- 113.Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, et al. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–1685. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- 114.Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, et al. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem. 2000;275:5379–5387. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- 115.Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 117.Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci USA. 2004;101:17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 119.Knowlton AA, Korzick DH. Estrogen and the female heart. Mol Cell Endocrinol. 2014;389:31–39. doi: 10.1016/j.mce.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vivar OI, Zhao X, Saunier EF, Griffin C, Mayba OS, Tagliaferri M, et al. Estrogen receptor beta binds to and regulates three distinct classes of target genes. J Biol Chem. 2010;285:22059–22066. doi: 10.1074/jbc.M110.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 122.Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC. Selective estrogen receptor-beta agonists repress transcription of proinflammatory genes. J Immunol. 2008;180:630–636. doi: 10.4049/jimmunol.180.1.630. [DOI] [PubMed] [Google Scholar]
- 123.Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, et al. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277:24353–24360. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- 124.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Prossnitz ER, Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol. 2014;389:71–83. doi: 10.1016/j.mce.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodeling in oophorectomized mRen2. Lewis rats. Cardiovasc Res. 2012;94:96–104. doi: 10.1093/cvr/cvs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tombran-Tink J, Mazuruk K, Rodriguez IR, Chung D, Linker T, Englander E, et al. Organization, evolutionary conservation, expression and unusual Alu density of the human gene for pigment epithelium-derived factor, a unique neurotrophic serpin. Mol Vis. 1996;2:11. [PubMed] [Google Scholar]
- 128.Leung KW, Cheung LW, Pon YL, Wong RN, Mak NK, Fan TP, et al. Ginsenoside Rb1 inhibits tube-like structure formation of endothelial cells by regulating pigment epithelium-derived factor through the oestrogen beta receptor. Br J Pharmacol. 2007;152:207–215. doi: 10.1038/sj.bjp.0707359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 130.Li C, Tang Y, Li F, Turner S, Li K, Zhou X, et al. 17beta-estradiol (betaE2) protects human retinal Müller cell against oxidative stress in vitro: evaluation of its effects on gene expression by cDNA microarray. Glia. 2006;53:392–400. doi: 10.1002/glia.20291. [DOI] [PubMed] [Google Scholar]
- 131.Mowa CN, Iwanaga T. Differential distribution of oestrogen receptor-alpha and -beta mRNAs in the female reproductive organ of rats as revealed by in situ hybridization. J Endocrinol. 2000;165:59–66. doi: 10.1677/joe.0.1650059. [DOI] [PubMed] [Google Scholar]
- 132.Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab. 2000;85:4835–4840. doi: 10.1210/jcem.85.12.7029. [DOI] [PubMed] [Google Scholar]
- 133.Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER){alpha} and ER{beta} null mice indicate a role for ER{beta} in follicular maturation. Endocrinology. 2005;146:2817–2826. doi: 10.1210/en.2004-1108. [DOI] [PubMed] [Google Scholar]
- 134.Lenie S, Smitz J. Estrogen receptor subtypes localization shifts in cultured mouse ovarian follicles. Histochem Cell Biol. 2008;129:827–840. doi: 10.1007/s00418-008-0408-9. [DOI] [PubMed] [Google Scholar]
- 135.Brandenberger AW, Tee MK, Jaffe RB. Estrogen receptor alpha (ER-alpha) and beta (ER-beta) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-beta in neoplastic tissues. J Clin Endocrinol Metab. 1998;83:1025–1028. doi: 10.1210/jcem.83.3.4788. [DOI] [PubMed] [Google Scholar]
- 136.Jarkovska K, Kupcova Skalnikova H, Halada P, Hrabakova R, Moos J, Rezabek K, et al. Development of ovarian hyperstimulation syndrome: interrogation of key proteins and biological processes in human follicular fluid of women undergoing in vitro fertilization. Mol Hum Reprod. 2011;17:679–692. doi: 10.1093/molehr/gar047. [DOI] [PubMed] [Google Scholar]
- 137.Twigt J, Steegers-Theunissen RP, Bezstarosti K, Demmers JA. Proteomic analysis of the microenvironment of developing oocytes. Proteomics. 2012;12:1463–1471. doi: 10.1002/pmic.201100240. [DOI] [PubMed] [Google Scholar]
- 138.Kampfer C, Saller S, Windschüttl S, Berg D, Berg U, Mayerhofer A. Pigment Epithelium Derived Factor (PEDF) and the human ovary: a role in the generation of ROS in granulosa cells. Life Sci. 2014;97:129–136. doi: 10.1016/j.lfs.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 139.Joham AE, Teede HJ, Hutchison SK, Stepto NK, Harrison CL, Strauss BJ, et al. Pigment epithelium-derived factor, insulin sensitivity, and adiposity in polycystic ovary syndrome: impact of exercise training. Obesity (Silver Spring) 2012;20:2390–2396. doi: 10.1038/oby.2012.135. [DOI] [PubMed] [Google Scholar]
- 140.Lecke SB, Morsch D, Spritzer PM. Circulating levels and subcutaneous adipose tissue gene expression of pigment epithelium-derived factor in polycystic ovary syndrome and normal women: a case control study. Reprod Biol Endocrinol. 2013;11:77. doi: 10.1186/1477-7827-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chuderland D, Ben-Ami I, Bar-Joseph H, Shalgi R. Pigment epithelium derived factor (PEDF) in the reproductive system. Reproduction. 2014b;148:R53–61. doi: 10.1530/REP-14-0251. [DOI] [PubMed] [Google Scholar]
- 142.Humaidan P, Quartarolo J, Papanikolaou EG. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94:389–400. doi: 10.1016/j.fertnstert.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 143.Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol. 2002;39:225–237. doi: 10.1016/s1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- 144.Chuderland D, Ben-Ami I, Kaplan-Kraicer R, Grossman H, Ron-El R, Shalgi R. The role of pigment epithelium-derived factor in the pathophysiology and treatment of ovarian hyperstimulation syndrome in mice. J Cli Endocrinol Metab. 2013b;98:E258–266. doi: 10.1210/jc.2012-3037. [DOI] [PubMed] [Google Scholar]
- 145.Tsuchiya T, Nakahama KI, Asakawa Y, Maemura T, Tanaka M, Takeda S, et al. The reduction in pigment epithelium-derived factor is a sign of malignancy in ovarian cancer expressing low-level of vascular endothelial growth factor. Gynecol Endocrinol. 2009;25:104–109. doi: 10.1080/09513590802549841. [DOI] [PubMed] [Google Scholar]
- 146.Rutherford T, Brown WD, Sapi E, Aschkenazi S, Muñoz A, Mor G. Absence of estrogen receptor-β expression in metastatic ovarian cancer. Obstet Gynecol. 2000;96:417–421. doi: 10.1016/s0029-7844(00)00917-0. [DOI] [PubMed] [Google Scholar]
- 147.Chan KK, Wei N, Liu SS, Xiao-Yun L, Cheung AN, Ngan HY. Estrogen receptor subtypes in ovarian cancer. A clinical correlation. Obstet Gynecol. 2008;111:144–151. doi: 10.1097/01.AOG.0000296715.07705.e9. [DOI] [PubMed] [Google Scholar]
- 148.Halon A, Nowak-Markwitz E, Maciejczyk A, Pudelko M, Gansukh T, Györffy B, et al. Loss of estrogen receptor beta expression correlates with shorter overall survival and lack of clinical response to chemotherapy in ovarian cancer patients. Anticancer Res. 2011;31:711–718. [PubMed] [Google Scholar]
- 149.Fujimoto J, Hirose R, Sakaguchi H, Tamaya T. Clinical significance of expression of estrogen receptor alpha and beta mRNAs in ovarian cancers. Oncology. 2000;58:334–341. doi: 10.1159/000012121. [DOI] [PubMed] [Google Scholar]
- 150.Johns A, Freay AD, Fraser W, Korach KS, Rubanyi GM. Disruption of estrogen receptor gene prevents 17 beta estradiol-induced angiogenesis in transgenic mice. Endocrinology. 1996;137:4511–4513. doi: 10.1210/endo.137.10.8828515. [DOI] [PubMed] [Google Scholar]
- 151.May K, Becker CM. Endometriosis and angiogenesis. Minerva Ginecol. 2008;60:245–254. [PubMed] [Google Scholar]
- 152.Weigelt B, Banerjee S. Molecular targets and targeted therapeutics in endometrial cancer. Curr Opin Oncol. 2012;24:554–563. doi: 10.1097/CCO.0b013e328354e585. [DOI] [PubMed] [Google Scholar]
- 153.Torry DS, Leavenworth J, Chang M, Maheshwari V, Groesch K, Ball ER, et al. Angiogenesis in implantation. J Assist Reprod Genet. 2007;24:303–315. doi: 10.1007/s10815-007-9152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson JA. Estrogen receptors alpha and beta in the rodent mammary gland. Proc Natl Acad Sci USA. 2000;97:337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cheng G, Butler R, Warner M, Gustafsson JÅ, Wilczek B, Landgren BM. Effects of short-term estradiol and norethindrone acetate treatment on the breasts of normal postmenopausal women. Menopause. 2013b;20:496–503. doi: 10.1097/GME.0b013e318276c4ea. [DOI] [PubMed] [Google Scholar]
- 156.Förster C, Mäkela S, Wärri A, Kietz S, Becker D, Hultenby K, et al. Involvement of estrogen receptor beta in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci USA. 2002;99:15578–15583. doi: 10.1073/pnas.192561299. Erratum in: Proc Natl Acad Sci USA 2006; 103:8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lattrich C, Juhasz-Boess I, Ortmann O, Treeck O. Detection of an elevated HER2 expression in MCF-7 breast cancer cells overexpressing estrogen receptor beta1. Oncol Rep. 2008;19:811–817. [PubMed] [Google Scholar]
- 158.Fitzgerald DP, Subramanian P, Deshpande M, Graves C, Gordon I, Qian Y, et al. Opposing effects of pigment epithelium-derived factor on breast cancer cell versus neuronal survival: implication for brain metastasis and metastasis-induced brain damage. Cancer Res. 2012;72:144–153. doi: 10.1158/0008-5472.CAN-11-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Broadhead ML, Choong PF, Dass CR. Efficacy of continuously administered PEDF-derived synthetic peptides against osteosarcoma growth and metastasis. J Biomed Biotechnol. 2012;2012:230298. doi: 10.1155/2012/230298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rüegg C, Hasmim M, Lejeune FJ, Alghisi GC. Antiangiogenic peptides and proteins: from experimental tools to clinical drugs. Biochim Biophys Acta. 2006;1765:155–177. doi: 10.1016/j.bbcan.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 161.Filleur S, Volz K, Nelius T, Mirochnik Y, Huang H, Zaichuk TA, et al. Two functional epitopes of pigment epithelial-derived factor block angiogenesis and induce differentiation in prostate cancer. Cancer Res. 2005;65:5144–5152. doi: 10.1158/0008-5472.CAN-04-3744. [DOI] [PubMed] [Google Scholar]
- 162.Mirochnik Y, Aurora A, Schulze-Hoepfner FT, Deabes A, Shifrin V, Beckmann R, et al. Short pigment epithelial-derived factor-derived peptide inhibits angiogenesis and tumor growth. Clin Cancer Res. 2009;15:1655–1663. doi: 10.1158/1078-0432.CCR-08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ek ET, Dass CR, Contreras KG, Choong PF. PEDF-derived synthetic peptides exhibit antitumor activity in an orthotopic model of human osteosarcoma. J Orthop Res. 2007b;25:1671–1680. doi: 10.1002/jor.20434. [DOI] [PubMed] [Google Scholar]
- 164.Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R, et al. Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther. 2008;324:1064–1072. doi: 10.1124/jpet.107.131318. [DOI] [PubMed] [Google Scholar]
- 165.Yang J, Chen S, Huang X, Han J, Wang Q, Shi D, et al. Growth suppression of cervical carcinoma by pigment epithelium-derived factor via anti-angiogenesis. Cancer Biol Ther. 2010;9:967–974. doi: 10.4161/cbt.9.12.11635. [DOI] [PubMed] [Google Scholar]
- 166.Wu Q, He S, Wei X, Shao B, Luo S, Guo F, et al. Synergistic antitumor effect of rAAV-mediated PEDF with hyperthermia on solid tumor. Hum Gene Ther. 2014;25:811–823. doi: 10.1089/hum.2013.150. [DOI] [PubMed] [Google Scholar]
- 167.He SS, Wu QJ, Gong CY, Luo ST, Zhang S, Li M, et al. Enhanced efficacy of combination therapy with adeno-associated virus-delivered pigment epithelium-derived factor and cisplatin in a mouse model of Lewis lung carcinoma. Mol Med Rep. 2014;9:2069–2076. doi: 10.3892/mmr.2014.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chuderland D, Hasky N, Ben-Ami I, Kaplan-Kraicer R, Grossman H, Shalgi R. A physiological approach for treating endometriosis by recombinant pigment epithelium-derived factor (PEDF) Hum Reprod. 2013c;28:1626–1634. doi: 10.1093/humrep/det027. [DOI] [PubMed] [Google Scholar]
- 169.Xu Z, Fang S, Zuo Y, Zhang Y, Cheng R, Wang Q, et al. Combination of pigment epithelium-derived factor with radiotherapy enhances the antitumor effects on nasopharyngeal carcinoma by downregulating vascular endothelial growth factor expression and angiogenesis. Cancer Sci. 2011;102:1789–1798. doi: 10.1111/j.1349-7006.2011.02013.x. [DOI] [PubMed] [Google Scholar]
- 170.Dass CR, Contreras KG, Dunstan DE, Choong PF. Chitosan microparticles encapsulating PEDF plasmid demonstrate efficacy in an orthotopic metastatic model of osteosarcoma. Biomaterials. 2007;28:3026–3033. doi: 10.1016/j.biomaterials.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 171.Ta HT, Dass CR, Larson I, Choong PF, Dunstan DE. A chitosan hydrogel delivery system for osteosarcoma gene therapy with pigment epithelium-derived factor combined with chemotherapy. Biomaterials. 2009;30:4815–4823. doi: 10.1016/j.biomaterials.2009.05.035. [DOI] [PubMed] [Google Scholar]