Abstract
Objective(s):
The neuropeptide calcitonin gene-related peptide (CGRP) has long been postulated to play an integral role in the pathophysiology of migraine. Earlier studies showed that CGRP can stimulate the synthesis and release of nitric oxide (NO) and cytokines from trigeminal ganglion glial cells. The purpose of this study was to determine the effect of 17β-estradiol in regulation of CGRP expression, inducible nitric oxide synthase (iNOS) activity, and NO and interleukin-1beta (IL-1β) release in cultured peripheral blood mononuclear cells (PBMCs) from patients with pure menstrual migraine and healthy individuals.
Materials and Methods:
This study was performed on twelve patients with pure menstrual migraine and twelve age-and sex-matched healthy individuals. PBMCs treated with 17β-estradiol for 24 hr at physiological and pharmacological doses. Gene expression was evaluated by real time-PCR. CGRP and IL-1β proteins in culture supernatant were determined by ELISA method. Activity of iNOS in PBMCs and total nitrite in the culture supernatant were measured by colorimetric assays.
Results:
Treatment with 17β-estradiol had a biphasic effect on expression of CGRP. We found that 17β-estradiol treatment at pharmacological dose significantly increases mRNA expression of CGRP in both groups (P<0.001), whereas at physiological dose it could significantly decrease CGRP mRNA expression (P<0.001), CGRP protein levels, IL-1β release, NO production and iNOS activity only in patient groups (P<0.05).
Conclusion:
Collectively, it appears that 17β-estradiol can exert protective effect on decrease of inflammation in migraine via decrease in levels of CGRP, IL-1β and iNOS activity; however, more studies are necessary in this regard.
Keywords: Calcitonin gene-related-peptide, Inducible nitric oxide-synthase, Neurogenic inflammation, Nitric oxide, Pure menstrual migraine, 17β-estradiol
Introduction
Migraine as a common neurovascular syndrome is associated with dysfunction of cranial nerves and blood vessels. It is characterized by intense unilateral, pulsatile, and debilitating headache attacks, lasting from 4 to 72 hr and accompanied by neurological and gastrointestinal symptoms (1). Migraine complications are responsible for substantial socio-economic burden. More importantly, migraine, according to the World Health Organization (WHO), has been placed on nineteenth rank among debilitating diseases (2, 3). In view of higher incidence of migraine in women in comparison with men, the understanding of molecular mechanism that is related to gender, seems essential in order to unravel migraine etiology and explore novel treatments (4). More than 50% of female migraineurs report an association between menstruation and migraine (5, 6). Pure menstrual migraine is defined as migraine that occurs only on day 1±2 of menstruation. It is generally accepted that abrupt decrease in female sex hormone levels in the late luteal phase and early follicular phase may be responsible for this phenomenon. The rapid decline of sex hormone levels, as a potential migraine trigger, could stimulate sensory fibers of the trigeminal nerve through dilation of cranial blood vessels (7-9). Notably, stimulation of trigeminal sensory nerve fibers can result in the release of nitric oxide (NO) (10) and certain neuropeptides such as calcitonin gene-related peptide (CGRP) (10). These events can exacerbate vasodilation and cause neurogenic inflammation. NO as a bioactive free radical, and CGRP as a 37-amino-acid neuropeptide, are the most effective dilators of blood vessels and is thought to play an important role in the development of inflammation and more importantly the migraine pathomechanism (11-13). NO is generated by the activity of various isoforms of nitric oxide synthase (NOS) including inducible NOS (iNOS), endothelial NOS (eNOS), and neuronal NOS (nNOS) (14). iNOS produces large amounts of NO that can cause tissue damage and cell death. NO and CGRP also stimulate the release of pro-inflammatory cytokines such as interleukin-1beta (IL-1β) (15, 16). Cytokines induce cyclooxygenas-2 (COX-2) in the spinal cord and dorsal root ganglia cells, thereby increasing pain sensitivity (17). There is compelling evidence that 17β-estradiol as an important female sex hormone can affect gene expression of CGRP and secretion of cytokines such as IL-1β and also inhibit inflammatory reaction (8, 18, 19). Since the estrogen receptors and the full complement of epigenetic enzymes and machinery presented in most tissues such as neurons are also found in PBMCs, we decided to use these cells as a model of trigeminal cells for evaluation of neurogenic inflammation by female sex hormone intervention (20-22).
Moreover, there is a great deal of uncertainty regarding the influence of 17β-estradiol on expression and activity of genes involved in inflammation. To our knowledge, no study thus far has evaluated a possible effect of 17β-estradiol on expression of CGRP, IL-1 β and iNOS as key components of migraine pathophysiology; thus, we aimed to investigate the effect of 17β-estradiol on CGRP gene expression, iNOS activity, and IL-1β release in patients with pure menstrual migraine and healthy individuals.
Materials and Methods
Participants
We recruited 12 patients with pure menstrual migraine and 12 healthy individuals (aged 25 to 45 years) from Sina hospital affiliated by Tehran University of Medical Sciences (TUMS), Tehran, Iran. The diagnosis of pure menstrual migraine was established by a neurologist according to the criteria of the International Classification of Headache Disorders (ICHD-II) [1]. The exclusion criteria for participation in the study were: (1) secondary headache and other neurological disease; (2) systemic diseases such as cardiovascular or acute infectious disease; (3) oral contraceptives, pregnancy, or lactation period; and (4) use of nonsteroidal anti-inflammatory drugs (NSAIDs). It should be noted that patients and healthy individuals are well matched regarding body mass index (BMI). This study was approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS), and written informed consent was obtained from all participants prior to participation. Human peripheral venous blood was obtained from healthy controls and patients with pure menstrual migraine. Blood sample collection was performed 2 days before and 3 days after the onset of the menses. Blood samples were collected in sterile sodium heparin-containing tubes.
Preparation and culture of PBMCs
PBMCs were isolated by Ficoll-Hypaque density centrifugation (Lympholyte-H; Cedarlane Laboratories, Hornby, Ontario, Canada). Whole blood was layered on top of Ficoll and centrifuged at 800 g for 40 min. After centrifugation, the interphase containing PBMCs was collected and washed twice in phosphate-buffered saline (PBS) (GIBCO; Invitrogen Laboratories, UK). Then cells were counted, and viability was determined by trypan blue exclusion test. After that, cells were resuspended in phenol red free RPMI 1640 medium (GIBCO; Invitrogen Laboratories, UK), 5% charcoal-dextran-stripped fetal bovine serum (FBS), and 1% penicillin/streptomycin solution (GIBCO; Invitrogen Laboratories, UK) at a final concentration of 2 × 106 cells/ml in 12-well flat-bottom culture plates. After 4 hr incubation at 37 °C in 5% CO2 humidified atmosphere, the cultures were treated with concentrations of 1×10-6 M and 1×10-8 M 17β-estradiol (Sigma-Aldrich) for 24 hr. We used a dose of 1×10-8 M 17β-estradiol to treat PBMCs, because this value falls near the mean±SEM concentration of estrogen detected in human serum at the maximum preovulatory (i.e., 2,393±356 pmol/l)(23). After incubation, PBMCs were collected in order to evaluate mRNA expression and iNOS activity. In addition, the cell-free supernatants were collected and stored at -70 °C until assay.
Determination of cell viability
The effect of 17β-estradiol on PBMCs viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma; 5 mg/ml in phosphate-buffered saline). Isolated PBMCs from a heparinized blood sample were seeded into a 96-well flat-bottom plate at a concentration of 2×105 cells/well in 200 μl phenol red-free RPMI 1640 supplemented with 1% penicillin and streptomycin, and 5% heat-inactivated FBS treated with charcoal. Then PBMCs were treated with 17β-estradiol at doses of 10-10, 10-9, 10-8, 10-7, 10-6, 10-5 mol/l in ethanol and incubated at 37 °C and 5% CO2 for 24 hr. After incubation, the supernatants were carefully removed, and 100 μl MTT reagents was added at a concentration of 0.5 mg/ml. Following incubation of the plate for 4 hr at 37 °C, 100 μl of dimethyl sulphoxide (DMSO) (Sigma–Aldrich)) was added. The cell viability was determined spectrophotometrically at 540 nm using a microplate reader (Lab systems MC340) after incubation of plate for 30 min at a dark place.
Quantitative real-time PCR
PBMCs (2×106 cells/well) seeded in 12-well plates were used for RNA extraction. RNA extraction was performed using Total RNA Extraction Miniprep kit (VIOGENE, Taiwan) according to manufacturer’s instructions. The concentration of RNA was determined by measuring the 260/280 nm absorbance ratio, and its quality was evaluated using agarose gel electrophoresis. Synthesis of first-strand cDNA was carried out using 1 µg RNA with cDNA Revert Aid First Strand cDNA Synthesis Kit (Thermo scientific, Fermentas, USA). The resulting cDNA was amplified using SYBR Green PCR Master Mix (Takara, Japan) by Rotor Gene real-time thermocycler (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. CGRP and β-actin primers were purchased from QIAGEN (Hilden, Germany). Relative gene expression was normalized to β-actin and calculated as 2-∆CT using the following formula: 2-(Ct target gene-Ct β-actin).
The standard curves were generated from the pooled cDNA of the assayed samples.
CGRP and IL-1β assay
CGRP was measured using an ELISA kit (MyBiosource) in supernatants according to recommended method by the kit manufacturer. Kit sensitivity, intra- and inter-assay coefficients of variability were 1.12 ng/l, <10% and <12%, respectively.
Quantitative assay of IL-1β release was performed using commercially available sandwich ELISA kits (Abcam). The minimum decidable dose of IL-1β was less than 0.3 pg/ml. Intra- and inter-assay coefficients of variability were <10% and <12%, respectively.
The evaluation of activity of NOS and iNOS
NOS activity in PBMCs was measured using the NOS assay kit (Oxford Biomedical Research) according to manufacturer’s instructions. The procedure is based on the conversion of L-arginine to L-citrulline by using a NADPH recycling system. NOS was assessed spectrophotometrically by measuring the accumulation of its stable degradation products, nitrate and nitrite. In this method, nitrate is converted to nitrite via the nitrate reductase, and then total nitrite was assayed by using Griess reagent. To perform this assay, PBMCs were lysed in an ice-cold homogenization buffer [HEPES (50 mM), EDTA (0.5 mM), dithiothreitol (1 mM), CaCl2 (2.5 mM), KCl (1.7 mM), MgCl2 (2 mM) and protease inhibitor cocktail (sigma), pH 7.4], and sonicated on crushed ice with two 30 sec bursts following these were passed by a 26-gauge needle (20 times) attached to a syringe. Following centrifugation, the supernatant was collected and the protein concentration was determined using Bradford assay (24). Then 70 µg proteins from lysates in a volume of 30 μl were used for NOS assay (calcium-dependent NOS) according to kit manufacturers’ instructions. iNOS activity (calcium-independent NOS) was measured in replicate samples by replacing CaCl2 with EDTA (1.7 mmol/l), because EDTA chelates calcium required for nNOS and eNOS activity. NOS and iNOS activities data were expressed as nmol/min/mg of protein.
Measurement of nitrite
Nitrite and nitrate are the stable products of NO. The spectrophotometric measurement of nitrite by Griess reaction is a well known method for the indirect determination of NO, which requires nitrate first be reduced to nitrite and then total nitrite determined by this method (25). Absorbance was measured at 540 nm by a microplate reader; nitrite concentration in culture supernatant was measured using sodium nitrite as the standard.
Measurement of 17β-estradiol
The blood containing sodium heparin was centrifuged at 1200×g for 10 min at 18 °C, and plasma was stored at −70 °C until analysis. The plasma concentrations of 17β-estradiol were measured by Elecsys 2010 autoanalyser (Hitachi, Japan) based on electrochemiluminecance method.
Statistical analysis
Mean, standard deviation and standard error of within and between groups were calculated using SPSS (IBM SPSS Statistics 22). Results were expressed as mean ± standard error of mean (SEM).
Real-time PCR data distributions were skewed, and a logarithmic transform was applied to normalize the distribution. All subsequent calculations were performed on the transformed data. Repeated measures ANOVA with Tukey’s HSD as post hoc test was performed to compare within-group. Non-parametric Wilcoxon signed-rank test and paired-sample T-Test were used to compare within-group differences. All statistical significance level (α) is set at less than 5% (P<0.05).
Results
Demographics and clinical characteristics
Table 1 shows the characteristics of the healthy participants and patients with pure menstrual migraine. As depicted in this table, there was no significant difference between these two groups with respect to age, height or the plasma levels of E2. We also found that the patients with pure menstrual migraine had higher weights and BMI compared with control participants.
Table 1.
The mean±standard deviation of the physiological and clinical characteristics of all participants
| Healthy controls n=12 | Patient n=12 | Ρ-value | |
|---|---|---|---|
| Age (years) | 31.92±5.92 | 35.00±6.02 | 0.22 |
| Weight (kg) | 55.5±5.95 | 58.5±5.98 | 0.23 |
| Height (cm) | 1.62±0.56 | 1.62±0.45 | 0.84 |
| BMI (kg/m2) | 21.15±1.74 | 22.42±1.97 | 0.11 |
| E2 (pg/ml) | 56.53±28.9 | 41.90±17.37 | 0.15 |
Cell viability
Results of MTT test in order to investigate the effects of 17β-estradiol on PBMCs viability are shown in Figure 1. We observed no cytotoxicity effect after 24 hr exposure to different doses of 17β-estradiol. The cell viability did not decline less than 97% under exposure to 17β-estradiol concentrations in comparison with untreated group. Therefore, we selected a physiologic concentration (10-8 M) and a pharmacologic concentration (10-6 M) for further experiments.
Figure 1.
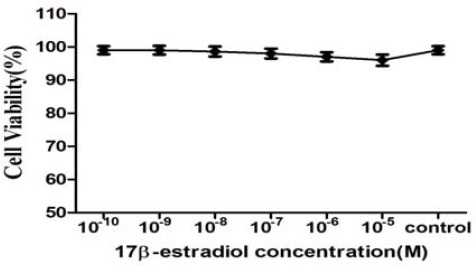
Assessment of PBMCs viability in response to 17β-estradiol (E2) treatment
17β-estradiol on mRNA expression of CGRP
Following treatment of PBMCs with pharmaco-logical dose of estrogen (1×10-6 M 17β-estradiol) in both groups, gene expression of CGRP was significantly increased (1.9 fold change in healthy participants vs 1.4 fold change in patient group, P<0.001), while physiological dose of 17β-estradiol (1×10-8M 17β-estradiol) significantly reduced CGRP mRNA level only in the patient group (Figure 2a). In case of CGRP mRNA expression, we found a significant difference after treatment of PBMCs with physiologic and pharmacologic doses of estrogen.
Figure 2.
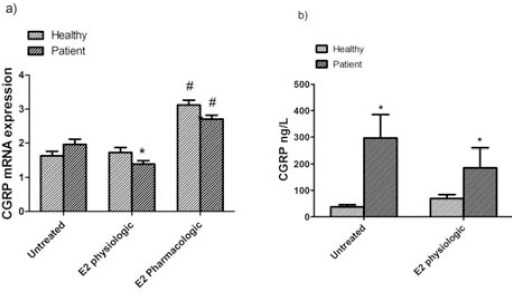
a) Expression of CGRP mRNA b) and CGRP concentrations in PBMCs of untreated and treated with 17β-estradiol (E2 physiologic) in healthy controls and pure menstrual migraine patients. Values are mean±standard error of mean (n=12) *P<0.05, #P<0.001
The effect of 17β-estradiol on release of CGRP
To assess the effect 17β-estradiol on CGRP release in PBMCs, we studied CGRP concentration in the supernatant of PBMCs after treatment with physiological dose of 17β-estradiol (Figure 2b). Accordingly, CGRP level was significantly decreased in patient group (Mean±SEM; untreated cells: 296.53±88.77 ng/ml; treated cells with 17β-estradiol: 185.2±74.27 ng/ml; P=0.046), whereas in healthy group, CGRP levels were significantly increased compared to cells without intervention (Mean±SEM; untreated cells: 37.5±8.1 ng/ml; treated cells with 17β-estradiol: 69.2±14.8 ng/ml; P=0.017). These findings were consistent with gene expression in PBMCs from patients.
Measurement of total NOS and iNOS enzyme activities
The effect of 17β-estradiol on activity of total and inducible NOS in PBMCs from both healthy and patient groups is shown in Figure 3a, b. After treatment with physiological dose, we found a borderline significant decrease in total NOS activity (P=0.084) in patients as compared to untreated PBMCs. Also, we found no significant difference concerning total NOS and iNOS activities before and after treatment with 17β-estradiol in healthy controls.
Figure 3.
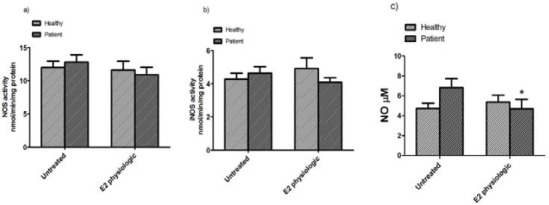
Total NOS activity a) and iNOS activity b) and total nitrite concentrations c) in PBMCs of untreated and treated with 17β-estradiol (E2 physiologic) in healthy subjects and pure menstrual migraine patients. Values are Mean ±standard error of mean (n=12) *P<0.05
The effect of 17β-estradiol on release of NO
The effect of 17β-estradiol on total nitrite in the culture supernatant of both healthy women and patients with pure menstrual migraine are shown in Figure 3c. The concentration of total nitrite in the culture supernatant of patients was borderline significantly higher compared to those in healthy controls (Mean±SEM; untreated: 6.82±0.91 μM and 4.74±0.51 μM; P=0.062, respectively). 17β-estradiol could significantly decrease total nitrite concentration in patient group (Mean±SEM; untreated: 6.82±0.91 μM; treated: 4.69±0.96 μM; P=0.026), while in healthy group, total nitrite concentrations were non-significantly increased compared to untreated PBMCs (Mean±SEM; untreated: 4.74±0.51; treated: 5.37±0.70; P=0.510).
The effect of 17β-estradiol on the secretion of IL-1 β
To evaluate the effect of physiological dose of 17β-estradiol on IL-1 β release, its concentrations in cell supernatants were measured in both groups. In patient group, IL-1β levels were significantly decreased (Mean±SEM; untreated cells: 64.73±7.62 pg/ml; PBMCs treated with 17β-estradiol: 59.98±7.81 pg/ml; P=0.042), whereas in control group, IL-1β levels were non-significantly increased compared to cells without intervention (Mean±SEM; untreated: 49.16±6.97 pg/ml; treated: 51.11±7.97 pg/ml; P=0.7) (Figure 4).
Figure 4.
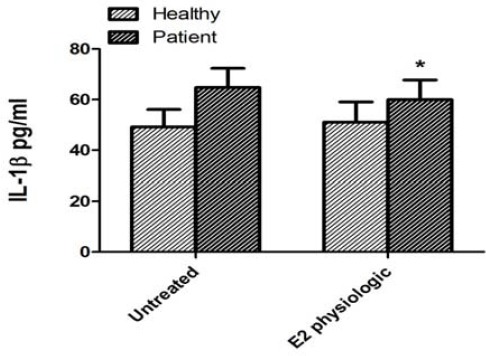
IL-1reconcentrations in PBMCs of untreated and treated with 17untreated a (E2 physiologic) in healthy subjects and pure menstrual migraine patients. Values are Mean ± standard error of mean (n = 12) *P<0.05
Discussion
There is increasing evidence showing the high incidence of migraine in women, and its attacks in menstrual cycle may be related to changes in levels of sex steroid hormones. Accumulating evidences suggested that neurogenic inflammation, which typified by vasodilation, release of pro-inflammatory mediators and plasma extravasation, plays a key role in migraine pathogenesis. In migraine, activation of the trigeminal nerves triggers neurogenic inflamma-tion via release of different neuropeptides such as CGRP (26), which released from trigeminal sensory nerve fibers at the site of stimulation (27). It is well documented that CGRP is also synthesized by other cells including lymphocytes (28), monocytes (29), and macrophages (30, 31). There is compelling evidence showing that plasma levels of CGRP increased in the jugular venous during migraine attacks (32, 33). CGRP, which widely distributed in the central and peripheral nervous systems (34, 35), is stored in perivascular nerve terminals surrounding most blood vessels. It is plausible that release of CGRP initiates cranial blood vessel dilatation and can lead to pain in an inflammation-dependent mechanism (36-38). CGRP homeostasis in the central nervous system is strongly influenced by sex steroids (8). Possible mechanism may be the estrogen withdrawal in the late luteal phase of the menstrual cycle (39). The results of a study revealed that there is an inverse relationship between migraine and urinary estrogen levels during the menstrual cycle (40). In this regard, another research also showed that estrogen withdrawal during the early post-partum period causes migraine headaches, (41).
Accordingly, Somerville used a single injection of 17β-estradiol as a preventive strategy in 6 women with menstrual migraine and observed that migraine attacks delay by this estrogen supplement (39). Similarly, it is shown that a subcutaneous implant of 17β-estradiol causes improvement of more than 80% in women with menstrual migraine.
On the other hand, several studies have reported that ovariectomy decreased the gene expression of CGRP, which was apparently normalized following estrogen administration (43-45), as well as in postmenopausal women undergoing hormone replacement therapy (HRT) (46). A similar study demonstrated that estrogen replacement increases the frequency and severity of migraine attacks (47). Our results regarding increased CGRP levels following treatment of PBMCs with pharmacological dose of 17β-estradiol in both healthy and patient groups, are partly in accordance with other studies in the dorsal ganglion in animal models (18, 48). The elevated expression of CGRP by the sudden drop in estrogen levels can provide a possible mechanism for onset of migraine headaches in women with menstrual migraine. In this study, for the first time, we observed that 17β-estradiol at the concentration of 10-8 M (physiologic dose) significantly decreased release of CGRP from PBMCs of women with pure menstrual migraine. This difference may arise from applied dose or route of estrogen administration, albeit more studies are necessary to explain exact reason. Also, the results of our study indicated that untreated CGRP protein concentrations of patient group are higher than CGRP gene expression levels. This discrepancy may be due to previous storage of CGRP in PBMCs, albeit we did not find evidence of stored CGRP in blood mononuclear cells.
Released CGRP from activated trigeminal nerves can cause the secretion of pro-inflammatory mediators (such as iNOS and IL-1β) from mast cells and also other mononuclear cells (49). IL-1β is synthesized and released via various cells including PBMCs, neuronal and glial cells. It is believed that IL-1β may modulate trigeminal ganglion (TRG) neuronal excitability via binding to interleukin type I receptor (IL-1RI) and also cause the generation of hyperalgesia (50, 51). Previous studies reported that 17β-estradiol can decrease production of IL-1β from mononuclear cells (23, 52, 53). In agreement with our findings, it is shown that estrogen has a regulatory role in cytokine release in macrophages and monocytes (54). In contrast, another study indicated no significant inhibition of IL-1 activity by 17β-estradiol (55). In this study, we observed that 17-β estradiol inhibits the spontaneous secretion of IL-1 β from PBMCs of patient group after treatment with physiological concentrations of this estrogen. In patient groups, concerning decrease of CGRP levels following treatment with17β-estradiol at dose of 10-8 mol/l, we also observed a significant decreasing response to this dose of estrogen for IL-1β.
These pro-inflammatory mediators intensify vasodilation and transmission of nociceptive information through increase in the synthesis and release of CGRP in a positive feedback loop that can strengthen inflammation and pain (36-38). The effects of CGRP may be mediated by activation of the CGRP receptor that coupled to activation of mitogen-activated protein kinases (MAPKs) (56, 57).
It is well-established that MAPK pathways regulate iNOS gene expression that produces a large amount of NO in the cells of nervous system including neuronal cells (10, 13, 58-60). NO is a bioactive free radical and a potent endogenous vasodilator, involved in inflammation, pain transmission, chronic pain, and hyperalgesia. Released NO in nerve fibers surrounding intracranial arteries probably plays a regulatory role in the release of CGRP (61, 62) and neurogenic inflammatory process. Hence, it seems that likely decrease in levels of CGRP may account for reduced production of NO possibly through regulation of iNOS activity. Although present findings showed a borderline significant decrease in iNOS activity after treatment with estrogen; however, experimental studies are warranted to determine exact molecular mechanism linking the production of CGRP to NO release in PBMCs. It is suggested that NO of endothelium-derived can be considered as a plausible mediator intracranial arterial dilation in migraine patients; therefore, the shear stress on the vessel walls can activate eNOS, which in turn increase the production of endothelial NO (63, 64). On the other hand, in some studies, possible role of NO and endothelial dysfunction in patients with migraine have not yet confirmed (64, 65).
Conclusion
Taken together, it appears that 17β-estradiol can exert protective effect on decrease of inflammation in migraine via decrease in levels of CGRP, IL-1β and iNOS activity, although further investigation is required to unravel obscure issues regarding effects of 17β-estradiol on other inflammatory components involved in migraine pathogenesis.
Acknowledgment
We are thankful of Tehran University of Medical Science, Tehran, Iran, for financial support of this project. The results reported in this paper were part of a student thesis supported by Tehran University of Medical Sciences, Tehran, Iran.
References
- 1.Bracci-Laudiero L, Aloe L, Caroleo MC, Buanne P, Costa N, Starace G, et al. Endogenous NGF regulates CGRP expression in human monocytes,and affects HLA-DR and CD86 expression and IL-10 production. Blood. 2005;106:3507–3514. doi: 10.1182/blood-2004-10-4055. [DOI] [PubMed] [Google Scholar]
- 2.World Health Report. Geneva: WHO; 2001. [Google Scholar]
- 3.Ruiz de Velasco I, Gonzalez N, Etxeberria Y, Garcia-Monco JC. Quality of life in migraine patients: a qualitative study. Cephalalgia. 2003;23:892–900. doi: 10.1046/j.1468-2982.2003.00599.x. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991;44:1147–1157. doi: 10.1016/0895-4356(91)90147-2. [DOI] [PubMed] [Google Scholar]
- 5.Couturier EG, Bomhof MA, Neven AK, van Duijn NP. Menstrual migraine in a representative Dutch population sample: prevalence, disability and treatment. Cephalalgia. 2003;23:302–308. doi: 10.1046/j.1468-2982.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin VT. Menstrual migraine: a review of prophylactic therapies. Curr Pain Headache Rep. 2004;8:229–237. doi: 10.1007/s11916-004-0057-1. [DOI] [PubMed] [Google Scholar]
- 7.MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354–361. doi: 10.1016/S1474-4422(04)00768-9. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Mehrotra S, Villalon CM, Perusquia M, Saxena PR, MaassenVanDenBrink A. Potential role of female sex hormones in the pathophysiology of migraine. Pharmacol Ther. 2007;113:321–340. doi: 10.1016/j.pharmthera.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis--part 2. Headache. 2006;46:365–386. doi: 10.1111/j.1526-4610.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Vause CV, Durham PL. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–8. doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. 2009;124:309–323. doi: 10.1016/j.pharmthera.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Vause CV, Durham PL. CGRP stimulation of iNOS and NO release from trigeminal ganglion glial cells involves mitogen-activated protein kinase pathways. J Neurochem. 2009;110:811–821. doi: 10.1111/j.1471-4159.2009.06154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 15.Cuesta MC, Quintero L, Pons H, Suarez-Roca H. Substance P and calcitonin gene-related peptide increase IL-1 beta, IL-6 and TNF alpha secretion from human peripheral blood mononuclear cells. Neurochem Int. 2002;40:301–306. doi: 10.1016/s0197-0186(01)00094-8. [DOI] [PubMed] [Google Scholar]
- 16.Reuter U, Bolay H, Jansen-Olesen I, Chiarugi A, Sanchez del Rio M, Letourneau R, et al. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001;124:2490–2502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- 17.Neeb L, Hellen P, Boehnke C, Hoffmann J, Schuh-Hofer S, Dirnagl U, et al. IL-1beta stimulates COX-2 dependent PGE(2) synthesis and CGRP release in rat trigeminal ganglia cells. PloS one. 2011;6:e17360. doi: 10.1371/journal.pone.0017360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mowa CN, Usip S, Collins J, Storey-Workley M, Hargreaves KM, Papka RE. The effects of pregnancy and estrogen on the expression of calcitonin gene-related peptide (CGRP) in the uterine cervix, dorsal root ganglia and spinal cord. Peptides. 2003;24:1163–1174. doi: 10.1016/j.peptides.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Pratt MA, Bishop TE, White D, Yasvinski G, Menard M, Niu MY, et al. Estrogen withdrawal-induced NF-kappaB activity and bcl-3 expression in breast cancer cells: roles in growth and hormone independence. Mol Cell Biol. 2003;23:6887–6900. doi: 10.1128/MCB.23.19.6887-6900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavin DP, Sharma RP. Chromatin from peripheral blood mononuclear cells as biomarkers for epigenetic abnormalities in schizophrenia. Cardiovasc Psychiatry Neurol. 2009:409562. doi: 10.1155/2009/409562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inui A, Ogasawara H, Naito T, Sekigawa I, Takasaki Y, Hayashida Y, et al. Estrogen receptor expression by peripheral blood mononuclear cells of patients with systemic lupus erythematosus. Clin Rheumatol. 2007;26:1675–1678. doi: 10.1007/s10067-007-0568-3. [DOI] [PubMed] [Google Scholar]
- 23.Polan ML, Kuo A, Loukides J, Bottomly K. Cultured human luteal peripheral monocytes secrete increased levels of interleukin-1. J Clin Endocrinol Metab. 1990;70:480–484. doi: 10.1210/jcem-70-2-480. [DOI] [PubMed] [Google Scholar]
- 24.NJ K. The Bradford method for protein quantitation. The protein protocols handbook. Springer; 2009. pp. 17–24. [Google Scholar]
- 25.Ansar MM, Ansari M. Nitric oxide involvement in pancreatic beta cell apoptosis by glibenclamide. Nitric Oxide. 2006;14:39–44. doi: 10.1016/j.niox.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Bellamy J, Bowen EJ, Russo AF, Durham PL. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci. 2006;23:2057–2066. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holzer P, Maggi CA. Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience. 1998;86:389–398. doi: 10.1016/s0306-4522(98)00047-5. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Xing L, Xing Y, Tang Y, Han C. Identification and characterization of immunoreactive calcitonin gene-related peptide from lymphocytes of the rat. J Neuroimmunol. 1999;94:95–102. doi: 10.1016/s0165-5728(98)00230-6. [DOI] [PubMed] [Google Scholar]
- 29.Bracci-Laudiero L, Aloe L, Caroleo MC, Buanne P, Costa N, Starace G, et al. Endogenous NGF regulates CGRP expression in human monocytes, and affects HLA-DR and CD86 expression and IL-10 production. Blood. 2005;106:3507–3514. doi: 10.1182/blood-2004-10-4055. [DOI] [PubMed] [Google Scholar]
- 30.Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Muller B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004;32:1715–1721. doi: 10.1097/01.ccm.0000134404.63292.71. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Zhang J. Heterogeneity of macrophages in injured trigeminal nerves: cytokine/chemokine expressing vs. phagocytic macrophages. Brain Behav Immun. 2012;26:891–903. doi: 10.1016/j.bbi.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocrine reviews. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- 33.van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 34.Kopp S. Neuroendocrine, immune, and local responses related to temporomandibular disorders. J Orofac Pain. 2001;15:9–28. [PubMed] [Google Scholar]
- 35.Pietrobon D. Migraine: new molecular mechanisms. Neuroscientist. 2005;11:373–386. doi: 10.1177/1073858405275554. [DOI] [PubMed] [Google Scholar]
- 36.Hargreaves R. New migraine and pain research. Headache. 2007;47(Suppl 1):S26–43. doi: 10.1111/j.1526-4610.2006.00675.x. [DOI] [PubMed] [Google Scholar]
- 37.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 38.Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25:179–183. doi: 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 39.Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355–365. doi: 10.1212/wnl.22.4.355. [DOI] [PubMed] [Google Scholar]
- 40.MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology. 2006;67:2154–2158. doi: 10.1212/01.wnl.0000233888.18228.19. [DOI] [PubMed] [Google Scholar]
- 41.Stein GS. Headaches in the first post partum week and their relationship to migraine. Headache. 1981;21:201–205. doi: 10.1111/j.1526-4610.1981.hed2105201.x. [DOI] [PubMed] [Google Scholar]
- 42.Magos AL, Zilkha KJ, Studd JW. Treatment of menstrual migraine by oestradiol implants. J Neurol Neurosurg Psychiatry. 1983;46:1044–1046. doi: 10.1136/jnnp.46.11.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gangula PR, Zhao H, Wimalawansa SJ, Supowit SC, DiPette DJ, Yallampalli C. Pregnancy and steroid hormones enhance the systemic and regional hemodynamic effects of calcitonin gene-related peptide in rats. Biol Reprod. 2001;64:1776–1783. doi: 10.1095/biolreprod64.6.1776. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal M, Puri V, Puri SP. Effects of estrogen on the serotonergic system and calcitonin gene-related peptide in trigeminal ganglia of rats. Ann Neurosci. 2012;19:151–157. doi: 10.5214/ans.0972.7531.190403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarajari S, Oblinger MM. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp Neurol. 2010;224:163–169. doi: 10.1016/j.expneurol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma QL, Zhou HY, Sun M. [Relationship between sex hormone levels and blood calcitonin gene-related peptide/endothelin-1 in postmenopausal women with coronary heart disease] Hunan Yi Ke Da Xue Xue Bao. 2001;26:146–148. [PubMed] [Google Scholar]
- 47.Martin V, Wernke S, Mandell K, Zoma W, Bean J, Pinney S, et al. Medical oophorectomy with and without estrogen add-back therapy in the prevention of migraine headache. Headache. 2003;43:309–321. doi: 10.1046/j.1526-4610.2003.03065.x. [DOI] [PubMed] [Google Scholar]
- 48.Gangula PR, Lanlua P, Wimalawansa S, Supowit S, DiPette D, Yallampalli C. Regulation of calcitonin gene-related peptide expression in dorsal root ganglia of rats by female sex steroid hormones. Biol Reprod. 2000;62:1033–1039. doi: 10.1095/biolreprod62.4.1033. [DOI] [PubMed] [Google Scholar]
- 49.Durham PL. CGRP-receptor antagonists--a fresh approach to migraine therapy? N Engl J Med. 2004;350:1073–1075. doi: 10.1056/NEJMp048016. [DOI] [PubMed] [Google Scholar]
- 50.Desson SE, Ferguson AV. Interleukin 1beta modulates rat subfornical organ neurons as a result of activation of a non-selective cationic conductance. J Physiol. 2003;550:113–122. doi: 10.1113/jphysiol.2003.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferri CC, Ferguson AV. Interleukin-1 beta depolarizes paraventricular nucleus parvocellular neurones. J Neuroendocrinol. 2003;15:126–133. doi: 10.1046/j.1365-2826.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 52.Hu SK, Mitcho YL, Rath NC. Effect of estradiol on interleukin 1 synthesis by macrophages. Int J Immunopharmacol. 1988;10:247–252. doi: 10.1016/0192-0561(88)90055-0. [DOI] [PubMed] [Google Scholar]
- 53.Polan ML, Daniele A, Kuo A. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil Steril. 1988;49:964–968. [PubMed] [Google Scholar]
- 54.Kramer PR, Kramer SF, Guan G. 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis and rheumatism. 2004;50:1967–1975. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- 55.Stock JL, Coderre JA, McDonald B, Rosenwasser LJ. Effects of estrogen in vivo and in vitro on spontaneous interleukin-1 release by monocytes from postmenopausal women. J Clin Endocrinol Metab. 1989;68:364–368. doi: 10.1210/jcem-68-2-364. [DOI] [PubMed] [Google Scholar]
- 56.Parameswaran N, Disa J, Spielman WS, Brooks DP, Nambi P, Aiyar N. Activation of multiple mitogen-activated protein kinases by recombinant calcitonin gene-related peptide receptor. Eur J Pharmacol. 2000;389:125–130. doi: 10.1016/s0014-2999(99)00874-2. [DOI] [PubMed] [Google Scholar]
- 57.Schaeffer C, Vandroux D, Thomassin L, Athias P, Rochette L, Connat JL. Calcitonin gene-related peptide partly protects cultured smooth muscle cells from apoptosis induced by an oxidative stress via activation of ERK1/2 MAPK. Biochim Biophys Acta. 2003;1643:65–73. doi: 10.1016/j.bbamcr.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Homma H, Takahashi T, Seki H, Ohtani M, Kondoh T, Fukuda M. Immunohistochemical localization of inducible nitric oxide synthase in synovial tissue of human temporomandibular joints with internal derangement. Arch Oral Biol. 2001;46:93–97. doi: 10.1016/s0003-9969(00)00086-8. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi T, Homma H, Nagai H, Seki H, Kondoh T, Yamazaki Y, et al. Specific expression of inducible nitric oxide synthase in the synovium of the diseased temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:174–181. doi: 10.1067/moe.2003.45. [DOI] [PubMed] [Google Scholar]
- 60.Nagai H, Kumamoto H, Fukuda M, Takahashi T. Inducible nitric oxide synthase and apoptosis-related factors in the synovial tissues of temporomandibular joints with internal derangement and osteoarthritis. J Oral Maxillofac Surg. 2003;61:801–807. doi: 10.1016/s0278-2391(03)00155-1. [DOI] [PubMed] [Google Scholar]
- 61.Holzer P, Jocic M. Cutaneous vasodilatation induced by nitric oxide-evoked stimulation of afferent nerves in the rat. Br J Pharmacol. 1994;112:1181–1187. doi: 10.1111/j.1476-5381.1994.tb13208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei EP, Moskowitz MA, Boccalini P, Kontos HA. Calcitonin gene-related peptide mediates nitroglycerin and sodium nitroprusside-induced vasodilation in feline cerebral arterioles. Circ Res. 1992;70:1313–1319. doi: 10.1161/01.res.70.6.1313. [DOI] [PubMed] [Google Scholar]
- 63.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 64.de Hoon JN, Smits P, Troost J, Struijker-Boudier HA, Van Bortel LM. Forearm vascular response to nitric oxide and calcitonin gene-related peptide: comparison between migraine patients and control subjects. Cephalalgia. 2006;26:56–63. doi: 10.1111/j.1468-2982.2005.00993.x. [DOI] [PubMed] [Google Scholar]
- 65.Silva FA, Rueda-Clausen CF, Silva SY, Zarruk JG, Guzman JC, Morillo CA, et al. Endothelial function in patients with migraine during the interictal period. Headache. 2007;47:45–51. doi: 10.1111/j.1526-4610.2006.00532.x. [DOI] [PubMed] [Google Scholar]


