Abstract
Objective(s):
Acrylamide (ACR) has many applications in different industries. ACR damages the central and the peripheral nervous system in human and animals. Importance of ACR-induced neurotoxicity encouraged researchers to find both different mechanisms involved in ACR neurotoxicity and potent neuroprotective agents. Therefore, this study was designed to investigate the protective effect of crocin, an active constituent of Crocus sativus L. (saffron) on ACR-induced neurotoxicity in Wistar rats.
Materials and Methods:
Animals were treated with ACR (50 mg/kg, IP) 11 days for induction neurotoxicity. Crocin (12.5, 25 and 50 mg/kg, IP) were used during treatment with ACR. At the end of treatment, gait score examination was performed. Then, rats were sacrificed and the severity of damage in brain tissue was determined using pathological tests. The level of malondialdehyde (MDA) and glutathione (GSH) content were evaluated in cerebral cortex and cerebellum to determine the role of oxidative stress in this model.
Results:
Exposure to ACR induced severe gait abnormalities and pathological changes, but administration of crocin markedly improved behavioral index and histopathological damages. The elevation of lipid peroxidation parallel with reduction of GSH level was observed in cerebral cortex and cerebellum following exposure to ACR. Treatment with crocin markedly decreased MDA level, while elevated GSH content in nervous system as compared to ACR-treated animals.
Conclusion:
The administration of crocin markedly improved behavioral and histopathological damages in Wistar rats exposed to ACR. Reduction of oxidative stress can be considered as an important mechanism of neuroprotective effects of crocin against ACR-induced toxicity.
Keywords: Acrylamide, Crocin, Gait score examination, Lipid peroxidation, Neurotoxicity, Saffron
Introduction
Polyacrylamides, high- molecular- weight polymers, are formed by the polymerization of acrylamide (ACR). These polymers are used in different industries such as water and wastewater management, soil coagulation, dye synthesis and in laboratories for gel electro-phoresis. In addition to industrial and laboratory uses, ACR forms when carbohydrate- rich foods are cooked in high temperature. Therefore, diet is another main source of environmental ACR exposure in human (1-3). The toxicity of ACR has been studied extensively in both experimental animals and human. It has been shown that exposure to ACR causes genotoxicity, and cellular damage in nervous and reproductive systems in animals. However, only neurotoxicity has been identified by epidemiological studies on human population (4). ACR as a potent neurotoxic agent damages the central and the peripheral nervous system. ACR induces ataxia, skeletal muscle weakness and weight loss in both occupationally exposed human and experimental animal models (3, 4).
An imbalance between the production and removal of free radicals and reactive oxygen species (ROS) increases oxidative stress that is implicated in pathogenesis of some neurodegenerative diseases (5). Furthermore, it has been reported that ACR-induced neurotoxicity in Wistar rats induced lipid peroxidation and reduced antioxidant capacity in nervous system (6, 7). Therefore, oxidative stress plays important role in ACR-induced toxicity (8, 9).
Also, in previous study we showed that ACR could induce ROS production and apoptosis in PC12 cells as an in vitro model of neurotoxicity (10).
Crocin is an active and important compound of Crocus sativus L. (saffron) (11). Crocin is shown to have high antioxidant activity in several in vitro and in vivo models. In both traditional use (12) and modern pharmacological studies, saffron extracts or its active constituents crocin and safranal are shown to have antitumor effects (13), radical scavenger properties (14), antidepressant (15-17), memory-improved (17, 18), anticonvulsant (19, 20), antinociceptive (21), antineuropathic (22, 23), hypotensive (24), anxiolytic and hypnotic (25, 26) and cardioprotective effects (27).
Crocin can reduce oxidative damage caused by ischemia-reperfusion injury in kidney (28) and skeletal muscle (29). This carotenoid increases cell viability in serum deprivation PC12 cells by inducing glutathione (GSH) synthesis, increasing the glutathione reductase (GR) and c-glutamylcysteinyl synthase (c-GCS) activities, and decreasing ceramide formation (30, 31). Further study indicated that crocin is more potent than α-tocopherol, against oxidative stress-induced death of neurons (30, 31). Crocin also reduced the haematological toxicity of diazinon and the augmentation of direct 8-iso-prostaglandin F (2 beta), TNF-α, and S100 beta levels by diazinon in rats (32, 33). Previously, we showed that crocin could decrease ACR-induced cytotoxicity in PC12 cells, especially via reduction of ROS production in the dose dependent manner (10).
Therefore, in the current study we decided to find the protective effect of crocin against ACR-induced neurotoxicity in Wistar rats with focus on oxidative stress evaluation in cerebral cortex and cerebellum.
Materials and Methods
Materials
ACR was purchased from Merck. Malondialdehyde tetrabutylammonium, reduced glutathione (GSH) and DTNB [5, 5’-dithiobis-(2-nitrobenzoic acid)] were obtained from Sigma. Vitamin E (Ampule 100 IU/ml, Osveh) was used for this study.
Crocin preparation
Stigmas of Crocus sativus L. were purchased from Novin Saffron (collected from Ghaen, Khorasan province, Northeast of Iran) and analyzed in accordance with the ISO/TS 3632-2. Crocin was extracted and purified according to the previously described method (34). The purity of crocin was 98%.
Experimental animals
Male Wistar rats, 230 to 250 g were housed in colony rooms with 12/12 hr light/dark cycle at 21±2 °C and had free access to food and water. All animal experiments were carried out in accordance with Mashhad University of Medical Sciences, Ethical committee Acts.
Experimental design
To induce neurotoxicity in Wistar rats, animals were exposed to ACR (50 mg/kg/day IP) for 11 days (3, 35, 36). Daily dose-rate and corresponding route of administration have been well characterized with respect to neuropathological expression and neurological deficits. For our study, rats were randomly divided into 7 groups (n=6 in each group) and the treatment was given as follow:
-
1)
Control, Normal saline
-
2)
ACR, 50 mg/kg
-
3)
ACR, 50 mg/kg + crocin 12.5 mg/kg (37)
-
4)
ACR, 50 mg/kg + crocin 25 mg/kg
-
5)
ACR, 50 mg/kg + crocin 50 mg/kg
-
6)
ACR, 50 mg/kg + vitamin E 200 mg/kg (7)
-
7)
crocin 50 mg/kg
ACR was administrated IP once a day for 11 days. Saline was given in the same way to control rats. Crocin and vitamin E administrated IP for 11 days once a day and three times a week, respectively.
The behavioral index (gait scores) examination
At the end of animal treatment, the gait scores were examined according to the methods described by LoPachin (3). Rats were placed in a clear plexiglass box and were observed for 3 min, and a gait score, from 1 to 4, was assigned; where 1= a normal, unaffected gait; 2= a slightly affected gait (foot splay, slight hindlimb weakness and spread); 3= a moderately affected gait (foot splay, moderate hindlimb weakness, moderate limb spread during ambulation,); and 4= a severely affected gait (foot splay, severe hindlimb weakness, dragging hindlimbs, inability to rear).
Histopathology assay
Tissue preparation
After gait score test, animals were sacrificed and the brain of each animal was removed completely and immediately fixed in 10% buffered formalin solution overnight. Then each brain was cut coronally and sliced. Each slice measuring about 2 to 3 mm processed by an automatic tissue processor (Shandon, England) in about 18 hr through the routine histopathological method, and further fixed in formalin, dehydrated in alcohol, cleared in xyline and finally paraffinized. Then paraffin blocks were prepared and sectioned at 3 to 5 µm thickness and the microscopic slides were stained by routine Hematoxyline and Eosin method.
Histopathologic examination
Slides were evaluated by the pathologist using the light microscope (Olympus.BX-40, Japan). Necrotic neurons were considered in all areas of the brain, and the amount of necrosis was estimated in a semiquantitive manner in the whole brain for each animal.
Tissue sampling
At the end of 11 days exposure, after behavioral index examination, rats were sacrificed and the brain tissues (cerebral cortex and cerebellum) were dissected, and then samples were snap-frozen in liquid nitrogen and stored at −80 °C until use.
Lipid peroxidation assay
Malondialdehyde (MDA) levels, as marker of lipid peroxidation, were measured in brain tissues. MDA reacts with thiobarbituric acid (TBA) as a thiobarbituric acid reactive substance (TBARS) to produce a pink colored complex, which has maximum absorbance at 532 nm.
Briefly, 3 ml phosphoric acid (1%) and 1 ml TBA (0.6%) were added to 0.5 ml of brain tissue homogenate 10% in KCl and the mixture was heated for 45 min in a boiling water bath. After cooling, 4 ml of n-butanol was added to the mixture and vortex-mixed for 1 min followed by centrifugation at 3000 g for 10 min. The organic layers were transferred to a fresh tube and absorbance was recorded at 532 nm (38). A calibration curve was plotted using malondialdehyde tetrabutylammonium. MDA levels were expressed as nmol/mg protein. The total protein contents of the supernatants were determined by the Bradford assay using bovine serum albumin as standard.
Reduced glutathione (GSH) assay
Brain GSH content was determined by the method of Moron et al (1979) with minor changes. Briefly, 300 µl of tissue homogenates were mixed with 300 µl of 10% tricolor acetic acid (TCA) and vortexed. After centrifugation at 2500 g for 10 min, supernatants were mixed with reaction mixtures containing 2 ml phosphate buffer (pH: 8) and 500 µl DTNB [5,5 ′ di thiobis-(2-nitrobenzoic acid)]. Within 10 min, the absorbance was measured at 412 nm using a spectrophotometer (Jenway 6105 uv/vis, UK) (39). GSH contents were determined from a standard curve produced using commercially available GSH. Levels of GSH were expressed as nmol/mg protein.
Statistical analysis
Results are expressed as mean±SD. Statistical analyses were performed with ANOVA followed by Tukey–Kramer test to compare the differences between the means. Differences were considered statistically significant when P<0.05. Statistical analysis for the gait abnormalities were performed with nonparametric test Kruskal-Wallis followed by Dunn’s multiple comparison test.
Results
Effect of ACR on the behavioral index (gait scores) in rats and protective effect of crocin
Exposure to ACR (50 mg/kg, IP) for 11 days led to progressive gait abnormalities in rats as shown in Figure 1. In animals that treated with combination of crocin (25 mg/kg and 50 mg/kg) and ACR gait abnormalities significantly reversed in the dose-dependent manner (P<0.05 and P<0.01 vs ACR treated group).
Figure 1.
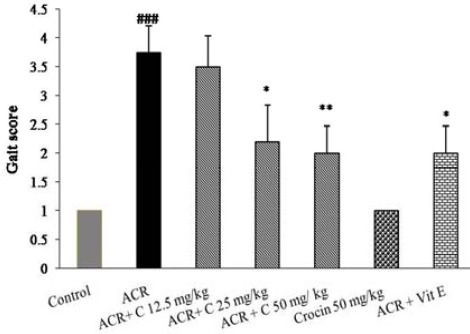
Effects of crocin on behavioral index (gait scores) in rats during the treatment with ACR (50 mg/kg, IP) for 11 days, Data are expressed as the mean±SD, (n=6). ###P<0.001 vs. control, * P<0.05, **P<0.01 vs. ACR treated animals
ACR: Acrylamide
Histopathological examination of brain tissues
Evaluation of brain tissues in control group exhibited normal cells (Figure 2A), but severe neuronal necrosis was observed in rats that were exposed to ACR. Histopathological evaluation of brain tissues exhibited the dark neurons in cerebral cortex, cerebellum, hippocampus and basal ganglia (Figure 2B, D, E and F). Treatment with crocin diminished the severity of necrosis to the extent that in some treated brains, no considerable necrosis was detectable (Figure 2C, Table 1).
Figure 2.
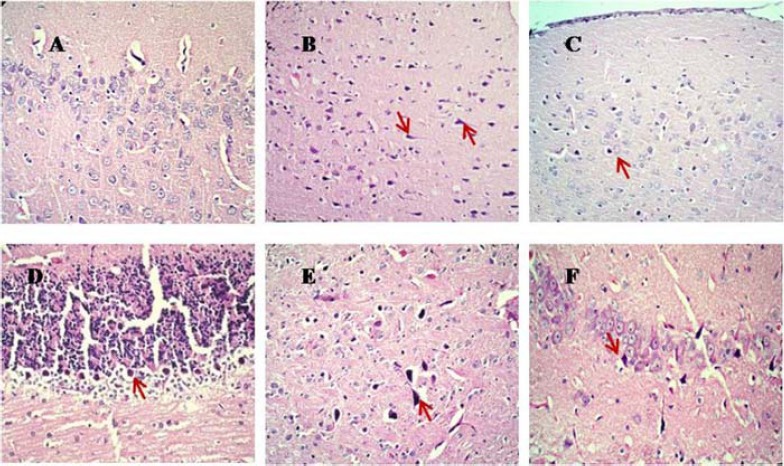
Histopathological alterations (Hematoxyline and Eosin staining by light microscope) of brain tissues in control and experimental groups of rats. Sections were visualized under light microscope at the magnification of × 200 for A, and × 100 for B, C, D, E and F). A, control rats showing normal histology in cerebral cortex. B, D, E and F, ACR- exposed rats showing the dark neurons in cerebral cortex, cerebellum, basal ganglia and hippocampus, respectively). C, ACR + Crocin 50 mg/kg group showing less numbers of dark neurons in cerebral cortex
ACR: Acrylamide
Table 1.
Effect of crocin on the histopathological changes in brain tissues following exposure to ACR. ###P<0.001 vs. Control, *** P<0.001 vs. ACR treated animals
| Group | % Necrosis |
|---|---|
| Control | 0 |
| ACR (50 mg/kg) | ### 100 |
| ACR + Crocin (12.5 mg/kg) | *** 70 |
| ACR + Crocin (25 mg/kg) | *** 60 |
| ACR + Crocin (50 mg/kg) | *** 30 |
| ACR + Vit E (200 IU/kg) | *** 30 |
| Crocin (50 mg/kg) | 0 |
ACR: Acrylamide
Effect of crocin on lipid peroxidation induced by ACR
Lipid peroxidation in the cerebral cortex and cerebellum was determined by measuring MDA content. Rats that were exposed to ACR showed a significant (P<0.001) increase in the level of MDA from 1.00 ± 0.31 to 4.04 ± 0.60 nmol/mg protein in cerebral cortex and from 1.26 ± 0.32 to 3.00 ± 0.41 nmol/mg protein in cerebellum tissue when compared to control rats (Figure 3A, B). Treatment with crocin at doses of 12.5, 25 and 50 mg/kg significantly decreased the level of MDA in cerebral cortex (P<0.001). In cerebellum tissue, crocin 12.5 mg/kg had no effect on MDA level, but crocin 25 and 50 mg/kg significantly (P<0.001) inhibited lipid peroxidation.
Figure 3.

Effect of crocin on lipid peroxidation in cerebral cortex (A) and cerebellum (B) following treatment with ACR. Animals were treated with different doses of crocin (12.5, 25 and 50 mg/kg) during treatment with ACR 50 mg/kg for 11 days. Data are expressed as the mean±SD, (n=6). ### P<0.001 vs. Control, ***P<0.001 vs. ACR treated animals
ACR: Acrylamide
Effect of crocin on GSH content in brain tissues following exposure to ACR
Exposure to ACR exhibited a significant decrease in GSH content in cerebral cortex and cerebellum (P<0.001) (Figure 4A, B). Crocin dose-dependently and significantly increased the levels of GSH in brain tissues compared to ACR treated rats. But, 12.5 mg/kg crocin could not recover GSH content in cerebral cortex and cerebellum. Treatment with 50 mg/kg crocin alone had no effect on the GSH content (P>0.05).
Figure 4.
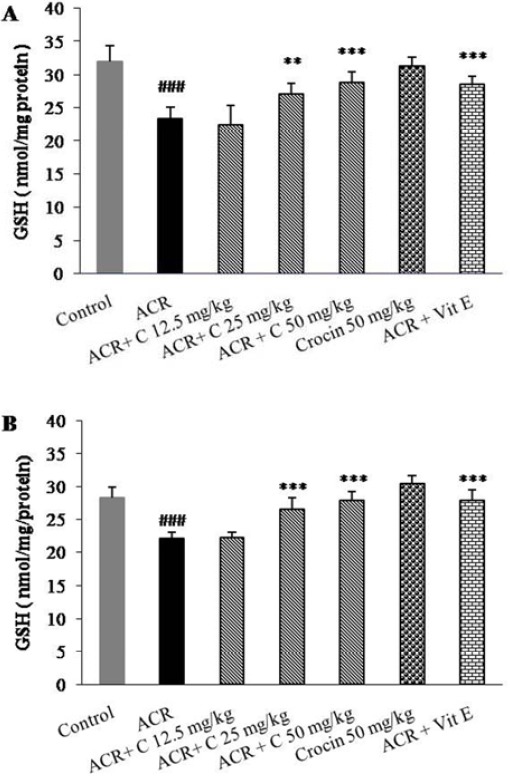
Effect of crocin on GSH content in cerebral cortex (A) and cerebellum (B) following treatment with ACR. Animals were treated with different doses of crocin (12.5, 25 and 50 mg/kg) during treatment with ACR 50 mg/kg for 11 days. Data are expressed as the mean ± SD, (n = 6). ### P<0.001 vs. Control, ** P<0.01, ***P<0.001 vs. ACR treated animals
ACR: Acrylamide
Discussion
In the previous study, we reported ACR induced cytotoxicity in PC12 cells, increased ROS generation and apoptotic cell death, but crocin protected cells from ACR-induced cytotoxicity by interrupting the cell death cascade through inhibition of intracellular ROS production (10). Based on these results from in vitro model, current study was designed to evaluate neuroprotective effect of crocin in Wistar rat.
Results showed that exposure to ACR markedly declined GSH contents while enhanced MDA levels in cerebral cortex and cerebellum. Crocin administration dose-dependently reduced lipid peroxidation through enhancement of GSH content in both cerebral cortex and cerebellum. Also, crocin recovered histopathological and behavioural damage, which was induced with ACR.
GSH plays an important role in maintaining cell integrity against exogenous and endogenous derived ROS (40). Previous studies indicated that ACR is detoxified by conjugation with GSH (41, 42). Gycidamime, as a metabolite of ACR, could also conjugate with GSH (3, 43). Our study clearly exhibited that exposure to ACR markedly decreased GSH content in cerebral cortex and cerebellum. Reduction of GSH may make the brain tissues more sensitive to the oxidative stress, because following ACR treatment, significant enhancement of MDA level was observed in the cerebral cortex and cerebellum, suggesting the augmentation of the lipid peroxidation. Because of crucial role of oxidative stress in ACR- induced cytotoxicity (6, 8), it is suggested that antioxidants could be considered as an alternative approach in modulation of ACR toxicity (9, 44, 45).
Numerous researches indicated the antioxidant effect of crocin in different conditions. Crocin attenuated lipid peroxidation in kidney (28) and skeletal muscle (29) during ischemia-reperfusion-induced oxidative damage in rats. Moreover, this carotenoid protected the brain against reperfusion-induced oxidative/nitrative injury after global cerebral ischemia in mice (46).
Crocin administration (12.5, 25 and 50 mg/kg) does-dependently increased GSH content in both cerebral cortex and cerebellum, markedly reduced MDA level as a marker of lipid peroxidation, and markedly declined gait abnormalities in experimental groups treated with crocin. Depletion of neural GSH level and enhancement of lipid peroxidation might be one of the primary events in ACR-induced neurotoxicity; therefore, potent antioxidants such as crocin can demonstrate neuroprotective effect in ACR-induced neurotoxicity through interrupting oxidative stress.
Vitamin E has been considered as a neuroprotective agent in many researches, and its protective effect has been widely believed to be due to its antioxidant activity (47). Recently, anti-apoptotic effect of vitamin E has been reported (48). Thus, in current study vitamin E was used as a positive control in protection against ACR- induced neurotoxicity. Results clearly exhibited that there is no difference between vitamin E and crocin (50 mg/kg) effect in inhibition of lipid peroxidation; therefore, crocin may be mentioned as a potent neuroprotective agent.
Conclusion
ACR induced gait abnormalities and histopathological damages via reduction of GSH contents and induction of lipid peroxidation in cerebral cortex and cerebellum. Crocin administration dose-dependently decreased ACR toxicity through suppression of lipid peroxidation and the elevation of GSH content.
Acknowledgment
Authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences, Mashhad, Iran, for financial support. Results described in this article are part of PhD thesis.
References
- 1.LoPachin RM. The changing view of acrylamide neurotoxicity. NeuroToxicol. 2004;25:617–630. doi: 10.1016/j.neuro.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Claus A, Carle R, Schieber A. Acrylamide in cereal products: A review. J Cereal Sci. 2008;47:118–133. [Google Scholar]
- 3.LoPachin RM. Acrylamide neurotoxicity: Neuro-logical, morhological and molecular endpoints in animal models. Adv Exp Med Biol. 2005;561:21–37. doi: 10.1007/0-387-24980-X_2. [DOI] [PubMed] [Google Scholar]
- 4.Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, et al. Acrylamide: Review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol. 2006;36:481–608. doi: 10.1080/10408440600851377. [DOI] [PubMed] [Google Scholar]
- 5.Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res in Toxicol. 2007;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 6.Zhu YJ, Zeng T, Zhu YB, Yu SF, Wang QS, Zhang LP, et al. Effects of acrylamide on the nervous tissue antioxidant system and sciatic nerve electrophysiology in the rat. Neurochem Res. 2008;33:2310–2317. doi: 10.1007/s11064-008-9730-9. [DOI] [PubMed] [Google Scholar]
- 7.Mehri S, Meshki MA, Hosseinzadeh H. Linalool as a neuroprotective agent against acrylamide-induced neurotoxicity in Wistar rats. Drug Chem Toxicol. 2014;21:1–5. doi: 10.3109/01480545.2014.919585. [DOI] [PubMed] [Google Scholar]
- 8.Yousef MI, El-Demerdash FM. Acrylamide-induced oxidative stress and biochemical perturbations in rats. Toxicology. 2006;219:133–141. doi: 10.1016/j.tox.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Lakshmi D, Gopinath K, Jayanthy G, Anjum S, Prakash D, Sudhandiran G. Ameliorating effect of fish oil on acrylamide induced oxidative stress and neuronal apoptosis in cerebral cortex. Neurochem Res. 2012;37:1859–1867. doi: 10.1007/s11064-012-0794-1. [DOI] [PubMed] [Google Scholar]
- 10.Mehri S, Abnous K, Mousavi S, Shariaty V, Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32:227–235. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Hosseinzadeh H, Nassiri-Asl M. Avicenna’s (Ibn Sina) the Canon of Medicine and saffron (Crocus sativus): a review. Phytother Res. 2013;27:475–483. doi: 10.1002/ptr.4784. [DOI] [PubMed] [Google Scholar]
- 13.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Det Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 15.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. J Med Plants. 2004;3:48–58. [Google Scholar]
- 16.Vahdati Hassani F, Naseri V, Razavi B, Mehri S, Abnous K, Hosseinzadeh H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. DARU. 2014;22:16. doi: 10.1186/2008-2231-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moshiri M, Vahabzadeh M, Hosseinzadeh H. Clinical applications of saffron (Crocus sativus) and its constituents: a review. Drug Res. 2015;65:287–95. doi: 10.1055/s-0034-1375681. [DOI] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H, Ziaei T. Effects of Crocus sativus stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J Med Plants. 2006;5:40–50. [Google Scholar]
- 19.Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–724. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinzadeh H, Sadeghnia HR. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of GABAergic and opioids systems. Phytomedicine. 2007;14:256–562. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Hosseinzadeh H, Shariaty VM. Anti-nociceptive effect of safranal, a constituent of Crocus sativus (saffron), in mice. Pharmacologyonline. 2007;2:498–503. [Google Scholar]
- 22.Amin B, Hosseinzadeh H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L. and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia. 2012;83:888–895. doi: 10.1016/j.fitote.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Mousavi SH, Tayarani NZ, Parsaee H. Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol. 2010;30:185–191. doi: 10.1007/s10571-009-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother Res. 2010;24:990–994. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 25.Hosseinzadeh H, Noraei NB. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother Res. 2009;23:768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 26.Rezaee R, Hosseinzadeh H. Safranal: From an aromatic natural product to a rewarding pharmacological agent. Iran J Basic Med Sci. 2013;16:12–26. [PMC free article] [PubMed] [Google Scholar]
- 27.Mehdizadeh R, Parizadeh MR, Khooei A-R, Mehri S, Hosseinzadeh H. Cardioprotective Effect of Saffron Extract and Safranal in Isoproterenol-Induced Myocardial Infarction in Wistar Rats. Iran J Basic Med Sci. 2013;16:56–63. [PMC free article] [PubMed] [Google Scholar]
- 28.Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Pharm Sci. 2005;8:387–393. [PubMed] [Google Scholar]
- 29.Hosseinzadeh H, Modaghegh MH, Saffari Z. Crocus sativus L. (saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid Based Complement Alternat Med. 2009;6:343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of α±tocopherol. Neurosci Lett. 2004;362:61–64. doi: 10.1016/j.neulet.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 31.Ochiai T, Soeda S, Ohno S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of PC-12 cells through sphingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem Int. 2004;44:321–330. doi: 10.1016/s0197-0186(03)00174-8. [DOI] [PubMed] [Google Scholar]
- 32.Hariri AT, Moallem SA, Mahmoudi M, Memar B, Hosseinzadeh H. Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: Protective effects of crocin and safranal. Food Chem Toxicol. 2010;48:2803–2808. doi: 10.1016/j.fct.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Hariri AT, Moallem SA, Mahmoudi M, Hosseinzadeh H. The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine. 2011;18:499–504. doi: 10.1016/j.phymed.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Hadizadeh F, Mohajeri S, Seifi M. Extraction and purification of crocin from saffron stigmas employing a simple and efficient crystallization method. Pak J Biol Sci. 2010;13:691–698. doi: 10.3923/pjbs.2010.691.698. [DOI] [PubMed] [Google Scholar]
- 35.Shukla PK, Khanna VK, Ali MM, Maurya RR, Handa SS, Srimal RC. Protective effect of Acorus calamus against acrylamide induced neurotoxicity. Phytother Res. 2002;16:256–260. doi: 10.1002/ptr.854. [DOI] [PubMed] [Google Scholar]
- 36.LoPachin RM, Ross JF, Reid ML, Das S, Mansukhani S, Lehning EJ. Neurological evaluation of toxic axonopathies in rats: Acrylamide and 2,5-hexanedione. NeuroToxicol. 2002;23:95–110. doi: 10.1016/s0161-813x(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 37.Razavi M, Hosseinzadeh H, Abnous K, Sadat Motamedahariaty V, Imenshahidi M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran J Basic Med Sci. 2012;16:64–72. [PMC free article] [PubMed] [Google Scholar]
- 38.Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 39.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 40.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 41.Tong GC, Cornwell WK, Means GE. Reactions of acrylamide with glutathione and serum albumin. Toxicol Lett. 2004;147:127–131. doi: 10.1016/j.toxlet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Awad ME, Abdel-Rahman MS, Hassan SA. Acrylamide toxicity in isolated rat hepatocytes. Toxicol In Vitro. 1998;12:699–704. doi: 10.1016/s0887-2333(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 43.Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem. 2003;51:4504–4526. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- 44.Alturfan AA, Tozan-Beceren A, Sehirli AO, Demiralp E, Sener G, Omurtag GZ. Resveratrol ameliorates oxidative DNA damage and protects against acrylamide-induced oxidative stress in rats. Mol Biol Rep. 2012;39:4589–4596. doi: 10.1007/s11033-011-1249-5. [DOI] [PubMed] [Google Scholar]
- 45.Mehri S, Shahi M, Razavi B, Vahdati Hassani F, Hosseinzadeh H. Neuroprotective effect of thymo-quinone in acrylamide-induced neurotoxicity in Wistar rats. Iran J Basic Med Sci. 2014;17:1007–1011. [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng YQ, Liu JX, Wang JN, Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 47.Onem G, Aral E, Enli Y, Oguz EO, Coskun E, Aybek H, et al. Neuroprotective effects of l-carnitine and vitamin E alone or in combination against ischemia-reperfusion injury in rats. J Surg Res. 2006;131:124–130. doi: 10.1016/j.jss.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Jin DP, Li C, Cong Y, Yang H, Zhang WX, Guan W, et al. Inhibitory effects of vitamin E on UVB-induced apoptosis of chicken embryonic fibroblasts. Cell Biol Inte. 2011;35:381–389. doi: 10.1042/CBI20090285. [DOI] [PubMed] [Google Scholar]


