Abstract
Objective(s):
Cutaneous Leishmaniasis is a common and endemic disease in Khorasan province in North-East of Iran. The pentavalant antimony (Sb V) is the mainstay of treatment that has many side effects and resistance to the drug has been reported. The microbicidal effect of ozone was proven in different microorganisms. Since there is no study in this respect and to achieve a low cost and effective treatment, we decided to evaluate the efficacy of ozone against promastigotes of Leishmania major, in vitro.
Materials and Methods:
Ozonated olive oil was prepared after production of ozone by bubbling ozone-oxygen gas produced by ozone generator through olive oil until it solidified. Promastigotes of L. major were cultivated in two phasic media. After calculation of the number of promastigotes, they were incubated with ozonated olive oil (0, 0.626, 0.938, 1.25, 2.5, 5, 10 mcg/ml) at 28 °c for 24 hr. Parasites survival percentage was evaluated using MTS and microscopic assay, and then compared with Glucantime and non-ozonated olive oil.
Results:
According to the results, there were significant differences in parasites survival percentage between ozonated olive oil and non-ozonated olive oil, at similar concentrations (P<0.001). Ozonated olive oil was more effective than Glucantime. According to MTS results, Glucantime and ozonated olive oil gel concentrations that are required to inhibit the growth of L. major promastigotes by 50% (IC50), were 165 and 0.002 mg/ml, respectively.
Conclusion:
Ozonated olive oil has in vitro activity against the promastigotes of L. major and this effect is dose dependent.
Keywords: In vitro, Leishmania major, Ozone, Promastigote
Introduction
Leishmaniasis is a group of infectious diseases that are caused by different species of parasites belonging to the Leishmania genus (1). Although leishmaniasis is estimated to cause the ninth largest disease burden among individual infectious diseases, it is largely neglected in considerations of tropical disease priorities (2, 3). Leishmaniasis is endemic in 15 out of 30 provinces of Iran (4) and a recent study showed that Iran is among the top ten countries with the highest estimated case counts that together account for 70 to 75% of global estimated Cutaneous Leishmaniasis (CL) incidence (5).
It is a complex disease manifesting as cutaneous, muco-cutaneous and visceral forms. Amongst these forms, CL is the most common type of the disease and could result in severe skin infection (6). The choice of the treatment is dependent on size, number, and location of lesions, Leishmania species and also the accessibility of treatment modalities (7).
Systemic agents against Leishmaniasis are expensive and limited to a few drugs with inconsistent efficacy and unacceptable side effects (8-10). There is not any treatment for all forms of the disease, so far. The first line of the treatment of CL in Iran is pentavalent antimony (SbV). Resistance to SbV containing drugs is now well established and it was found to occur in some regions (11-13). Few alternatives to SbV are available and the resistant parasites are cross-resistant to some of them (14). So, an effective topical drug would be valuable if it offers a safe and unsupervised treatment at a reasonable cost. Various forms of topical treatment serve as alternative drugs in the control of leishmaniasis (15).
Ozone, an allotropic form of oxygen in nature, has a molecular weight of 48 and a density of one and a half times that of oxygen, and consists of a large overload of energy in its molecule. It has been used in human medical practice since long to kill bacteria, fungi, some protozoa, to inactivate viruses and to control hemorrhages (16-19). Related clinical trials have shown little side effects following topical ozone therapy (20-22).
Since no previous study had been done to evaluate the efficacy of ozone therapy on leishmaniasis, we decided to determine the effect of olive oil, ozonated olive oil and meglumine antimoniate (Glucantime) on promastigotes of L. major, in vitro, compare their anti-leishmanial activity and determine the most effective dose of ozonated olive oil on promastigotes of L. major.
Materials and Methods
This basic interventional study was done at Research Center for Cutaneous Leishmaniasis. Here, the efficacy of ozonated olive oil and Glucantime against promastigotes of L. major was evaluated and was compared with each other.
Chemicals preparation
-
a)
Olive Oil (food grade), was obtained from Tarom industrial company, north of Iran.
Olive oil (150 g) was warmed in water bath at 30 °C. Oxygen gas containing about 200 ppm ozone was then bubbled through the olive oil at a rate of 1.0 l/min for 50 hr to give ozonated olive oil as vaseline with the distinctive odor of ozone. The ozonated olive oil was stored in a refrigerator. Then 3-4 drops Tween 80 was added as an emulsifier. This product was diluted using Phenol red-free RPMI 1640 medium supplemented with 5% PBS and was tested at the concentrations of 0, 0.626, 0.938, 1.25, 2.5, 5, 10 mcg/ml.
-
b)
Positive control: Glucantime (0, 45, 120, 150, 180 and 240 mg/ml) was prepared after dilution of Glucantime ampoule (1.5 g/5 ml, Aventis, France) as same as ozonated olive oil.
-
c)
Olive oil as a negative control: Olive oil was mixed with 3-4 drops of Tween 80 as an emulsifier. Then, the mixture was diluted as mentioned above to prepare different concentrations.
Parasite culture
A number of 3×106 promastigotes of L. major (MRHO/IR/75/ER) were injected subcutaneously into the tails of BALB/c mice. Splenectomy was done and amastigotes in mice spleen were cultured in Novy-MacNeal-Nicolle (N.N.N) medium containing Agar (4 mg/100 ml) and defibrinated rabbit blood (10%). After transforming from amastigotes form, the promastigotes were incubated in RPMI 1640 (HIMED IA; AT 028) supplemented with 100 units/ml penicillin, streptomycin 100 μg/ml and 20% FBS at 28 °C (23).
Seven days later, the parasites were transformed to a culture flask containing 5-10 ml complete culture medium. The parasites were cultured for 2-3 days and used when they were at the stationary growth phase.
Promastigotes survival assay
At first, 200 μl of promastigotes suspension at the density of 1× 10 7 parasite/ml was transferred to the wells of a 96-well plate and incubated at 28 °C for 24 hr. Then, incubation was continued in the presence of different concentrations of olive oil, ozonated olive oil or Glucantime for 3 hr. After 24 hr, promastigotes survival percentage was assessed using MTS solution ([4, 5-dimethylthiazol-2-yl]-5-(3-carboxymethoxyphenyl)-2H-tetrazolium, inner salt). Optical density of the samples was read by an ELISA Micro plate Reader (USA, STAT FAX 2100) at 492 nm and parasite survival was calculated for all samples in comparison with the control. Also, under similar conditions, the number of alive promastigotes treated with olive oil, ozonated olive oil and Glucantime was also determined using a hemocytometer slide under a light microscope in a separate experiment seri and the promastigotes survival percentage was calculated relative to control group. Since Glucantime with MTS produces a color like the color of reduced MTS produced by alive parasites, we subtracted its optical absorption from all experimental groups treated with Glucantime.
Statistical analysis
Statistical analysis was performed using SPSS software version 16 by Kolmogorov-Smirnov, One way ANOVA and Tukey statistical tests and Independent sample t-test. The P-values of lower than 0.05 were considered significant. IC50 value (the concentration that is required to inhibit the growth of L. major promastigotes by 50%) was calculated using linear regression analysis or linear interpolation for Glucantime and ozonated olive oil gel.
Results
Figures 1 and 2 show that increasing concentrations of ozonated olive oil and Glucantime led to a decrease in parasite survival and had an increasing leishmanicidal activity against L. major promastigotes which was significantly different among different concentrations (P<0.001). Olive oil alone had no significant leishmanicidal activity. Comparison of data related to similar concentrations of olive oil and ozonated olive oil revealed significant differences between the two groups (P<0.05) (Figure 1). The anti-leishmanial activity at the highest concentration of ozonated olive oil was significantly more than that of Glucantime (P<0.001).
Figure 1.
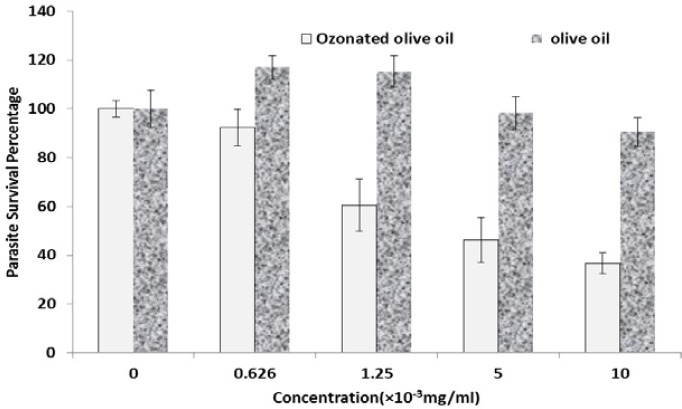
Comparison of mean parasite survival percentage in similar concentrations of ozonated olive oil and olive oil using MTS method. Data are shown as Mean±SD
Figure 2.
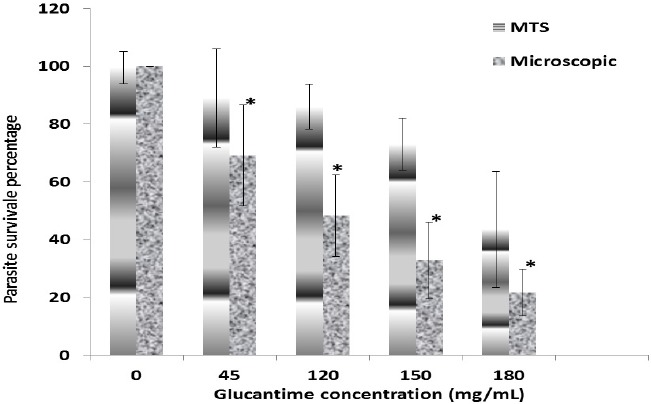
Promastigotes survival percentage after incubation with different concentrations of Glucantime. Data was obtained by microscopy and MTS assays
Considering mean alive promastigotes determined by MTS method and light microscope, there were significant differences among different concentrations of Glucantime and some concentrations of ozonated olive oil (P≤0.001 and P=0.01, respectively). As it has been shown in Figures 2 and 3, on the basis of the microscopic observations and MTS assay data, IC50 were 120 mg/ml and 165 mg/ml for Glucantim and almost 2 and 3.5 mcg/ml for ozonated olive oil.
Figure 3.
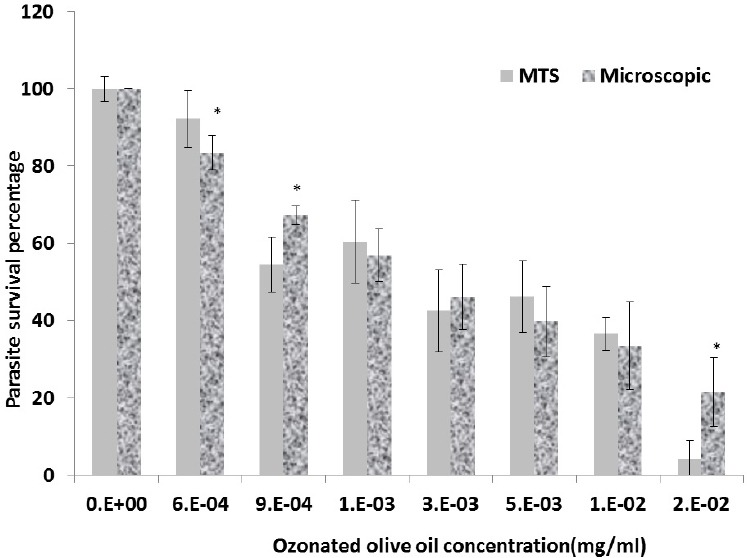
Promastigotes survival after incubation with different concentrations of ozonated olive oil as evaluated by microscopy and MTS assays. Data are expressed as Mean±SD
Discussion
Treatment of leishmaniasis is still dependant on pentavalent antimonial drugs, but the emergence of drug-resistant parasites shows treatment failures (11-13). So, seeking for a new inexpensive more effective safer drug which is preferably administered topically is a logical aim.
Ozone has been shown to be a powerful and reliable anti-microbial agent against bacteria, fungi, protozoa, and viruses. In a randomized study, ozonated sunflower oil was shown to be more effective than ketoconazole in the treatment of onychomycosis (22). Ozonated olive oil has germicidal action and was used in the treatment of tinea pedis (24). Inactivation of other pathogenic fungi like Candida albicans and Aspergillus nigerwas reported by Coronel et al (25). Also, ringworm infection caused by Trichophyton, Microsporum, and Epidermophyton species was studied by Gupta et al (26). Strong bactericidal activity of ozonated water against bacteria in plaque biofilm has been frequently reported in dentistry for improvement of periodontal diseases, chronic gingival disorders and oral hygiene (27). Moreover, Sechi et al noted an interesting antimicrobial activity for ozonated sunflower oil against Mycobacterium (28).
With respect to the toxic effects of ozone and its compounds, since there is no study on anti-leishmanial activity, we decided to evaluate the efficacy of this easy and low cost drug on promastigotes of L. major. In this study, ozonated olive oil was used against L. major, in-vitro. Ozonated water and ozonated olive oil are ideal delivery systems as they have the capacity to entrap and then release oxygen/ozone (27).
According to our results, ozonated olive oil was effective against promastigotes of L. major at different concentrations. Conspicuously, in comparison with Glucantime as positive control, mean alive promastigotes percentage following treatment with the highest concentration of ozonated olive oil was significantly less.
The mechanism that explains how ozone decreases the number of leishmania parasite has not been studied yet. But it is generally accepted that the oxidant potential of ozone induces the destruction of cell walls and cytoplasmic membranes of bacteria and fungi. The reaction of ozone with olive oil occurs almost exclusively with c-c double bonds present in unsaturated fatty acids and produces different toxic products such as several oxygenated compounds, hydroperoxide, polyperoxides, aldehydes, ozonide and diperoxides that might be responsible for wide antimicrobial activity of ozonated olive oil (29-31).
During this process, ozone attacks glycoproteins, glycolipids, and other amino acids and inhibits and blocks the enzymatic control system of the cell. The degradation of nucleic acid was parallel to what happened to enzymatic activities. This leads to an increasing in membrane permeability that plays a key role in cell viability, leading to prompt functional cessation. It lets ozone molecules penetrating to the cell resulting in the microorganism death (32).
The wide availability of olive oil makes ozonated olive oil an inexpensive accessible anti-microbial agent and hopefully an alternative (or adjunctive) treatment of cutaneous leishmaniasis.
Conclusion
The results showed anti-leishmanial effect of ozonated olive oil. We recommend more studies on intracellular amastigotes and in infected animal models.
Acknowledgment
We are grateful to the Research Council of Mashhad University of Medical Sciences for providing the financial grant and Mrs. Soodmand for her kind efforts during this project. The results presented in this work have been taken from Dr Fatemeh Abbasi’s thesis with the ID number 6660.
References
- 1.Desjeux P. Leishmaniasis: Current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, Sachs JD. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 2006;3:e102. doi: 10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez PJ, Remme JH, Buss P, Alleyne G, Morel C, Breman JG. Combating tropical infectious diseases: report of the Disease Control Priorities in Developing Countries Project. Clin Infect Dis. 2004;38:871–888. doi: 10.1086/382077. [DOI] [PubMed] [Google Scholar]
- 4.Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J. 2004;10:591–599. [PubMed] [Google Scholar]
- 5.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klaus SN, Frankenburg S, Ingber A. Epidemiology of cutaneous alaeishmaniasis. Clin Dermatol. 1999;17:257–260. doi: 10.1016/s0738-081x(99)00043-7. [DOI] [PubMed] [Google Scholar]
- 7.Global Health-Division of Parasitic Diseases and Malaria. Resources for health professionals. C. 2010. [updated 2010 Nov 2; cited 2012 Dec 2]. Available at: http://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html .
- 8.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 9.Coler RN, Reed SG. Second-generation vaccines against leishmaniasis. Trends Parasitol. 2005;21:244–249. doi: 10.1016/j.pt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Soto J, Toledo JT. Oral miltefosine to treat new world cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:7. doi: 10.1016/S1473-3099(06)70665-X. [DOI] [PubMed] [Google Scholar]
- 11.Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. Resistance to antimony and treatment failure in human leishmania (viannia) infection. J Infect Dis. 2006;193:1375–1383. doi: 10.1086/503371. [DOI] [PubMed] [Google Scholar]
- 12.Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 2006;3:e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pourmohammadi B, Motazedian MH, Handjani F, Hatam GH, Habibi S, Sarkari B. Glucantime efficacy in the treatment of zoonotic cutaneous leishmaniasis. Southeast Asian J Trop Med Public Health. 2011;42:502–508. [PubMed] [Google Scholar]
- 14.Hadighi R, Boucher P, Khamesipour A, Meamar AR, Roy G, Ouellette M, et al. Glucantime-resistant Leishmania tropica isolated from Iranian patients with cutaneous leishmaniasis are sensitive to alternative antileishmania drugs. Parasitol Res. 2007;101:1319–1322. doi: 10.1007/s00436-007-0638-0. [DOI] [PubMed] [Google Scholar]
- 15.Kivçak B, Mert T, Ertabaklar H, Balcioğlu IC, Ozensoy Töz S. In vitro activity of Arbutus unedo against Leishmania tropica promastigotes. Turkiye Parazitol Derg. 2009;33:114–115. [PubMed] [Google Scholar]
- 16.Sunnen G. The utilization of ozone for external medical applications. J Adv Med. 1998;1:159–174. [Google Scholar]
- 17.Gupta AK, Brintnell WC. Sanitization of contaminated footwear from onychomycosis patients using ozone gas: a novel adjunct therapy for treating onychomycosis and tinea pedis? J Cutan Med Surg. 2013;17:243–249. doi: 10.2310/7750.2012.12068. [DOI] [PubMed] [Google Scholar]
- 18.Lin YC, Wu SC. Effects of ozone exposure on inactivation of intra- and extracellular enterovirus 71. Antiviral Res. 2006;70:147–153. doi: 10.1016/j.antiviral.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Skalska K, Ledakowicz S, Perkowski J, Sencio B. Germicidal properties of ozonated sunflower oil. Ozone-Sci Eng. 2009;31:232–237. [Google Scholar]
- 20.Bocci V, Aldinucci C. Biochemical modifications induced in human blood by oxygenation-ozonation. J Biochem Mol Toxicol. 2006;20:133–138. doi: 10.1002/jbt.20124. [DOI] [PubMed] [Google Scholar]
- 21.Travagli V, Zanardi I, Silvietti A, Bocci V. A physicochemical investigation on the effects of ozone on blood. Int J Biol Macromol. 2007;41:504–511. doi: 10.1016/j.ijbiomac.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Menendez S, Re L, Falcon L, Argote MB, Mendez I, Fernandez D, et al. Safety of topical Oleozon® in the treatment of Tinea pedis: phase IV clinical trial. Int J Ozone Ther. 2008;7:55–59. [Google Scholar]
- 23.Sazgarnia A, Zabolinejad N, Layegh P, Rajabi O, Berenji F, Javidi Z, et al. Antileishmania activity of liposomal clarithromycin against leishmania major promastigotes. Iran J Basic Med Sci. 2012;15:1005–1014. [PMC free article] [PubMed] [Google Scholar]
- 24.Menéndez S, Falcón L, Simón DR, Landa N. Efficacy of ozonized sunflower oil in the treatment of tinea pedis. Mycoses. 2002;45:329–332. doi: 10.1046/j.1439-0507.2002.00780.x. [DOI] [PubMed] [Google Scholar]
- 25.Coronel B, Duroselle P, Behr H, Moskovtchenko JF, Freney J. In situ decontamination of medical wastes using oxidative agents: a 16-month study in a polyvalent intensive care unit. J Hosp Infect. 2002;50:207–212. doi: 10.1053/jhin.2002.1188. [DOI] [PubMed] [Google Scholar]
- 26.Gupta AK, Brintnell W. Ozone gas effectively kills laboratory strains of Trichophyton rubrum and Trichophyton mentagrophytes using an in vitro test system. J Dermatolog Treat. 2014;25:251–255. doi: 10.3109/09546634.2012.714456. [DOI] [PubMed] [Google Scholar]
- 27.Meena A, Trivedi HP, Gupta M, Parvez Sh, Likhyani L. Therapeutic applications of ozonated products. Int J Dent Clin. 2011;3:68–69. [Google Scholar]
- 28.Sechi LA, Lezcano I, Nunez N, Espim M, Duprè I, Pinna A, et al. Antibacterial activity of ozonized sunflower oil (Oleozon) J Appl Microbiol. 2001;90:279–284. doi: 10.1046/j.1365-2672.2001.01235.x. [DOI] [PubMed] [Google Scholar]
- 29.Mohammadi Z, Shalavi S, Soltani MK, Asgary S. A review of the properties and applications of ozone in endodontics: an update. Iran Endod J. 2013;8:40–43. [PMC free article] [PubMed] [Google Scholar]
- 30.Ledwa O. PhD. Thesis, National center for scientific research. Havana city: [Google Scholar]
- 31.Neveen SI, Geweel Y. Antifungal activity of ozonized olive oil (oleozone) Int J Agri Biol. 2006;8:670–675. [Google Scholar]
- 32.Azarpazhooh A, Limeback H. The application of ozone in dentistry: a systematic review of literature. J Dent. 2008;36:104–116. doi: 10.1016/j.jdent.2007.11.008. [DOI] [PubMed] [Google Scholar]


