Abstract
Objective(s):
There are supportive evidences about the possible role of latent Toxoplasma. gondii infections on the behavior and neurologic functions, such as increased dopamine levels in the brain. The aim of this study was to examine anti-toxoplasma activity of aripiprazole that is an atypical anti-psychotic drug in mice.
Materials and Methods:
Mice were randomly divided into four groups, including; control, vehicle, aripiprazole 10 mg/kg, and aripiprazole 20 mg/kg. The mice were inoculated intraperitoneally with mice brain suspension containing tissue cysts. At the end of second month, the number of cysts was counted in smears prepared from brain homogenate by optical microscope.
Results:
There was no significant difference between mean logarithms of brain cyst numbers of aripiprazole groups compared with control.
Conclusion:
Results indicate that in aripiprazole groups, the brain cystogenesis was not decrease. Further study needs to investigate the role of anti-psychotic drugs on T. gondii.
Keywords: Aripiprazole, Cysts, Toxoplasma gondii
Introduction
Toxoplasma gondii is a cosmopolitan obligate intracellular protozoan zoonosis which infects warm-blooded vertebrates (1). In immunocompetent hosts, cellular invasion of proliferative tachyzoites slows usually after a short period of acute phase of infection; afterwards, the parasite survives as a latent infection throughout life in tissues of hosts, especially in brain without remarkable inflammation around tissue cysts (2). The brain latent infections of T. gondii are considered one of the probable causative agents of chronic neurologic disorders with unknown etiology. Epidemiological studies have shown that seroprevalence of T. gondii is higher in patients with schizophrenia (3) in comparison to controls. Moreover, behavior and personality alterations have attributed to the chronic infections of T. gondii (4). Experimentally, there are evidences that support probable role of latent T. gondii infections in behavior and neurologic functions, such as the increasing of dopamine levels in the brain (5).
Evidence shows that some of anti-psychotic and anti-schizophrenic drugs have in vitro anti-toxoplasma activity. Valproic acid, an anticonvulsant mood-stabilizer (6), and fluphenazine and thioridazine, typical anti-psychotics (7) inhibit replication of T. gondii RH strain tachyzoites in cell culture (8). Our aim in this study was to investigate in vivo anti-toxoplasma activity of aripiprazole, which is an atypical anti-psychotic drug.
Materials and Methods
Parasite
Avirulent Tehran strain of T. gondii was kindly prepared by the Department of Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Iran. The strain was isolated in Iran from the lymph node aspirate of a lymphadenopathy patient (9). Mice were purchased from Razi vaccine and serum research institute, Karaj, Iran.
Animals
A total of 62 male BALB/c mice (20-25 g) were housed in groups of five per cage under standard laboratory conditions. They were kept at a constant room temperature (21±2 °C) under a normal 12L:12D regimen with free access to food and water. All animal experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) in such a way as to minimize the number of animals and their suffering.
Drugs
Powder of aripiprazole (Tamin pharmaceutical investment company, Iran) was dissolved in tween 80 and administrated IP to mice.
Treatment
Mice were randomly divided into four groups, including; Control (n=11), tween 80 as vehicle (n=16), aripiprazole 10 mg/kg (n=17), and aripiprazole 20 mg/kg (n=18). The mice were inoculated IP with 0.5 ml of mice brain suspension containing 50 tissue cysts.
Vehicle and aripiprazole were injected one day after inoculation of parasites and every other day for the first ten days and every two days until the end of the first month. Control mice did not receive any drugs.
At the end of the second month after inoculation, the mice were anesthetized with intraperitoneal injections of ketamine/xylazine (60 mg/kg and 6 mg/kg, respectively). Mice were sacrificed under anesthesia, and their brains were quickly removed. Then, brain homogenate was provided separately from whole brain of each mouse in 2 ml of saline. The number of cysts was counted in smears prepared from 200 µl of brain homogenate with 100× and 400× magnifications of optical microscope.
Data analysis
For normalizing the initial data, natural logarithm transformation was used. Data analysis was through Kolmogorov-Smirnov test, Analysis of Variance (ANOVA) and Tukey post hoc. A level of P<0.05 was considered significant.
Results
In our study, some of the mice died in weeks 1–3 after inoculation of brain suspension containing tissue cysts. Ten mice in each group were randomly examined for counting T. gondii brain cysts. Overall, the cysts were observed in brains of all mice; however, there was a remarkably intra-group and inter-group variation in numbers of cysts (Table 1).
Table 1.
Frequency of tissue cysts of Toxoplasma gondii in 200 µl of brain homogenate in different treatment groups of mice
| Mice number in each group | Number of cysts |
|||
|---|---|---|---|---|
| Control | Arip (LD) | Arip (HD) | Tween 80 | |
| 1 | 1 | 60 | 322 | 36 |
| 2 | 10 | 51 | 39 | 27 |
| 3 | 16 | 45 | 41 | 689 |
| 4 | 10 | 31 | 27 | 50 |
| 5 | 27 | 16 | 32 | 4 |
| 6 | 2175 | 17 | 30 | 49 |
| 7 | 37 | 40 | 31 | 9 |
| 8 | 77 | 9 | 748 | 7 |
| 9 | 44 | 6 | 89 | 15 |
| 10 | 26 | 81 | 12 | 70 |
| Mean±SEM | 242.3±215.8 | 35.6±7.7 | 137.1±73.8 | 95.6±66.3 |
Arip: aripiprazole; LD: Low dose (10 mg/kg); HD: High dose (20 mg/kg); Tween 80 as vehicle
As the numbers of cysts varied, the data was normalized. Normalized data analysis showed no significant difference between mean±SEM of brain cyst numbers in the two doses of aripiprazole compared to the control and vehicle groups, which is shown on a logarithmic scale in Figure 1.
Figure 1.
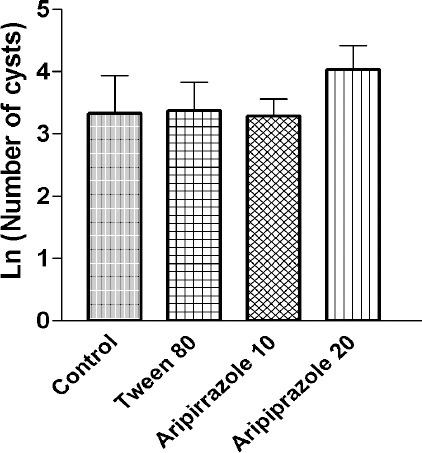
Mean±SEM logarithm of brain cysts numbers of Toxoplasma gondii in 200 µl of brain homogenate in different treatment groups of mice
Discussion
In our study, aripiprazole had no inhibitory effect on brain cystogenesis of T. gondii in vivo. The results corresponds with results of Goodwin et al who showed valproic acid is ineffective on brain cysts of T. gondii in mice (10). On the other hand, these findings are inconsistent with in vitro results of other studies (6, 11) which showed valproic acid has an inhibitory effect against tachyzoites of T. gondii in cell culture. In other studies of Goodwin et al, thioridazine and fluphenazine decreased proliferation of T. gondii tachyzoites in vitro (7).
Experimentally and clinically, anti-Toxoplasma effect of anti-psychotic drugs has not been demonstra-ted to date. There is a main difference in design of our study and Goodwin et al (10). In the present study, aripiprazole was injected in acute phase of infection in order to prevent tissue cystogenesis in mice, whereas, they administrated valproic acid orally in latent phase of infection for elimination of the tissue cysts. In the acute phase of toxoplasma infection, the bradyzoites convert to rapidly dividing tachyzoites that are sensitive to the anti-Toxoplasma drugs. In contrast, in the latent phase of infection, the proliferative tachyzoites converted to slowly dividing bradyzoites enclosed by cyst wall, which can persist for long-time in host tissue and are impervious against the anti-Toxoplasma drugs. Therefore, evidence obtained in our study is a strong support for the results of the previous study (10): anti-psychotic drugs have no anti-Toxoplasma effect in vivo. Also, brain cystogenesis capacity of T. gondii shows a remarkable variation in mice experimentally infected with this parasite, as observed in the present study and others (12).
The mechanism of action of anti-toxoplasmosis drugs is established. But, this mechanism about anti-psychotic drugs is unclear. In this study, we have used aripiprazole, an atypical anti-psychotic drug, which unlike typical anti-psychotic drugs, is a partial D2-receptor agonist and also has therapeutic efficacy through its 5-HT 2A antagonism and possibly 5-HT 1A partial agonism (13).
On the other hand, an increase in intracellular calcium occurs in tachyzoites of T. gondii when they attach to their host cells and this increase is required for invasion. Based on the results, it seems that initial attachment of tachyzoites to host cells is followed by calcium signaling (14) and this interaction was also inhibited by calcium channel blockers (verapamil) and calmodulin antagonists such as trifluoperazine and calmidazolium (15). Previously, it was reported that clozapine as a prototype agent in the atypical class, was not calmodulin antagonist and it is suggested that some of its action may be dependent on calmodulin-activated kinase activity (7, 16).
Furthermore, clozapine in protecting host cell against tachyzoites of T. gondii was not effective in vitro (7). It is possible that aripiprazole similar to clozapine is not antagonist of calmodulin to inhibit entry of tachyzoites to host cells. On the other hand, the mechanism of clozapine and aripiprazole in the atypical group are not same. It was shown that the ratio of affinity for D2 receptors to affinity for 5-HT2A receptors for aripiprazole is medium and this ratio for clozapine is very low (16). Also, in contrast to clozapine, aripiprazole as a blocker of 5-HT2A receptors could reduce intracellular Ca2+ levels in rat pituitary cell line (17). Thus, it seems that aripiprazole with chemical structure of dihydrocarbostyril has different effects on Ca2+ intracellular.
Conclusion
Results show that aripiprazole groups could not decrease the brain cystogenesis. Further studies need to clear the role of differently structured anti-psychotic drugs in treatment of T. gondii.
Acknowledgment
This work was supported by Qazvin University of Medical Sciences. The authors are thankful to the Vice Chancellor of Research of Qazvin University of Medical Sciences for their financial support. The authors are kindly appreciate Dr H Keshavarz, and Dr S Shojaee, Department of Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences for preparing an avirulent Tehran strain of T. gondii. The results reported in this paper were part of the thesis of N Samadzadeh.
References
- 1.Montoya JG, Boothroyd JC, Kovacs JA. Toxoplasma gondii. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 7th ed. Philadelphia: Churchill Livigstone; 2010. pp. 3495–3526. [Google Scholar]
- 2.Dubey JP. Tissue cyst tropism in Toxoplasma gondii: a comparison of tissue cyst formation in organs of cats, and rodents fed oocysts. Parasitology. 1997;115:15–20. doi: 10.1017/s0031182097008949. [DOI] [PubMed] [Google Scholar]
- 3.Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38:642–647. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegr J. Influence of latent Toxoplasma infection on human personality, physiology and morphology: pros and cons of the Toxoplasma-human model in studying the manipulation hypothesis. J Exp Biol. 2013;216:127–133. doi: 10.1242/jeb.073635. [DOI] [PubMed] [Google Scholar]
- 5.Stibbs HH. Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann Trop Med Parasitol. 1985;79:153–157. doi: 10.1080/00034983.1985.11811902. [DOI] [PubMed] [Google Scholar]
- 6.Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62:237–244. doi: 10.1016/s0920-9964(02)00357-2. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin DG, Strobl JS, Lindsay DS. Evaluation of five antischizophrenic agents against Toxoplasma gondii in human cell cultures. J Parasitol. 2011;97:148–151. doi: 10.1645/GE-2536.1. [DOI] [PubMed] [Google Scholar]
- 8.Asgari Q, Keshavarz H, Shojaee S, Motazedian MH, Mohebali M, Miri R, et al. In vitro and in vivo potential of RH strain of Toxoplasma gondii (Type I) in tissue cyst forming. Iran J Parasitol. 2013;8:367–375. [PMC free article] [PubMed] [Google Scholar]
- 9.Ghorbani M, Samii AH. Toxoplasmic lymphadenitis in Iran. J Trop Med Hyg. 1973;76:158–160. [PubMed] [Google Scholar]
- 10.Goodwin DG, Strobl J, Mitchell SM, Zajac AM, Lindsay DS. Evaluation of the mood-stabilizing agent valproic acid as a preventative for toxoplasmosis in mice and activity against tissue cysts in mice. J Parasitol. 2008;94:555–557. doi: 10.1645/GE-1331.1. [DOI] [PubMed] [Google Scholar]
- 11.Strobl JS, Cassell M, Mitchell SM, Reilly CM, Lindsay DS. Scriptaid and suberoylanilide hydroxamic acid are histone deacetylase inhibitors with potent anti-Toxoplasma gondii activity in vitro. J Parasitol. 2007;93:694–700. doi: 10.1645/GE-1043R.1. [DOI] [PubMed] [Google Scholar]
- 12.Berenreiterová M, Flegr J, Kuběna AA, Němec P. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS One. 2011;6:e28925. doi: 10.1371/journal.pone.0028925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meltzer H. Anti-psychotic Agents and Lithium. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and clinical pharmacology. New York: McGraw-Hill; 2010. pp. 362–383. [Google Scholar]
- 14.Vieira MC, Moreno SN. Mobilization of intracellular calcium upon attachment of Toxoplasma gondii tachyzoites to human fibroblasts is required for invasion. Mol Biochem Parasitol. 2000;106:157–162. doi: 10.1016/s0166-6851(99)00182-6. [DOI] [PubMed] [Google Scholar]
- 15.Pezzella-D’Alessandro N, Le Moal H, Bonhomme A, Valere A, Klein C, Gomez-Marin J, et al. Calmodulin distribution and the actomyosin cytoskeleton in Toxoplasma gondii. J Histochem Cytochem. 2001;49:445–454. doi: 10.1177/002215540104900404. [DOI] [PubMed] [Google Scholar]
- 16.Ninan I, Jardemark KE, Liang X, Wang RY. Calcium/calmodulin-dependent kinase II is involved in the facilitating effect of clozapine on NMDA- and electrically evoked responses in the medial prefrontal cortical pyramidal cells. Synapse. 2003;47:285–294. doi: 10.1002/syn.10175. [DOI] [PubMed] [Google Scholar]
- 17.Stark AD, Jordan S, Allers KA, Bertekap RL, Chen R, Mistry Kannan T, et al. Interaction of the novel anti-psychotic aripiprazole with 5-HT1A and 5-HT 2A receptors: functional receptor-binding and in vivo electrophysiological studies. Psychopharmacology (Berl) 2007;190:373–382. doi: 10.1007/s00213-006-0621-y. [DOI] [PubMed] [Google Scholar]


