Abstract
Over the past two decades, healthcare-associated exposure has increasingly been proved to be a means of hepatitis C virus (HCV) transmission, especially in hemodialysis units. The prevalence of HCV among hemodialysis patients is known to be several times greater than that of the general population of the United States and chronic HCV infection is associated with significant morbidity and mortality among these patients. During 2008–2011, HCV infection outbreaks were identified in multiple US hemodialysis facilities, resulting in at least 46 new HCV infections among hemodialysis patients. These outbreaks, linked to infection control breaches, also highlight the failure of some facilities to follow established guidelines for routine HCV antibody (anti-HCV) screening and response to new HCV infection among hemodialysis patients. Current national guidelines recommend screening of hemodialysis patients for anti-HCV on facility admission and, for susceptible patients, on a semiannual basis.
Here, we seek to underscore the importance of compliance with national recommendations for anti-HCV screening of hemodialysis patients and actions to be taken in the event of possible HCV transmission within a hemodialysis facility. These include general steps to ensure that: hemodialysis patients are routinely screened for anti-HCV to facilitate early detection of new infections; newly infected patients are informed of the change in their HCV status and undergo clinical evaluation; and public health officials are notified of new HCV infections in a timely manner. We then focus on the need to assess infection control practices at the facility, with particular attention given to safe handling of injectable medications, hand hygiene and disinfection practices.
In the absence of a vaccine, routine screening and adherence to standard infection control practices will remain the key strategies for preventing HCV transmission in hemodialysis units.
Keywords: Hepatitis C Virus (HCV), Hemodialysis, Screening, Infection Control, Medication, Bloodborne Pathogen
Background
Hepatitis C virus (HCV) infection affects an estimated 2.7–3.9 million persons (1.0–1.5%) in the United States (1) and is associated with potentially serious health consequences, including liver cirrhosis and hepatocellular carcinoma (2). Identifying new HCV infections is often difficult, as most newly-infected patients tend to be asymptomatic (2–4). The risk of acquiring HCV infection is known to be high among injection-drug users (3, 4), since the virus is transmitted efficiently through intravenous exposure (5). However, healthcare-associated exposure has increasingly been linked to HCV transmission over the past two decades in the United States (6). Transmission of HCV in a variety of healthcare settings, including hemodialysis units, has been well documented (7–10).
Maintenance hemodialysis patients can be at increased risk of exposure to bloodborne pathogens, such as HCV, in the course of receiving treatment owing to several factors, including the need for repeated and prolonged access to the bloodstream and concurrent treatment of multiple patients in the same area. The prevalence of HCV among US hemodialysis patients (approximately 8–10%) (11–13) is also known to be several times higher than the general US population (1). Several outbreaks of HCV have occurred in hemodialysis units across the US, triggering notification of hundreds of patients. Of 13 healthcare-associated HCV outbreaks in the United States during 2008–2011 that were reported to the United States Centers for Disease Control and Prevention (CDC), five (38.5%) occurred in hemodialysis facilities, with 46 new HCV infections documented (14). While the exact mechanism of HCV transmission could not be ascertained in each facility where an outbreak occurred, several breaches in infection control practices, particularly improper injection-medication practices and suboptimal cleaning and disinfection, were identified. Investigations using molecular analysis of the viruses from affected patients, together with epidemiologic data, have demonstrated the spread of infection among patients treated in the same facility (15). These findings indicate that despite the availability of prevention guidelines (16), HCV transmission among patients receiving hemodialysis remains a problem. In the course of investigating outbreaks, some of the affected facilities were noted to be non-adherent with established guidelines for screening and managing hemodialysis patients for new HCV infection (17, 18). Because most new HCV infections are asymptomatic (2–4), routine screening of hemodialysis patients is crucial for the prompt identification of new infections and outbreaks among these patients.
CDC has developed and disseminated recommendations addressing practices to help prevent the transmission of bloodborne pathogens and bacterial infections in hemodialysis units (16). With respect to the detection of HCV transmission, routine screening of hemodialysis patients for antibody to HCV (anti-HCV) is recommended on facility entry (at the time of first admission), and subsequently, for anti-HCV susceptible patients, on a semiannual basis. The guidelines also recommend monthly alanine transaminase (ALT) testing for hemodialysis patients, to facilitate early detection of new HCV infections and assist in understanding the likely period of exposure. ALT elevations occur in a majority of new HCV infections and often precede HCV seroconversion (19–24). Routine HCV antibody screening for hemodialysis patients is also recommended by Kidney Disease Improving Global Outcomes (KDIGO) (25), and is included in the Department of Health and Human Services National Action Plan to Prevent Healthcare-Associated Infections (HAI) (26), which identifies HCV screening as a priority recommendation for advancing the prevention of healthcare-associated infections in end-stage renal disease facilities.
This paper outlines steps that should be taken by hemodialysis facilities to 1) ensure that new HCV infections are not occurring among patients receiving treatment within the hemodialysis unit; 2) detect new infections, if they occur, and manage patients in a timely manner; and 3) forestall or interrupt potential intra-facility transmission of the virus. The actions to be taken include identification of both acute hepatitis C infection (i.e., persons with signs and symptoms of inflammation of the liver, such as jaundice, nausea, anorexia and fever, often accompanied by elevated liver enzyme levels) and HCV seroconversion (i.e., identified change in serologic status from anti-HCV negative to positive, with or without symptoms of acute hepatitis C infection) among patients. Specific guidance is also provided on how to manage HCV screening results and assess infection control practices in the context of possible HCV transmission within the facility.
Managing Hepatitis C Virus Seroconversions
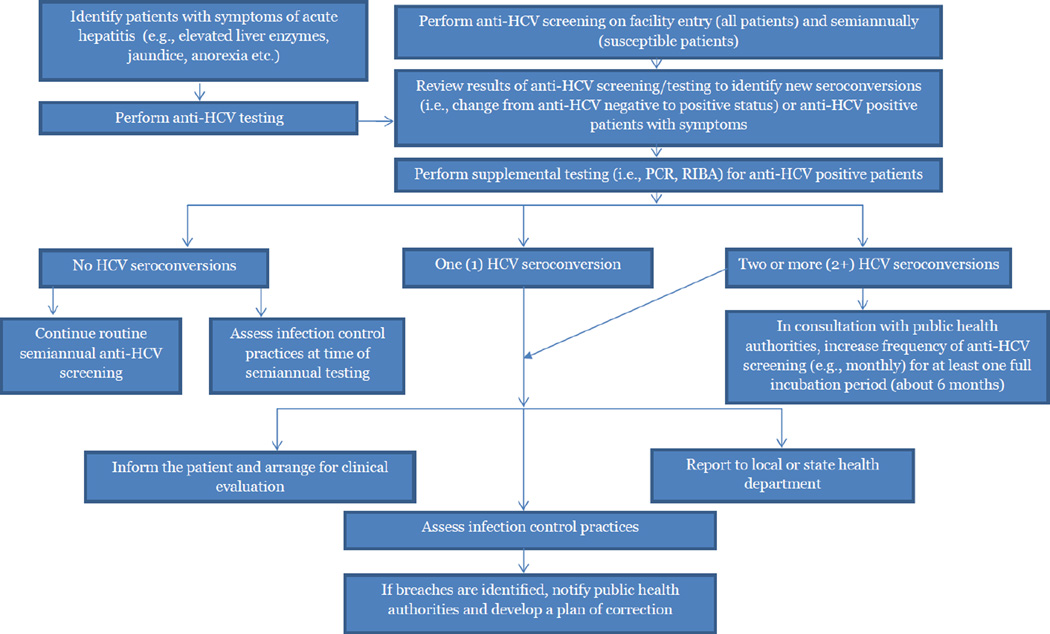
General Steps (Figure 1)
Figure 1.
Algorithm for Managing Hepatitis C Virus Screening Results and Cases In Hemodialysis Facilities
All hemodialysis facilities should establish a policy for screening patients for anti-HCV. Screening should occur on facility admission and subsequently, for HCV susceptible patients, on a semiannual basis. The intent of this practice is to establish each patient’s baseline anti-HCV status (i.e., at the time of first entry to the facility), and by routinely comparing susceptible patients’ baseline status to the results of their semiannual HCV screening, help providers to identify if an HCV seroconversion (i.e., a new HCV infection) occurs. In addition, patients should undergo monthly screening for serum ALT levels (16). The presence of symptoms consistent with acute hepatitis C infection or an unexplained elevation in ALT level should prompt HCV testing (27), whether or not six months have passed since the patient’s last anti-HCV test.
Patients who are anti-HCV positive on facility admission (based on admission testing, known history of infection, or documentation of prior positive test result) should be considered already infected, i.e., non-susceptible, and do not require continued routine anti-HCV screening. However, such patients should be evaluated and managed by appropriate clinical specialists. In the event that an HCV seroconversion or acute hepatitis C infection is identified in a previously susceptible patient (i.e., a patient who was anti-HCV negative on facility entry), the following steps should be taken:
Confirm HCV Status
The first step should be to confirm that the positive anti-HCV test result represents a new HCV infection. To accomplish this, all previous anti-HCV screening records for the patient should be reviewed to ensure there was no previously documented positive test result. In the event that there was no previous positive anti-HCV test result for the patient, i.e., this represents the first known positive anti-HCV test result, the patient should then undergo supplemental laboratory testing using a more specific test such as nucleic acid testing, i.e., polymerase chain reaction (PCR), and/or recombinant immunoblot assay (RIBA) to prevent reporting of a false-positive result (16, 28). If the supplemental test is positive, the patient should be considered infected with HCV. If the supplemental test is negative, then the anti-HCV positive test results should be considered a false-positive and the patient considered uninfected and susceptible to HCV infection. Routine anti-HCV screening should continue for such a patient on a semiannual basis (16). If the supplemental test is indeterminate, then the presence of a new HCV infection in such a patient cannot be established. In such cases, additional testing as well as monitoring of the patient’s liver enzymes (i.e., serum ALT) should be performed (28, 29).
In making a determination of which supplemental test to use, facilities should note that in recent years RIBA has been available on a limited basis, and nucleic acid testing, i.e., PCR, is now widely available and routinely used to confirm current viremia in infected patients (3). Because only a small proportion of hemodialysis patients have viremia in the absence of antibodies (12), it is not necessary to screen all patients initially with a nucleic acid test. A table to aid with the interpretation of HCV screening results has been developed by CDC (28), and is subject to updates to reflect changing testing practices and test availability.
Inform the Patient and Arrange for Clinical Evaluation
Owing to the benefits of preventive services and early clinical intervention (30), as well as the related morbidity and mortality associated with HCV infection in persons with renal disease (3, 30), a clinician from the hemodialysis facility should inform the patient if they have been found to have HCV infection. The patient should undergo clinical evaluation by an appropriate medical specialist (e.g., hepatologist, infectious disease specialist) for lifestyle counseling, monitoring, and possible treatment (3). Prompt identification of new HCV infection, referral to care, and early treatment – where indicated – have been shown to improve patient outcomes (30–33).
Report the Case(s) to the Health Department
Once a new, confirmed HCV infection at a hemodialysis facility has been identified, it should be promptly reported to the local or state health department (29). The Council of State and Territorial Epidemiologists (CSTE) recommends that healthcare providers and institutions report all cases of acute hepatitis C infection to public health authorities to facilitate disease surveillance and outbreak detection (34). Moreover, many states have laws requiring the reporting of outbreaks to public health authorities and reporting of viral hepatitis seroconversions to public health authorities is required by regulation as outlined in the Centers for Medicare & Medicaid Services ESRD Program Interpretative Guidance (35). Facilities reporting new HCV infections should also be aware that reporting such infections does not necessarily imply healthcare-associated transmission of the virus, but the practice offers the opportunity to receive the assistance of public health experts in evaluating the possibility of such transmission as well as establishing the presence of additional patient risk factors for HCV infection. Health departments help to: establish the patient’s past HCV testing history through review of public health surveillance records and hepatitis registry data; provide expertise in conducting patient interviews to identify the presence of other potential healthcare exposures or behavioral risk factors for HCV infection; initiate necessary epidemiologic investigations; and provide guidance and expertise on assessing infection control practices at the facility.
If public health evaluation of newly infected patients rules out behavioral risk factors and other opportunities for HCV exposure outside the dialysis facility, the possibility of healthcare-associated transmission should be given strong consideration. This is particularly important when infection control breaches are identified at the facility (as outlined in the following section) or when more than one new HCV infection is identified in a facility. In these instances, other at-risk patients at the facility should be notified and counseled on the need for testing (36).
Assess Infection Control Practices at the Facility
When one or more new HCV infections are identified, evaluating the potential for HCV transmission within the facility is important. A thorough infection control assessment should be conducted. Infection control remains the primary strategy for prevention of healthcare-associated HCV transmission and isolation of HCV-positive patients is not recommended in hemodialysis units (16).
The infection control assessment could be conducted by an infection preventionist in an affiliated hospital or a hemodialysis facility staff member with expertise in infection control. The latter could be a registered nurse (RN) with infection control training, in line with the proposal contained in the CMS Conditions for Coverage for End-Stage Renal Disease Facilities (37). If the facility does not have access to healthcare personnel with the proper training or certification, an infection preventionist could be hired on a consultant basis. The health department can be a resource to help in identifying such individuals and should also have the opportunity to guide or participate in infection control assessments in the setting of possible transmission.
Outlined below are the steps to be taken when conducting an infection control assessment in the context of identifying one or more patients with new HCV infection at the facility. These steps should also be undertaken routinely; for example, to coincide with periods when semiannual anti-HCV screening is conducted for patients at the facility, even in the absence of new HCV infection, to ensure ongoing compliance with best practices for infection control and prevention. When infection control breaches are noted, a plan of correction and a system for ensuring compliance with the plan should be developed within the dialysis unit. This might include educating staff to reinforce proper practices.
CDC has developed a free continuing education course on infection prevention that might be a helpful resource (38). The one-hour self-guided course is targeted at outpatient hemodialysis healthcare personnel, including nurses and technicians, and addresses basic infection control concepts and recommendations, such as medication safety and hand hygiene practices.
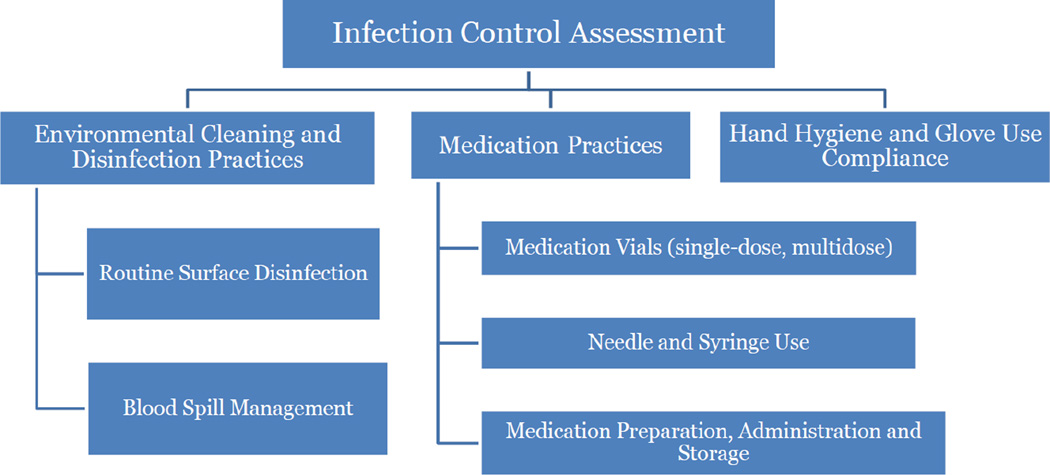
Infection Control Assessment (Figure 2)
Figure 2.
Guidance on Assessing Key Infection Control Practices in the Setting of Possible HCV Transmission in a Hemodialysis Unit
Medication Preparation and Administration
Transmission of bloodborne viruses and other pathogens has occurred in a variety of healthcare settings, including hemodialysis units, when healthcare personnel have failed to use aseptic technique and follow safe injection practices when preparing and administering parenteral medications. Improper medication practices have been identified as an independent risk factor for increased HCV prevalence in hemodialysis facilities (12). Unsafe practices that have resulted in HCV transmission in healthcare settings include: preparing medications in contaminated areas, using the same syringe to administer medication to more than one patient, and reusing a syringe to access a medication container and then using leftover contents from that container for additional patients (6).
Prepare Medications in a Clean Area Designated for Parenteral Medication Preparation and Storage
To minimize the risk of intra-facility transmission of HCV and other bloodborne pathogens, a clean area should be designated for parenteral medication preparation and storage (16). (The term “medications” should be interpreted to include intravenous fluids and saline flushes.) This area should be clearly delineated from all potentially contaminated areas and equipment in the dialysis unit and should be appropriately organized for the purpose of medication preparation (i.e., having bins of sterile, individually wrapped syringes and needles at hand, along with appropriate waste containers for needles and syringes). Medications should not be kept or prepared at the dialysis station as this is considered a contaminated area. Preferably, a separate clean room should be designated and used for medication preparation and storage, as this constitutes the safest approach with lowest likelihood of cross-contamination during medication preparation.
Once medications have been drawn, they should be delivered to each individual patient station separately. Mobile medication carts should not be used to deliver medications to patient stations, nor should medications move from one station to another, as this has been associated with HCV transmission in dialysis units (12, 15).
Use a Sterile Syringe and Sterile Needle for Each Injection and Each Entry into a Medication Container
Needles and syringes are sterile items intended for single use. A sterile needle and sterile syringe should be used for each injection and each entry into a medication container. Reuse of needles and syringes either for more than one patient or to reenter a medication container constitutes an improper and dangerous medication practice, which has been linked to the transmission of bloodborne pathogens, including HCV (39, 40).
While the danger associated with reusing needles is often easy to appreciate, several healthcare providers still underestimate the risk of bloodborne pathogen transmission associated with reusing syringes (6, 39). Contamination of syringes – after their use to administer a medication – has been well demonstrated, and the risk of backflow of blood and contamination of the syringe remains present even with the use of intervening lengths of IV tubing or valves (between the injection port and the patient) (6, 41). Changing the needle or maintaining positive pressure on the syringe plunger do not protect against contamination of the syringe (6). In the event that a contaminated syringe is used to access a medication container, the container also becomes contaminated and this could lead to the transmission of infections to a large number of patients if contents of that container are used for additional patients (42).
To assess compliance with safe injection practices, staff should be directly observed when preparing and administering medications using a standardized assessment tool (43, 44). Unsafe practices should be immediately corrected. Further, reuse of syringes, either for more than one patient or to access shared medications, should be reported immediately to public health authorities as this unsafe practice should result in notification and bloodborne-pathogen testing of potentially exposed patients.
Hand Hygiene Compliance and Glove Use
Bloodborne pathogens and other microorganisms may be transmitted directly from the hands of healthcare personnel to patients, or indirectly from patient to patient through the hands of healthcare personnel, if hand hygiene is not performed or is not done using the proper technique (45, 46). Hand hygiene refers to activities, such as handwashing, the use of an alcohol-based hand rub, and surgical antisepsis, which are intended to remove microorganisms from the hands (47).
Several improper practices have been noted among healthcare personnel with respect to hand hygiene and glove use. Healthcare personnel sometimes fail to perform hand hygiene before performing procedures such as preparation or administration of parenteral medications (48). Others have been observed wearing a fresh pair of gloves without performing hand hygiene after removal of used gloves, caring for more than one patient while wearing the same pair of gloves, or accessing clean supplies with contaminated gloves (15, 17). All the aforementioned practices increase the potential for disease transmission and should be corrected if observed during an infection control assessment.
Due to the inherent potential for exposure to blood and body fluids in dialysis units, healthcare personnel are required to wear gloves during routine patient-care activities at the dialysis station (16). The gloves are intended to provide protection for healthcare personnel; they are not intended as a substitute for proper hand hygiene. Hand hygiene should be performed whenever contact with the patient or with individual surfaces at the treatment station is anticipated. Also, hand hygiene should be performed after gloves are removed. Inadvertent contact with body fluids and just before performing an aseptic procedure are other examples of when hand hygiene is indicated (49).
In addition to audits performed during an assessment of suspected HCV transmission, hemodialysis facilities should routinely monitor staff adherence to proper hand hygiene and glove changing practices by performing hand hygiene and glove-use audits at least monthly. When performed, audits should be conducted in a way that directly measures compliance among individual healthcare workers whenever hand hygiene is indicated over a specified time period (47, 50). For example, a two-hour audit could be performed on the main treatment floor of the dialysis unit to assess hand hygiene compliance levels among healthcare personnel before and after performing procedures on patients. Such an audit will quantify the total number of times hand hygiene is properly performed over the number of times it was indicated in the course of carrying out patient-care activities (50). Similar methods could be used to assess glove use practices amongst healthcare personnel (50, 51). The services of an external auditor, such as a certified infection preventionist, could be solicited to carry out the audit. Audits should be performed at different times of the day, across shifts and during changeover periods. In the setting of potential HCV transmission, special attention should be paid to lapses in hand hygiene occurring between periods of caring for different patients and working at different patient stations (e.g., healthcare personnel fails to perform hand hygiene after direct contact with a patent or surfaces at that patient’s treatment station and prior to direct contact with another patient).
Environmental Cleaning and Disinfection Practices
HCV is capable of surviving in the environment and remaining viable for at least 16 hours (52) and has been isolated from contaminated surfaces and equipment in healthcare settings (53, 54). Therefore, suboptimal surface cleaning and disinfection in healthcare settings could result in transmission of HCV (15). Cleaning and disinfection involves not only surfaces within the environment, but also shared medical equipment, such as blood glucose meters and blood pressure cuffs. Surfaces and equipment should be considered contaminated following use or once they have come into contact with a patient, even in the absence of visible blood or soiling. It is a common misconception that surfaces or equipment are not contaminated when there is no visible blood or soiling. Microscopic quantities of blood or other traces of contamination may be sufficient to transmit infections.
To create a safe environment for providing patient care, all hemodialysis units should be cleaned and disinfected using recommended agents. All surfaces in the dialysis station should be routinely disinfected after a patient receives treatment and before commencing treatment for a new patient. To facilitate thorough cleaning and disinfection and reduce the opportunity for cross-contamination, healthcare personnel should ensure that the patient who has just received treatment has left the dialysis station before commencing routine disinfection of that station. Several HCV outbreaks have shown an epidemiologic link between incident and prevalent HCV cases who received treatment on sequential shifts at the same dialysis station or treatment section. This observation highlights the role of proper disinfection of the station during station turnover to prevent the transmission of bloodborne pathogens in dialysis units. Whenever feasible, there should be a distinct shift changeover period (i.e., separation in patient treatment shifts) devoted to cleaning and disinfection activities, as well as preparing the dialysis stations for patients on the next shift (15). Disinfection should be performed using an EPA-registered hospital disinfectant (55) at the dilution specified by the manufacturer. A single step involving mechanical wiping is sufficient for routine disinfection of surfaces, except in the event of visible soil. When there is visible soil, a cleaning step (which may occur in multiple phases, depending on the amount of soil), should precede disinfection of surfaces. Shared equipment, such as blood glucose meters and blood pressure cuffs, should be cleaned and disinfected between each patient use, following the manufacturer’s instructions.
To help ensure compliance with recommended practices, the facility should have in place written protocols that delineate clinical and housekeeping staff roles and responsibilities with respect to cleaning and disinfection of the environment. This should include guidance for how clinical staff should manage blood spills.
To assess compliance of environmental cleaning and disinfection practices with recommended guidelines, a staff member at the dialysis facility with certification in infection control should observe cleaning and disinfection practices on the treatment floor. The staff member should be attentive to all the steps involved in the process and ensure that the proper agents and techniques are employed in the process. In particular, attention should be devoted to observing the rinsing/wiping and drying of external surfaces of the dialysis machine and priming buckets between treatment sessions, to ensure that all surfaces are adequately cleaned and disinfected. It is preferable for bleeding patients onto the dialysis machine to not be practiced, as it can lead to blood-contamination of priming buckets. In the setting of suspected HCV transmission, pay particular attention to hurried station turnover procedures or other processes that could facilitate cross-contamination or incomplete disinfection of surfaces at the treatment station. The CDC has developed an environmental disinfection checklist to help facilitate assessment of facility cleaning and disinfection practices (56).
Multiple HCV Seroconversions
Healthcare-associated transmission of HCV – even in the event of a single confirmed case – constitutes an urgent public health situation, which should be thoroughly investigated and appropriately responded to (6, 16, 39), using the steps outlined above. Identification of multiple (i.e., two or more) new HCV infections at the same dialysis facility may represent ongoing transmission or signal a wider breach in infection control practices. For example, in 2008, three new HCV seroconversions occurring within weeks of each other were identified in a hemodialysis unit in New York (18). Further investigation of patients with HCV infection at the facility revealed there were six additional patients with HCV seroconversions that occurred at the facility from 2001 to 2008. HCV infection in four patients was subsequently linked by epidemiologic and laboratory investigation to previously infected patients at the same facility. Numerous infection control breaches were noted at the facility during the response to the outbreak, including failures to implement routine HCV screening and review of results. This example illustrates prolonged patient-to-patient transmission of HCV resulting from the facility’s failure to review and act on HCV screening test results in real time.
Due to the potential for ongoing transmission and risks to patient safety, additional steps are recommended for detection when more than one new HCV infection occurs within a dialysis facility. In such instances, the frequency of anti-HCV screening should be increased (e.g., implement monthly or quarterly screening) until no new infections have been identified for a period of six months (one full incubation period) (16). Aggressive infection control practices and ongoing monitoring of adherence should also be instituted in such facilities. These decisions should be made in consultation with public health authorities.
Summary
Hemodialysis patients bear a substantial healthcare burden by virtue of having comorbidities, immunosuppression and the need for regular invasive treatment procedures. Healthcare providers should make every effort to ensure that no additional burden, in the form of preventable infections, is imposed on these patients as a consequence of receiving care. Hemodialysis facilities need to ensure that all necessary measures are taken to protect patients from acquiring infections such as HCV in the course of receiving treatment. To accomplish this, facilities should ensure that they adhere to CDC’s existing guidelines and recommendations for the prevention of healthcare-associated infections among hemodialysis patients. Specific attention should be focused on ensuring compliance with recommended anti-HCV screening policies and standard infection control practices. The HCV status of every dialysis patient at the time they are admitted to a facility should be known, and subsequent routine screening is needed to identify changes in serologic status. Such measures will facilitate early detection of new HCV infections and ensure prompt intervention. If patients with new HCV infection are identified, notification of public health authorities, who can assist with evaluating the potential for HCV transmission, is warranted. Infection control assessments, performed in conjunction with public health authorities, to identify practices known to be related to HCV transmission should specifically focus on a) medication preparation and administration, b) hand hygiene and glove use, and c) environmental cleaning and disinfection practices. There is no vaccine at present to prevent HCV infection; hence, there is a strong need to establish a program of staff infection prevention and control education and consistently reinforce proper infection control practices.
Box 1: Best Practices in Preventing Healthcare-Associated Transmission of Hepatitis C Virus (HCV) in Hemodialysis Units.
Practices for Detecting HCV Infection
Screen all patients for possible HCV infection (anti-HCV antibody testing) on facility admission
For susceptible patients, perform anti-HCV antibody testing semiannually and serumalanine transaminase (ALT) testing monthly
Inform newly infected patients of the change in their HCV status and arrange for clinical evaluation/management
Report new HCV infections to the local health department in a timely manner
Infection Control Practices
Designate a separate clean area for medication preparation and storage
Do not prepare medications at the dialysis station or in areas of the treatment floor that have potential to become contaminated
Do not deliver medications to patient stations in mobile carts
Ensure that a new needle and syringe is used for each injection and entry into a medication container
Perform hand hygiene before and after contact with patients and prior to performing procedures
Ensure that gloves are changed and hand hygiene is performed when moving from one patient to the next and before accessing clean supplies
Disinfect each dialysis station after patient treatment is completed and before seating the next patient
Footnotes
Add CDC Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH. Hepatitis C: the clinical spectrum of disease. Hepatology. [Consensus Development Conference Review] 1997;26( 3 Suppl 1):15S–20S. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- 3.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2009;49(4):1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis. 1997;1(3):559–568. vi–vii. doi: 10.1016/s1089-3261(05)70321-4. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 6.Perz JF, Thompson ND, Schaefer MK, Patel PR. US outbreak investigations highlight the need for safe injection practices and basic infection control. Clin Liver Dis. 2010;14(1):137–151. doi: 10.1016/j.cld.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Petrosillo N, Gilli P, Serraino D, Dentico P, Mele A, Ragni P, Puro V, Casalino C, Ippolito G. Prevalence of infected patients and understaffing have a role in hepatitis C virus transmission in dialysis. Am J Kidney Dis. 2001;37(5):1004–1010. doi: 10.1016/s0272-6386(05)80017-4. [DOI] [PubMed] [Google Scholar]
- 8.Thompson ND, Novak RT, Datta D, Cotter S, Arduino MJ, Patel PR, Williams IT, Bialek SR. Hepatitis C virus transmission in hemodialysis units: importance of infection control practices and aseptic technique. Infect Control Hosp Epidemiol. 2009;30(9):900–903. doi: 10.1086/605472. [DOI] [PubMed] [Google Scholar]
- 9.Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998–2008. Ann Intern Med. 2009;150(1):33–39. doi: 10.7326/0003-4819-150-1-200901060-00007. [DOI] [PubMed] [Google Scholar]
- 10.Okuda K, Hayashi H, Kobayashi S, Irie Y. Mode of hepatitis C infection not associated with blood transfusion among chronic hemodialysis patients. J Hepatol. 1995;23(1):28–31. doi: 10.1016/0168-8278(95)80307-6. [DOI] [PubMed] [Google Scholar]
- 11.Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Seminars in dialysis. 2005;18(1):52–61. doi: 10.1111/j.1525-139X.2005.18108.x. [DOI] [PubMed] [Google Scholar]
- 12.Shimokura G, Chai F, Weber DJ, Samsa GP, Xia GL, Nainan OV, Tobler LH, Busch MP, Alter MJ. Patient-care practices associated with an increased prevalence of hepatitis C virus infection among chronic hemodialysis patients. Infect Control Hosp Epidemiol. 2011;32(5):415–424. doi: 10.1086/659407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, Rayner HC, Greenwood RN, Akiba T, Young EW. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335–2342. doi: 10.1111/j.1523-1755.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) [cited 2012 November 15];Healthcare-associated hepatitis B and C outbreaks reported to the Centers for Disease Control and Prevention (CDC) in 2008–2011. Available from: http://www.cdc.gov/hepatitis/Outbreaks/HealthcareHepOutbreakTable.htm.
- 15.Patel PR, Thompson ND, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis. 2010;56(2):371–378. doi: 10.1053/j.ajkd.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50(RR-5):1–43. [PubMed] [Google Scholar]
- 17.Mbaeyi C, Agarwal A, Belflower R, Mercedes L, Tuttle J, Kolhe P, Xia G, Ramachandran S, Khudyakov Y, Hu D, Thompson N, Patel P, Arduino M, Nielsen C, Schaefer M, Wise M. Outbreak of hepatitis C virus infections in an outpatient dialysis facility—Georgia. 61st Annual Epidemic Intelligence Service (EIS) Conference; April 16 – 20, 2012; Atlanta, GA. 2011. [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) Hepatitis C virus transmission at an outpatient hemodialysis unit---New York, 2001–2008. MMWR Morbid Mortal Wkly Rep. 2009;58(8):189–194. [PubMed] [Google Scholar]
- 19.Chan TM, Lok ASF, Cheng IKP, Chan RT. Prevalence of hepatitis C virus infection in hemodialysis patients: a longitudinal study comparing the results of RNA and antibody assays. Hepatology. 1993;17(1):5–8. [PubMed] [Google Scholar]
- 20.Sampietro M, Salvadori S, Corbetta N, Badalamenti S, Graziani G, Fiorelli G. Single-tube reverse transcription and heminested polymerase chain reaction of hepatitis C virus RNA to detect viremia in serologically negative hemodialysis patients. Int J Clin Lab Res. 1995;25(1):52–54. doi: 10.1007/BF02592578. [DOI] [PubMed] [Google Scholar]
- 21.Stuyver L, Claeys H, Wyseur A, Van Arnhem W, De Beenhouwer H, Uytendaele S, Beckers J, Matthijs D, Leroux-Roels G, Maertens G, De Paepe M. Hepatitis C virus in a hemodialysis unit: molecular evidence for nosocomial transmission. Kidney Int. 1996;49(3):889–895. doi: 10.1038/ki.1996.122. [DOI] [PubMed] [Google Scholar]
- 22.Schröter M, Feucht H-H, Schäfer P, Zöllner B, Laufs R. High percentage of seronegative HCV infections in hemodialysis patients: the need for PCR. Intervirology. 1997;40(4):277–278. doi: 10.1159/000150558. [DOI] [PubMed] [Google Scholar]
- 23.Le Pogam S, Le Chapois D, Christen R, Dubois F, Barin F, Goudeau A. Hepatitis C in a hemodialysis unit: molecular evidence for nosocomial transmission. J Clin Micro. 1998;36(10):3040–3043. doi: 10.1128/jcm.36.10.3040-3043.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alter HJ, Jett BW, Polito AJ, Farci P, Melpolder JC, Shih JW-K, Shimizu Y, Purcell R. Analysis of the role of hepatitis C virus in transfusion-associated hepatitis. In: Hollinger FB, Lemon SM, Margolis H, editors. Viral hepatitis and liver disease. Baltimore, MD: Williams & Williams; 1991. pp. 396–402. [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of Hepatitis C in chronic kidney disease. Kidney Int. 2008;73(Suppl 109):S1–S99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 26.Department of Health & Human Services. [cited 2012 November 15];National Action Plan to Prevent Healthcare-Associated Infections: Roadmap to Elimination. doi: 10.1097/MLR.0000000000000030. Available from: http://www.hhs.gov/ash/initiatives/hai/esrd.html. [DOI] [PubMed]
- 27.Sacks-Davis R, VAN Gemert C, Bergeri I, Stoove M, Hellard M. Identifying newly acquired cases of hepatitis C using surveillance: a literature review. Epidemiol Infect. 2012;140(11):1925–1934. doi: 10.1017/S0950268812001033. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. [cited 2012 November 27];Reference for interpretation of hepatitis C virus (HCV) test results. Available from: http://www.cdc.gov/hepatitis/HCV/PDFs/hcv_graph.pdf.
- 29.Centers for Disease Control and Prevention (CDC) [cited 2012 August 24];Guideline for viral hepatitis surveillance and case management. 2005 Available from: http://www.cdc.gov/hepatitis/Statistics/SurveillanceGuidelines.htm.
- 30.Centers for Disease Control and Prevention (CDC) Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Morb Mortal Wkly Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 31.Gupta S, Singh R. Analysis of the virus dynamics model reveals that early treatment of HCV infection may lead to the sustained virological response. PLoS One. 2012;7(7):e41209. doi: 10.1371/journal.pone.0041209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunnari G, Montineri A, Portelli V, Savalli F, Fatuzzo F, Cacopardo B. The use of peginterferon in monotherapy or in combination with ribavirin for the treatment of acute hepatitis C. Eur Rev Med Pharmacol Sci. 2012;16(8):1013–1016. [PubMed] [Google Scholar]
- 33.Sherman KE. Therapeutic approach to the treatment-naive patient with hepatitis C virus genotype 1 infection: a step-by-step approach. Clin Infect Dis. 2012;55(9):1236–1241. doi: 10.1093/cid/cis628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Council of State & Territorial Epidemiologists (CSTE) [cited 2012 November 14];Public health reporting and national notification for acute hepatitis C. Available from: http://www.cste.org/ps2009/09-ID-40.pdf. [Google Scholar]
- 35.Centers for Medicare & Medicaid Services. [cited 2012 November 15];ESRD program interpretative guidance. Available from: http://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/downloads/SCletter09-01.pdf.
- 36.Dudzinski DM, Hébert PC, Foglia MB, Gallagher TH. The disclosure dilemma--large-scale adverse events. N Engl J Med. 2010;363(10):978–986. doi: 10.1056/NEJMhle1003134. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Medicare & Medicaid Services. Medicare and Medicaid programs: conditions for coverage for end-stage renal disease facilities: Final rule. Federal register. 2008;73(73):20369–20484. [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) [cited 2012 August 27];Continuing Education Course: Infection prevention in dialysis settings. Available from: http://www.cdc.gov/dialysis/provider/CE/infection-prevent-outpatient-hemo.html.
- 39.Alter MJ. Healthcare should not be a vehicle for transmission of hepatitis C virus. J Hepatol. 2008;48(1):2–4. doi: 10.1016/j.jhep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Williams IT, Perz JF, Bell BP. Viral hepatitis transmission in ambulatory health care settings. Clin Infect Dis. 2004;38(11):1592–1598. doi: 10.1086/420935. [DOI] [PubMed] [Google Scholar]
- 41.Trepanier CA, Lessard MR, Brochu JG, Denault PH. Risk of cross-infection related to the multiple use of disposable syringes. Can J Anaesth. 1990;37(2):156–159. doi: 10.1007/BF03005462. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention (CDC) Acute hepatitis C virus infections attributed to unsafe injection practices at an endoscopy clinic--Nevada, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(19):513–517. [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. [cited 2012 December 16];Injection Safety Checklist. Available at: http://www.cdc.gov/injectionsafety/PDF/SIPC_Checklist.pdf.
- 44.Centers for Disease Control and Prevention. [cited 2013 March 11];Infection prevention checklist for outpatient settings: minimum expectations for safe care. Available from: http://www.cdc.gov/HAI/settings/outpatient/checklist/outpatient-care-checklist.html.
- 45.Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfurayh O, Sabeel A, Al Ahdal MN, Almeshari K, Kessie G, Hamid M, Dela Cruz DM. Hand contamination with hepatitis C virus in staff looking after hepatitis C-positive hemodialysis patients. Am J Nephrol. 2000;20(2):103–106. doi: 10.1159/000013565. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm Rep. 2002;51(RR-16):1–45. [PubMed] [Google Scholar]
- 48.Shimokura G, Weber DJ, Miller WC, Wurtzel H, Alter MJ. Factors associated with personal protection equipment use and hand hygiene among hemodialysis staff. Am J Infect Control. 2006;34(3):100–107. doi: 10.1016/j.ajic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. [cited 2013 March 11];Your 5 Moments for Hand Hygiene. Available from: http://www.who.int/gpsc/5may/Your_5_Moments_For_Hand_Hygiene_Poster.pdf.
- 50.Centers for Disease Control and Prevention (CDC) [cited 2012 November 14];Protocol for Hand Hygiene and Glove Use Observations. Available from: http://www.cdc.gov/dialysis/prevention-tools/Protocol-hand-hygiene-glove-observations.html.
- 51.Centers for Disease Control and Prevention (CDC) [cited 2012 November 14];Guide to Hand Hygiene Opportunities in Hemodialysis. Available from: http://www.cdc.gov/dialysis/PDFs/collaborative/Hemodialysis-Hand-Hygiene-Observations.pdf.
- 52.Kamili S, Krawczynski K, McCaustland K, Li X, Alter MJ. Infectivity of hepatitis C virus in plasma after drying and storing at room temperature. Infect Control Hosp Epidemiol. 2007;28(5):519–524. doi: 10.1086/513727. [DOI] [PubMed] [Google Scholar]
- 53.Girou E, Chevaliez S, Challine D, Thiessart M, Morice Y, Lesprit P, Tkoub-Scheirlinck L, Soing-Altrach S, Cizeau F, Cavin C, André M, Dahmanne D, Lang P, Pawlotsky JM. Determinant roles of environmental contamination and noncompliance with standard precautions in the risk of hepatitis C virus transmission in a hemodialysis unit. Clin Infect Dis. 2008;47(5):627–633. doi: 10.1086/590564. [DOI] [PubMed] [Google Scholar]
- 54.Froio N, Nicastri E, Comandini UV, Cherubini C, Felicioni R, Solmone M, Di Giulio S, Petrosillo N. Contamination by hepatitis B and C viruses in the dialysis setting. Am J Kidney Dis. 2003;42(3):546–550. doi: 10.1016/s0272-6386(03)00787-x. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention (CDC) [cited 2012 August 27];Guideline for disinfection and sterilization in healthcare facilities. 2008 Available from: http://www.cdc.gov/hicpac/pdf/guidelines/disinfection_nov_2008.pdf.
- 56.Centers for Disease Control and Prevention. Routine Disinfection of the Dialysis Station: User Checklist. Available from: (TBD)…. [Google Scholar]