Abstract
In elderly patients presenting with confusion and recurrent falls, conditions including infection, acute stroke, acute coronary events and electrolyte abnormalities have to be strongly considered as diagnostic possibilities. ECG is a non-invasive test that often serves as a useful tool in suggesting the underlying electrolyte disturbance. However, ECG must be interpreted with caution as it can, at times, be misleading, as in this case of hypercalcaemia seen by us. We discuss the different ECG findings in hypercalcaemic situations.
Background
In elderly patients presenting with confusion and recurrent falls, conditions including infection, acute stroke, acute coronary events and electrolyte abnormalities have to be strongly considered as diagnostic possibilities. ECG is a basic non-invasive test that often serves as a useful tool in determining the underlying electrolyte disturbance. However, ECG must be interpreted with caution as it can be misleading, as in this case of hypercalcemia seen by us.
Case presentation
A 79-year-old African-American man presented to the emergency room, with increasing lethargy and confusion for 1 day. This was associated with generalised weakness, nausea and recurrent falls of 2 days duration. His family reported that there was no prior history of seizures. He did not have any chest pain, breathlessness, fever, abdominal discomfort or vomiting. He also had a 4.5 kg loss of weight over 2 months. He was known to have a history of hypertension, glaucoma and prostate cancer, which was in remission. He drank alcohol on occasion, but was never a smoker. He never used any illicit drugs. His age-appropriate cancer screening was up-to-date and no abnormalities were reported.
On examination, he was drowsy, but responsive to voices, and he appeared extremely dehydrated. He was oriented only to self. He was afebrile, the blood pressure was 155/92 mm Hg and heart rate 120 bpm. His cardiac, lung and abdominal examination were unremarkable. Neurological examination did not show any focal deficits, he had normal reflexes.
Investigations
The initial ECG showed sinus tachycardia and was indicating ST elevations in V1–V4 (figure 1A, B). Laboratory tests were remarkable for leucocytosis (13 000 cells/mcL); serum creatinine of 2.3 mg/dL (baseline was 1.3 mg/dL); and serum calcium level of 19.4 mg/dL. He had had a recent CT scan of the thorax, which showed a 2 cm nodule in the lungs. CT of the brain was normal. Troponins were trended and shown to be consistently elevated at 0.08 ng/mL (reference range <0.03 ng/mL).
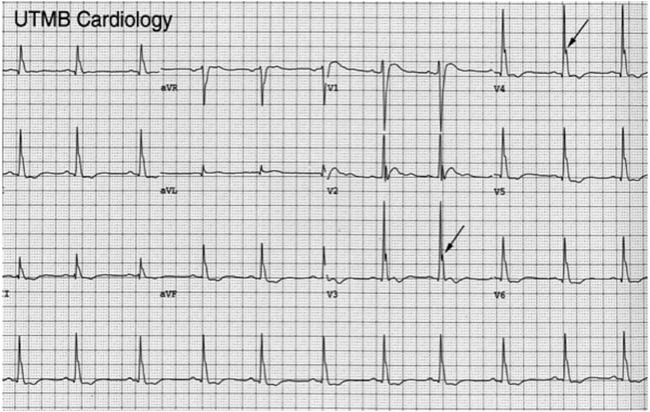
Figure 1.
(A and B) ECG of the patient on admission showing sinus tachycardia, normal PR and QRS. Q-aT interval was 200 ms, with no identifiable ST segment; serum calcium level was 19.4 mg/dL.
Differential diagnosis
Though ECG was initially thought to be suggesting ST elevation myocardial infarction (STEMI), a review after seeing the high-serum calcium levels clarified that ECG changes were all due to acute hypercalcaemia. A closer look at the ECG revealed that the Q-aT interval was 200 ms with no identifiable ST segment, T waves were upright with normal amplitude, sinus tachycardia, normal PR and QRS duration. There were no J waves or signs of left ventricular hypertrophy (figure 1A, B). All these findings were consistent with hypercalcaemia.
Furthermore, the patient reported no cardiovascular or respiratory symptoms and denied any chest pain/discomfort. Troponin levels were monitored and were consistently at 0.08 ng/dL, reassuringly without an upward trend. These findings suggested that acute coronary syndrome was unlikely.
Treatment
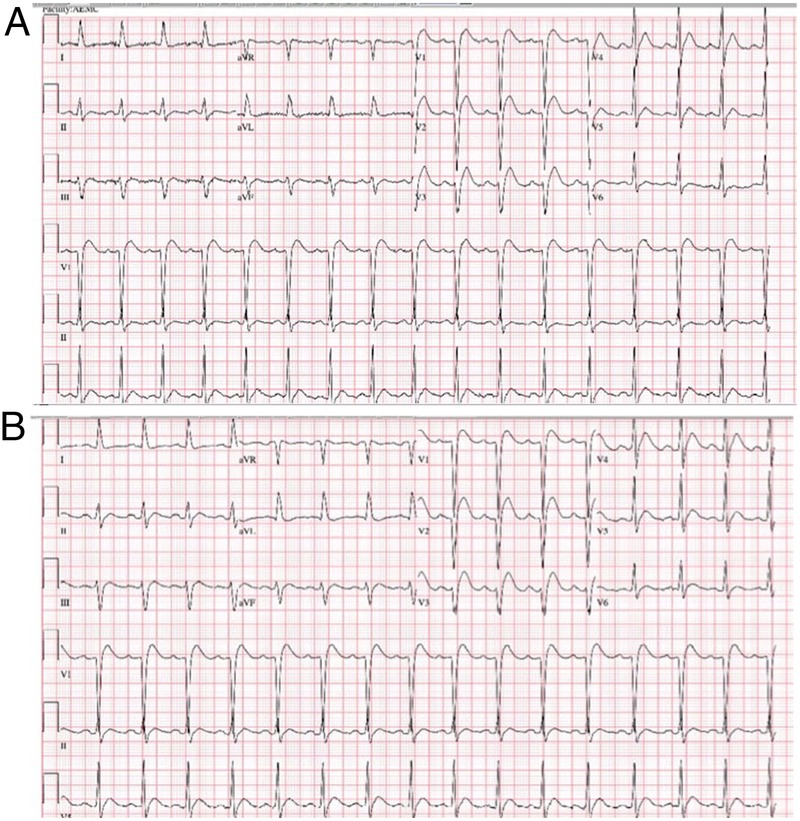
The patient was managed with aggressive intravenous fluids, calcitonin and pamidronate. The ECG features reverted to some extent after 4 days once the serum calcium returned to near-normal levels (figure 2).
Figure 2.

ECG on day 4 showing near normalisation of earlier changes; serum calcium near normal.
The laboratory and radiological work up excluded malignancy but revealed an elevated parathyroid hormone (PTH) level at 824 pg/mL (reference range 8.2–83.5 pg/mL). Sestamibi scintigraphy with 26mCi of technetium 99 m revealed a parathyroid adenoma posterior to the right thyroid lobe (figure 3A, B). Ultrasound of the neck revealed a 2 cm hypoechoic mass posterior to the right thyroid lobe (figure 4).
Figure 3.
(A) Sestamibi Scintigraphy with 26 mCi of Technetium 99 m Sestamibi revealed a parathyroid adenoma posterior to the mid right thyroid lobe on early images. (B) Delayed unzoomed and zoomed images confirm abnormal retention in the right parathyroid adenoma.
Figure 4.

Ultrasound of the neck revealed a 2 cm hypoechoic mass posterior to right thyroid lobe (arrow).
Outcome and follow-up
The parathyroid adenoma was successfully excised without complications. The ECG features reverted to normalcy.
Discussion
Hypercalcaemia can occur due to a number of causes but severe hypercalcaemia (serum calcium level of more than >13 mg/dL), as seen in our patient, most commonly results from primary hyperparathyroidism or malignancy.1 Other causes of elevated calcium are less common and usually not considered until malignancy and parathyroid disease are ruled out. Primary hyperparathyroidism (PHPT) is due to the unregulated overproduction of PTH resulting in abnormal calcium homeostasis. In approximately 85% of cases, primary hyperparathyroidism is caused by a single adenoma. In 15% of cases, multiple glands are involved (ie, either multiple adenomas or hyperplasia). Rarely, primary hyperparathyroidism is caused by parathyroid carcinoma. Hypercalcaemia is a common metabolic disorder seen in malignancy of the lung, breast, kidney, multiple myeloma or haematological system diseases. Hypercalcaemia is the most common life-threatening metabolic disorder associated with neoplastic diseases, occurring in an estimated 10–20% of all adults with cancer. X-linked hypophosphataemia, familial hypocalciuric hypercalcaemia, lithium-associated hypercalcemia, vitamin D intoxication, Milk-alkali syndrome/milk-drinker’s syndrome or Burnett’s syndrome, cardiac sarcoidosis and others granulomatous disorders such as berylliosis, tuberculosis, leprosy and coccidioidomycosis, and histoplasmosis, tertiary hyperparathyroidism, postrenal transplant and at initiation of chronic haemodialysis, immobilisation, hypophosphataemia (total parenteral nutrition) and various other causes can also result in hypercalcaemia.2
The diagnosis of hypercalcaemia, defined as serum calcium level of more than 10.5 mg/dL, is often made incidentally in asymptomatic patients. Hypercalcaemia can manifest in multiple organs, resulting in a wide array of neuromuscular, gastrointestinal, renal, skeletal or cardiovascular symptoms and, more often, non-specific symptoms such as anorexia, nausea, polydipsia, fatigue, confusion, restlessness and muscle weakness.
Characteristic ECG changes described in hypercalcaemia are shortened QT interval, prolonged PR, lengthened QRS interval, flattened or inverted T waves and variable degrees of heart block.3
QT interval changes
Shortened QoT, QaT and QeT intervals are measured from the beginning of the QRS complex to the origin (O), apex (A) and the end (E) of the T wave, respectively4 (figure 5). The corrected QT intervals (QTc), particularly the QaTc interval, are more reliable indicators of clinical hypercalcaemia.5 In a study by So et al of 13 patients of hypercalcaemia, in 77% of the patients, the QT-interval was shortened. A good, clinically useful correlation between the QT-interval and the serum calcium-concentration could be established in their study.6 In another study by Saikawa et al, the sensitivity of QoTc, QaTc and QeTc in predicting high-serum calcium concentration is 83%, 57% and 39%, and specificity is 100%, 100% and 89%, respectively. These observations suggest that QT intervals can serve as an indicator of high-serum calcium concentration and that the QoTc seems to be a good indicator of the three QTc’s.7
Figure 5.
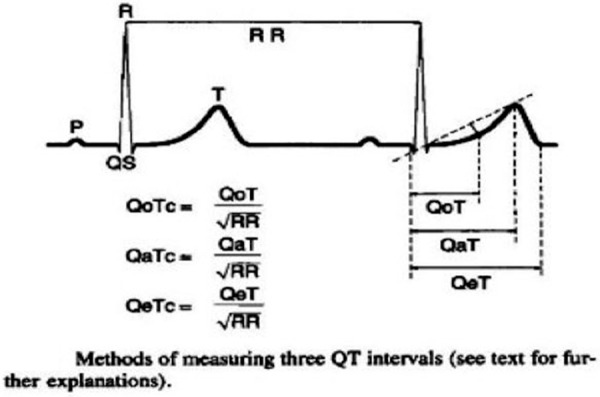
Diagram depicting the method of measuring the three QT intervals: QoT, QaT, QeT. (Reproduced with permission from John Wiley and Sons.)7
ST segment changes
Reduction of ST segment length (duration) or near absence of the ST segment is a common abnormality on ECG. When ST segment is present, it can, rarely, be depressed or elevated and may even mimic acute myocardial infarction.8 Littmann also reported 16 cases from medical records of 14 years, presenting in a similar pattern. This rare electrocardiographic pattern can cause diagnostic and therapeutic challenges and these authors opined that absence of concurrent T wave and Q waves rather than the length of QT interval may give a clue.9 Apparent ST elevation may be misleading, as in our patient. However, the shortened QTc interval and absent ST segment were remarkable changes in ECG that strongly suggest hypercalcaemia (figures 1A, B and 2).
In true STEMI, ST elevation is seen with concave, convex or obliquely straight morphology with normal QT interval. Benign early repolarisation produces modest ST elevations with concave morphology, especially in V2–V5 without reciprocal ST depressions in an asymptomatic patient. There may be slurring or notching at J point. In early pericarditis, there will be diffuse ST elevations with pTa segment depression. Brugada syndrome can also produce ST elevation with coving with T inversion in right chest leads (figures 6).
Figure 6.
ECG showing ST elevation with coving with T inversion in right chest leads in a patient of Brugada syndrome. (Reproduced with permission from Brugada.24)
PR and QRS intervals
PR or PQ interval is prolonged in some cases. The PQ interval tends to be prolonged in the case of hypercalcaemia, but the change is statistically insignificant.7 At very high levels, the QRS interval may lengthen.
Os born waves
A prominent and positive J point level, called a J wave (Os-born wave), has also been described in hypercalcaemia, in addition to hypothermia or use of thiazide diuretics10 11 (figure 7).
Figure 7.
ECG depicting typical Os-born waves; which may be seen in severe hypercalcaemia, hypothermia or following use of thiazide diuretics. (Reproduced with permission from Otero and Lenihan.11)
T waves
T waves can flatten or invert in hypercalcaemia. Flattened or biphasic T waves are prominent in moderate to severe hypercalcaemia, mimicking those seen in myocardial ischaemia. Changes in T wave morphology, polarity and amplitude either appear with development of hypercalcaemia or disappear with normalisation of serum calcium level. In addition to shortening of the QT interval, severe to extreme hypercalcaemia can cause development of inverted, biphasic or notched T waves with a marked decrease in amplitude of T waves. The proximal limb of the T wave acutely slopes to its peak and the ST segment may not be apparent12–14 (figure 8).
Figure 8.

ECG in a case of hypercalcaemia showing acutely sloping proximal limb of the T wave with no apparent ST segment. (Reproduced with permission from Wagner and Wang.25)
Miscellaneous manifestations
Varying degrees of AV blocks may develop. Symptomatic sinus node dysfunction is rarely observed. Tachy–brady syndrome in hypercalcaemia has been reported earlier.15 16 Primary hyperparathyroidism patients lacked the circadian rhythm of the low frequency to high frequency ratio, suggesting an increased sympathetic drive to the heart at night-time on Holter monitor. A modulation of the adrenergic control of circulation seems to be associated with hypercalcaemia and/or chronic PTH excess, but its biological relevance needs further investigation.17 18 Other electrophysiological manifestations described include Heart Rate Variability and increased premature ventricular complexes, ventricular tachycardia or atrial ectopics, but these are not consistently seen and could be due to other associated abnormalities.
A concurrent electrolyte abnormality poses a further challenge on ECG interpretation as the typical ECG pattern of hypercalcaemia is lost. For example, the electrocardiographic abnormalities in combined hypercalcaemia and hypokalaemia are: absence of the ST segment, prolonged T wave, a shortened Q-aTc interval and prominent U wave19 (figure 9). When hypermagnesaemia is additionally detected in combination, P wave changes, tendency for prolongation of P-R interval, prolongation of QRS complex, infra-His conduction disturbances, tendency for broadening and inversion of T wave, and the appearance of a prominent U wave are reported. Hypomagnesaemia normalises the QaTc interval, which is usually shortened by isolated hypercalcaemia.20
Figure 9.

ECG in a patient with hypercalcaemia with hypokalaemia showing absence of the ST segment, prolonged T wave, a shortened Q-aTc interval and a prominent U wave. (Reproduced with permission from Chia and Thai.9)
In our patient, shortened QTc interval and near-absence of the ST segment with ST elevation were the conspicuous changes in ECG when he had serum calcium of 19.4 mg/dL. Our patient presented with a history of repeated falls, dehydration and drowsiness, but had no remarkable physical findings. His ECG on admission showed sinus tachycardia, normal PR and QRS, Q-T interval of 200 ms, no identifiable ST segment and upright T wave of normal amplitude (figures 1A, B and 2). Before looking at the biochemical parameters, myocardial ischaemia was a consideration, but due to the absence of chest pain and normal troponins it was an unlikely diagnosis. The serum calcium level in this patient was grossly elevated at 19.4 mg/dL. The association of shortened QT interval, clinical background and laboratory results can resolve the diagnostic dilemma, as occurred in our case.
Treating the underlying cause, a low calcium diet and adequate hydration are all part of the primary treatment of hypercalcaemia.
Management
The degree and rate of rise of hypercalcaemia, renal function and symptoms dictate the choice of further management. Most patients of PHPT have modest levels of hypercalcaemia are often asymptomatic.
In absence of large-scale randomised trials, no standard recommendations are available for the management of PHPT, though the evidence favours parathyroidectomy as the only definitive therapy for symptomatic patients.21
The fourth International workshop on asymptomatic PHPT suggested that asymptomatic patients with the following criteria may be offered surgery:22
Serum calcium 1 mg/dL or more above upper limit of normal.
Estimated-glomerular filtration rate <60 mL/min.
Bone density >2.5 SDs below peak bone mass or previous asymptomatic vertebral fracture.
Twenty-four hour urinary calcium >400 mg/day.
Nephrolithiasis or nephrocalcinosis.
Age <50 years.
In severe hypercalcaemia with serum calcium concentration >14 mg/dL (especially in cases associated with malignancy), or an acute rise with marked change in sensorium, a three pronged approach is advocated: volume expansion, calcitonin and bisphosphonates. Guidelines issued by the American Society of Endocrinologists for the management of severe hypercalcaemia23 stress on initial rehydration using intravenous 0.9% saline 4–6 L in 24 h, carefully avoiding fluid-overload in the elderly and those with renal dysfunction. Saline therapy is maintained to ensure urine output of about 100–150 mL/h. Routine use of loop diuretics is not helpful. After rehydration, intravenous bisphosphates (Zoledronic acid 4 mg over 15 min or pamidronate 30–90 mg at 20 mg/h or Iandronic acid) are administered, which act by inhibiting the calcium release from the bones; serum calcium levels should be periodically monitored. Steroids, calcitonin and calcimimetics are considered as second-line drugs. Calcitonin decreases bone resorption and increases renal excretion. It is started at 4 IU/kg intramuscularly or subcutaneously every 12 h. Since tachyphylaxis develops early, calcitonin is followed by one of the bisphosphonates. For malignancy-induced hypercalcaemia, zolidronic acid is considered the drug of choice. Denosumab, a monoclonal antibody that prevents calcium release from the bones, has been evaluated in recent trials and is used in refractory cases and in patients with chronic kidney disease. Rarely, parathyroidectomy is considered in acute severe refractory hypercalcaemia. Haemodialysis with calcium-free dialysis fluid is used as a last resort for very severe and refractory cases.
Learning points.
In the absence of a typical presentation of acute coronary syndrome, an apparent ST elevation on ECG must alert us to think of non-ischemic causes for the ST elevation.
Clinicians should consider hypercalcemia as a cause of ST elevation on ECG, particularly in the absence of symptoms/cardiac markers suggestive of acute coronary syndrome.
ST elevation can be from ventricular aneurysm or pericarditis, yet measuring QT interval in these conditions serves no purpose.
Use of QTc interval can help with diagnosis of hypercalcemia. Measuring QT interval on ECG should be a routine bedside practice.
Management of severe hypercalcemia entails volume expansion, calcitonin and bisphosphanates.
Acknowledgments
The authors thank Dr Huyen Tran, MD, Phys-Radiology: Nuclear, for her help with Nuclear imaging pictures of the parathyroid adenoma.
Footnotes
Contributors: SP was responsible for preparation of the manuscript. YKL was responsible for preparation of the manuscript and editing, and approval of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ariyan CE, Sosa JA. Assessment and management of patients with abnormal calcium. Crit Care Med 2004;32:S146–54. 10.1097/01.CCM.0000117172.51403.AF [DOI] [PubMed] [Google Scholar]
- 2.Shane E, Dinaz I. Hypercalcemia: pathogenesis, clinical manifestations, differential diagnosis and management. In: Favus MJ, ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6th edn Vol 26 Philadelphia: Lippincott. Williams and Wilkins, 2006:176. [Google Scholar]
- 3.Khosla S. Hypercalcaemia and hypocalcaemia. In: Longo DL, Fauci AS, Kasper DL et al. Harrison's principles of internal medicine. 18th edn New York, NY: McGraw-Hill, 2012: Chapter 46;360–2. [Google Scholar]
- 4.Rayner HC, Hasking DJ. Hyperparathyroidism associated with severe hypercalcemia and myocardial calcification despite minimal bone disease. BMJ (Clin Res Ed) 1986;293:1277–8. 10.1136/bmj.293.6557.1277-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcaemia. Clin Cardiol 1988;11:395–400. 10.1002/clc.4960110607 [DOI] [PubMed] [Google Scholar]
- 6.So CS, Batrice L, Volger E [Electrocardiographic changes in electrolyte imbalance. Part 2: alterations in serum calcium] Med Klin 1975;70:1966–8. [PubMed] [Google Scholar]
- 7.Saikawa T, Tsumabuki S, Nakagawa M. QT intervals as an index of high serum calcium in hypercalcaemia. Clin Cardiol 1988;11:75–8. 10.1002/clc.4960110205 [DOI] [PubMed] [Google Scholar]
- 8.Nishi SP, Barbagelata NA, Atar S et al. Hypercalcemia-induced ST-segment elevation mimicking acute myocardial infarction. J Electrocardiol 2006;39:298–300. 10.1016/j.jelectrocard.2005.10.015 [DOI] [PubMed] [Google Scholar]
- 9.Littmann L, Taylor L, Brearley WD. ST-segment elevation: a common finding in severe hypercalcemia. J Electrocardiol 2007;40:60–2. 10.1016/j.jelectrocard.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 10.Carrillo-Esper R, Limon-Camacho L, Vallejo-Mora HL et al. Non-hypothermic J wave in subarachnoid haemorrhage. Cir Cir 2004;72:125–9. [PubMed] [Google Scholar]
- 11.Otero J, Lenihan DJ. The ‘normothermic’ Osborn wave induced by severe hypercalcaemia. Tex Heart Inst J 2000;27:316–17. [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas PS, Carmichael KA, Palevsky PM. Extreme hypercalcaemia and electrocardiographic changes. Am J Cardiol 1984;54:674–5. 10.1016/0002-9149(84)90274-1 [DOI] [PubMed] [Google Scholar]
- 13.Ahmed R, Yano K, Mitsuoka T et al. Changes in T wave morphology during hypercalcaemia and its relation to the severity of hypercalcaemia. J Electrocardiol 1989;22:125–32. 10.1016/0022-0736(89)90081-2 [DOI] [PubMed] [Google Scholar]
- 14.Nirenburg DW, Ransil BJ. Q-aTc interval as a clinical indicator of hypercalcaemia. A J Cardiol 1979;44:243–8. 10.1016/0002-9149(79)90312-6 [DOI] [PubMed] [Google Scholar]
- 15.Shah AP, Lopez A, Wachsner RY et al. Sinus node dysfunction secondary to hyperparathyroidism. J Cardiovasc Pharmacol Ther 2004;9:145–7. 10.1177/107424840400900209 [DOI] [PubMed] [Google Scholar]
- 16.Carpenter C, May ME. Case report: cardiotoxic calcemia. Am J Med Sci 1994;307:43–4. 10.1097/00000441-199401000-00008 [DOI] [PubMed] [Google Scholar]
- 17.Barletta G, De Feo ML, Del Bene R et al. Cardiovascular effects of parathyroid hormone: a study in healthy subjects and normotensive patients with mild primary hyperparathyroidism. J Clin Endocrinol Metab 2000;85:1815–21. 10.1210/jcem.85.5.6514 [DOI] [PubMed] [Google Scholar]
- 18.Lazzari R, Pellizzari F, Brusaferri A et al. [Hypercalcemia and changes in cardiac rhythm]. Minerva Med 1991;82:255–7. [PubMed] [Google Scholar]
- 19.Chia BL, Thai AC. Electrocardiographic abnormalities in combined hypercalcemia and hypokalaemia—case report. Ann Acad Med Singapore 1998;27:567–9. [PubMed] [Google Scholar]
- 20.Mosseri M, Porath A, Ovsyshcher I et al. Electrocardiographic manifestations of combined hypercalcaemia and hypermagnesemia. J Electrocardiol 1990;23:235–41. 10.1016/0022-0736(90)90162-U [DOI] [PubMed] [Google Scholar]
- 21.AACE/AAES Task Force on Primary Hyperparathyroidism. The American association of clinical endocrinologists and the American association of endocrine surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract 2005;11:49–54. 10.4158/EP.11.1.49 [DOI] [PubMed] [Google Scholar]
- 22.Bilezikian JP, Brandi ML, Fastell R et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab 2014;99:3561 10.1210/jc.2014-1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Society guidelines on acute hypercalcaemia- Emergency Guidance. http://www.endocrinology.org (accessed 24 Jul 2015).
- 24.Brugada P. The Brugada syndrome. Springer's e-book: figure 1/chapter-19. In: Gussak I, Antzelevitch C, eds. Cardiac repolarisation. Bridging basic and clinical science. Totowa, NJ: Humana Press, 2003:427–446. [Google Scholar]
- 25.Wagner GS, Wang TY. Miscellaneous conditions. In: Marriot HGL, Marriott's practical electrocardiography. 12th edn figure 13–19 PA: Wolters Kluwer/Lippincott Williams and Wilkins, 2013:246. [Google Scholar]