Abstract
Objective
Brain-derived neurotrophic factor (BDNF) is investigated in depression related to medical disorders and its secretion is influenced by epigenetic factors. We investigated the association between BDNF promoter methylation and depression following mastectomy for breast cancer.
Methods
In total, 309 patients with breast cancer were evaluated 1 week after mastectomy, and 244 (79%) were followed up 1 year later. Depression was diagnosed (major or minor depressive disorder) according to DSM-IV criteria and depression severity was estimated by Montgomery-Asberg Depression Rating Scale (MADRS). We assessed BDNF promoter methylation using leukocyte DNA. The effects of BDNF methylation on depression diagnosis and severity were investigated using multivariate logistic and linear regression models, respectively. The two-way interaction between BDNF methylation and the val66met polymorphism on depression was also evaluated using multivariate logistic regression models.
Results
Higher BDNF methylation was independently associated with depression diagnosis and with more severe symptoms at both 1 week and 1 year after mastectomy. No significant methylation-genotype interactions were found.
Conclusion
A role for BDNF in depression related to breast cancer was supported. Indeed, the association between depression and BDNF methylation may be useful for identifying patients who are at high risk for depression and for suggesting directions for promising drug research.
Keywords: Breast cancer, Depression, BDNF, DNA methylation, Epigenetics
INTRODUCTION
Breast cancer, which accounts for 23% of all cancers, is the leading cause of malignancy in women.1 The advances in diagnostic and treatment modalities in breast cancer have led to prolonged survival of people with this disease up to 89%.2 Hence, breast cancer is now conceptualized as a chronic illness, and survivors are likely to suffer from psychological distress, including depression.3 In fact, studies show that many breast cancer survivors experience depression and that 48% suffer from depression during the first year after diagnosis.4 Comorbid depression is, in turn, significantly associated with a poorer quality of life5 as well as with negative outcomes such as poor treatment adherence,6 impaired physical and cognitive functioning,7,8 and cancer progression or survival.9 Therefore, it is important to understand the etiology of depression associated with breast cancer.
The pathogenesis of depression in patients with breast cancer is influenced by a complex interplay between biological factors, including hormonal, inflammatory, and genetic mechanisms,7,10,11 and psychological factors, including bodily disfigurement and impaired sexual functioning.12,13 Recent evidence suggests that genetic predispositions are important in the etiology of depression that is precipitated by medical conditions, which act as environmental risk factors.14 One candidate contributor to depression in patients with breast cancer is brain-derived neurotrophic factor (BDNF), a member of the neurotrophic factor family, which is critical for the growth, survival, and differentiation of neuronal cells as well as for neuronal plasticity.15 BDNF is located on chromosome 11p14.1 and has several polymorphic markers, including a single-nucleotide polymorphism (SNP) at nucleotide 196G/A, which results in an amino acid substitution (valine to methionine) at codon 66 (val66met) of the pro-BDNF molecule. This SNP affects the intracellular processing and secretion of BDNF, and the met allele is associated with reduced activity-dependent secretion of BDNF.16 The met allele has been associated with depression after stroke,17 diabetes,18 and coronary artery disease.19 With respect to depression in patients with breast cancer, significant associations between the met allele and prevalent and persistent depression were previously reported in our longitudinal study.10
BDNF expression is also regulated by epigenetic chromatin remodeling, including DNA methylation, histone acetylation, and other chemical alterations in gene promoter regions. In the central nervous system (CNS), DNA methylation of cytosines in cytosine-guanine (CpG) dinucleotides is regarded as a representative component of broader epigenetic modification at a given locus.20 An increase in CpG methylation at promoter regions on the BDNF gene was reported to be correlated with decreased synthesis of BDNF in the neurons.21 Furthermore, increased BDNF methylation status was correlated with depression in the general population22 and in stroke patients.23 Based on these findings, we hypothesized that BDNF hypermethylation is associated with depression in patients with breast cancer. To our knowledge, no investigation of the role of BDNF promoter methylation and its interaction with BDNF polymorphism in depressed patients with cancer has been conducted. This study aimed to investigate whether BDNF promoter methylation status is associated with depression at 1 week and 1 year after breast surgery in a cohort of Korean women with breast cancer and to analyze these data in the context of the status of the BDNF polymorphism.
METHODS
Study overview and participants
This analysis was performed as a component of a larger parent study investigating mental disorders in patients with breast cancer using a naturalistic prospective design. Details about the design have been published elsewhere.10 In brief, participants were consecutively recruited from all women with breast cancer undergoing breast surgery at the Breast and Endocrine Tumor Clinic of Chonnam National University Hwasun Hospital, Hwasun, South Korea. Assessments were made at 1 week and 1 year after the breast surgery to investigate both acute and chronic outcomes. The recruitment period for the initial baseline assessment lasted from March 2008 to May 2009, and recruitment for the follow-up evaluation occurred 1 year thereafter.
All women with breast cancer undergoing breast surgery at the study site were approached regarding participation. Inclusion criteria were 1) confirmed breast cancer by histological examination, 2) ability to complete the necessary investigations and questionnaires, and 3) capacity to understand the objective of the study and provide informed consent. Exclusion criteria were 1) secondary breast cancer, 2) benign breast tumor, 3) male sex, and 4) any of the following comorbid neuropsychiatric conditions, which were identified by interview or medical record review: dementia, Parkinson's disease, brain tumor, epilepsy, psychosis, and alcohol or substance dependence. This study was approved by the Chonnam National University Hwasun Hospital Institutional Review Board. All participants provided written informed consent.
BDNF DNA methylation analysis and genotyping
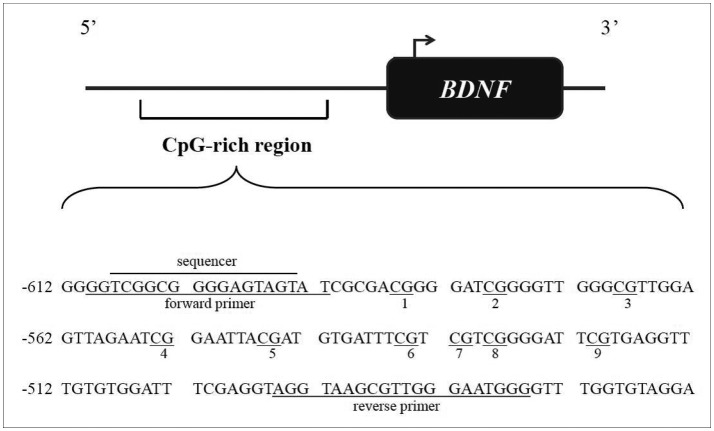
The DNA methylation status of the BDNF promoter was investigated using blood samples obtained from the subsample of participants who provided consent for this procedure. The correlation between gene expression in peripheral tissue and physiologically meaningful gene expression in the brain is not clear. However, it has been shown that BDNF may cross the blood-brain barrier and that postnatal platelet BDNF shows changes similar to those in the brain,24 suggesting parallel changes in BDNF at the blood and brain levels. Additionally, many previous studies have suggested an association between BDNF methylation status in peripheral blood and psychiatric symptoms, such as depression.22,25 The BDNF promoter region for analyzing methylation status is presented in Figure 1. These data have been deposited in GenBank (accession number: BankIt1568919 BDNF JX848620). As in other studies,25 a CpG-rich region of the promoter between -612 and -463 relative to the transcriptional start of exon VII, including nine CpG sites, was analyzed. This region was chosen because it was previously investigated in relation to antenatal depressed mood.25 Genomic DNA (1 µg) was extracted from leukocytes using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer's suggested protocol and was bisulfite treated using the EpiTech Bisulfite Kit (Qiagen) according to the manufacturer's protocol. A 135-bp fragment of BDNF promoter was amplified by polymerase chain reaction (PCR) from bisulfite-treated DNA using the forward and reverse primers designated in Figure 1. PCR conditions were 95℃ for 15 minutes, followed by 45 cycles of 95℃ for 15 seconds, 57℃ for 30 seconds, and 72℃ for 15 seconds, with a final extension of 5 minutes at 72°C. PCR products were sequenced using the PSQ 96M Pyrosequencing system (Biotage) according to the manufacturer's protocol with the sequencing primers designated in Figure 1. The methylation percentage at each CpG region was quantified using Pyro Q-CpG software, version 1.0.9 (Biotage). As the BDNF promoter methylation percentages at CpG sites 2, 6, and 8 were 100% in all participants and the results including the three CpG did not differ from those excluding the sites. Thus, the values of these three sites were excluded from the analyses. The individual methylation percentages at the remaining six CpG sites and their average values were used in the analyses. For genotyping, PCR and the PCR-based restriction fragment length polymorphism assays were performed. The primer sequences used were the forward primer 5'-ACTCTGGAGAGCGTGAATGG-3' and the reverse primer 5'-ACTACTGAGCATCACCCTGGA-3'. The amplification conditions were pre-denaturation at 95℃ for 5 minutes, followed by 40 cycles consisting of denaturation at 95℃ for 30 seconds, 62℃ for 30 seconds, and 72℃ for 30 seconds, post-elongation at 72℃ for 5 minutes, and a final maintenance step at 4°C. The PCR products were digested at 37℃ with the corresponding restriction enzyme (Eco72I), and gel electrophoresis was used to detect the 196G (val: 99- and 72-bp fragments) and 196A (met: 171-bp fragment) alleles. The genotype was categorized as val/val, val/met, and met/met.
Figure 1. BDNF promoter regions for analyzing methylation status. The CpGs are underlined and numbered. Forward and backward primers and sequencer are designated. Numbering of the gene sequence is relative to the transcriptional start site. BDNF: brain-derived neurotrophic factor, CpG: cytosines in cytosine-guanine.
Evaluations of depression
Depression was diagnosed at 1 week and 1 year after breast surgery using the Mini-International Neuropsychiatric Interview (MINI), a structured diagnostic psychiatric interview based on DSM-IV that identifies individuals with major or minor depression.26 As cases of major depression were too rare in this sample to analyze separately, they were combined with those of minor depression to form a single category. In addition, depression severity was measured with Montgomery-Asberg Depression Rating Scale (MADRS), consisted of 10 items with a total score ranging from 0-60.27 The MADRS excludes somatic symptoms of depression which may be difficult to differentiate from physical consequences of breast cancer.
Demographic and clinical characteristics
Information was obtained on age, duration of formal education, current employment (present or absent), marital status (married or divorced/separated), religious affiliation (yes or no), living alone or not, and personal and family histories of depression. The presence of the following 10 common and generally chronic health problems28 was evaluated: arthritis or rheumatism; eyesight problems; hearing difficulty or deafness; persistent cough, breathlessness, difficulty breathing, or asthma; high blood pressure; heart disease or angina; gastric or intestinal problems; diabetes; renal diseases; and liver diseases. Positive responses were summed to generate an overall scale. Body mass index (BMI) (kg/m2), estrogen and progesterone receptor status, tumor size (longest diameter at histology after surgery in mm), presence or absence of metastatic axillary lymph nodes, time since cancer diagnosis (months), chemotherapy history, and surgery type (breast conserving or mastectomy) were also recorded. Status as relapse or first episode was recorded. Breast cancer staging was performed after surgery by an endocrine surgeon using the American Joint Committee on Cancer Staging System for breast cancer.29
Statistical analyses
The dependent variables were depression at 1 week and 1 year after breast surgery. Sociodemographic and clinical characteristics as well as genotype distribution were compared between participants with and without depression using t-tests, χ2 tests, or Fisher's exact tests, as appropriate, at the two evaluations. The BDNF promoter methylation percentages at six CpG sites and their average values were compared by depression status using t-tests. The individual effects of BDNF promoter methylation percentages on depression in patients with breast cancer at the two evaluations were tested using multivariate logistic regression models after adjustment for those sociodemographic and clinical characteristics that were significantly associated with depression at 1 week or 1 year after breast surgery (p<0.05). We also examined the relationship between BDNF methylation percentages and MADRS scores among those diagnosed with depression. Because BDNF methylation percentages were positively skewed, they were log-transformed. The associations between these values both at baseline and at 1 year after breast surgery were assessed using a linear regression model after adjustment for those sociodemographic and clinical characteristics that were significantly associated with MADRS scores at 1week or 1 year after breast surgery (p<0.05). To evaluate the potential interactive effects of BDNF promoter methylation and genotype on depression related to breast cancer, the following two analyses were performed: 1) the BDNF methylation percentages of three BDNF genotypes were compared using analysis of variance; and 2) two-way interactions between BDNF methylation percentages and genotype were estimated using multivariate logistic regression models; As seven comparisons (six CpG sites and the average value) were performed for the BDNF methylation percentages analyses, Bonferroni corrections were applied to maintain an overall type I error rate of 0.05 against the multiple comparisons. All analyses were repeated after excluding participants with a personal history of depression to determine whether BDNF methylation affected depression only after breast cancer. Analyses were repeated after excluding participants treated with antidepressants, as this may affected both depression severity and DNA methylation status. Statistical analyses were performed using SPSS 18.0 software.
RESULTS
Recruitment
Recruitment at baseline and sample follow-up are presented in Supplementary Figure 1 (in the online-only Data Supplement). Of the 366 potentially eligible and consecutively enrolled women with breast cancer undergoing breast surgery, 336 (92%) consented to participate in the study. Of these, 309 (92%) agreed to provide blood samples for genotyping, and this group formed the baseline sample. We found no significant differences between participants and non-participants with respect to any demographic and clinical characteristics (p>0.20). The mean (SD) time between breast surgery and evaluation was 4.5 (1.2) days. Of the 309 participants, 74 (23.9%) were diagnosed with depression (11 with major depression and 63 with minor depression). At the 1-year follow-up, 244 (79%) were re-examined. Those who were present at follow-up did not differ significantly from those who were absent with respect to any baseline demographic or clinical characteristic (p>0.05).
Demographic and clinical characteristics
Depression was diagnosed in 44 (18.0%) of the 244 followed-up participants (five with major depression and 39 with minor depression). In the analyzed sample, the mean (SD; range) age was 50.8 (9.7; 25-80) years, and the mean (SD) duration of education was 10.4 (3.9) years. A total of 89 (28. 8%) participants were currently employed, 250 (80.9%) were married, 240 (77.7%) reported a religious affiliation, and 25 (8.1%) were living alone. The mean (SD) number of chronic health problems was 0.4 (0.8), and the mean (SD) BMI was 24.1 (3.4). A personal history of depression was reported by 24 (7.8%) participants, and a family history of depression was reported by four (1.3%) participants. Breast cancer was present for the first time in 293 (94.8%) participants, and the mean (SD) time since tumor detection was 2.5 (7.7) months. With respect to treatment, 201 (65.0%) received breast-conserving surgery, and 220 (71.2%) received chemotherapy. The mean (SD) tumor size was 18.5 (11.9) mm. The tumor stage was 0 in 29 (9.4%), I in 105 (34.0%), II in 122 (39.5), III in 43 (13.9%), and IV in 10 (3.2%) participants. These characteristics are compared by depression status at 1 week and 1 year after breast surgery in online (Supplementary Table 1 in the online-only Data Supplement). Participants with depression at baseline or at follow-up were more likely to report personal and family histories of depression and to have the met/met allele compared with those without depression (p<0.05).
BDNF promoter methylation status by depression status in patients with breast cancer
We analyzed the overall average BDNF promoter methylation percentage as well as the percentage at each CpG site according to depression status at 1 week and 1 year after breast surgery (Table 1). After Bonferroni corrections, depression at both 1 week and 1 year after breast surgery was significantly associated with a higher average BDNF promoter methylation percentage and a higher percentage at CpG site 9. The results of multivariate logistic regression analyses examining the effects of BDNF promoter methylation on depression in patients with breast cancer are presented in Table 2. After adjustment for personal and family histories of depression and BDNF genotype, a higher percentage at CpG site 9 was independently associated with depression at both 1 week and 1 year after breast surgery. After applying the Bonferroni correction, the strength of the association between depression at 1 week after breast surgery and higher methylation percentage at CpG site 9 and between depression at 1 year after mastectomy and higher average methylation and higher methylation at CpG site 9 remained significant.
Table 1. BDNF promoter methylation percentages by depression.
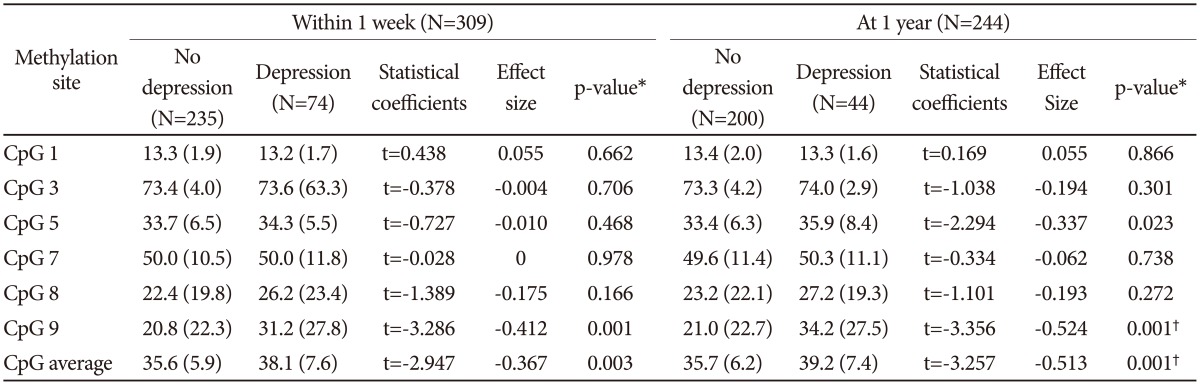
*p-value using t-tests, †statistically significant after Bonferroni's correction. BDNF: brain-derived neurotrophic factor, CpG: cytosines in cytosine-guanine
Table 2. Multi-varitate analyses of examining the individual effects of BDNF methylation percentages on depression.
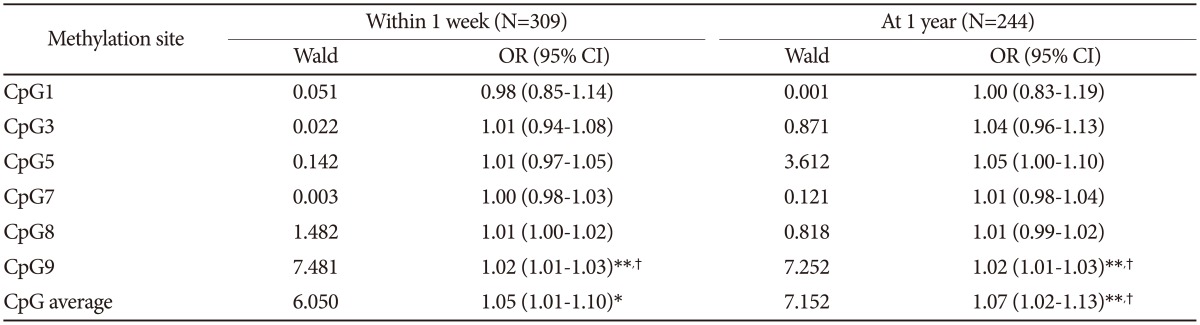
All analyses are adjusted for personal and family history of depression, and BDNF genotype. *p-value<0.05, **p-value<0.01, †statistically significant after Bonferroni's correction. BDNF: brain-derived neurotrophic factor, CpG: cytosines in cytosine-guanine
Association between BDNF promoter methylation status and depression severity in those with depression diagnoses
The mean (SD) MADRS score of the 73 participants with depression was 15.9 (6.2) within 1week after breast surgery and 16.5 (6.6) among the 44 participants with depression 1 year after breast surgery. MADRS scores were significantly associated with personal and family histories of depression on both occasions, Associations of the log-transformed BDNF methylation percentages with the MADRS scores at baseline and follow-up are summarized in Table 3. After applying Bonferroni corrections, baseline MADRS scores were significantly associated with higher methylation percentage at CpG site 8 and 9 and with average methylation percentage after adjusting for personal and family histories of depression. Additionally, more severe MADRS scores at follow-up period were significantly correlated with higher individual methylation percentages at CpG site 9 and a higher average methylation percentage in the same adjusted models. Scatter plots for the associations between average BDNF methylation percentage and MADRS scores at both baseline and follow-up are presented in Supplementary Figure 2 (in the online-only Data Supplement).
Table 3. Linear regression analyses of the associations between log-transformed BDNF promoter methylation percentages (CpGs) and scores on Montgomery-Asberg Depression Rating Scale.
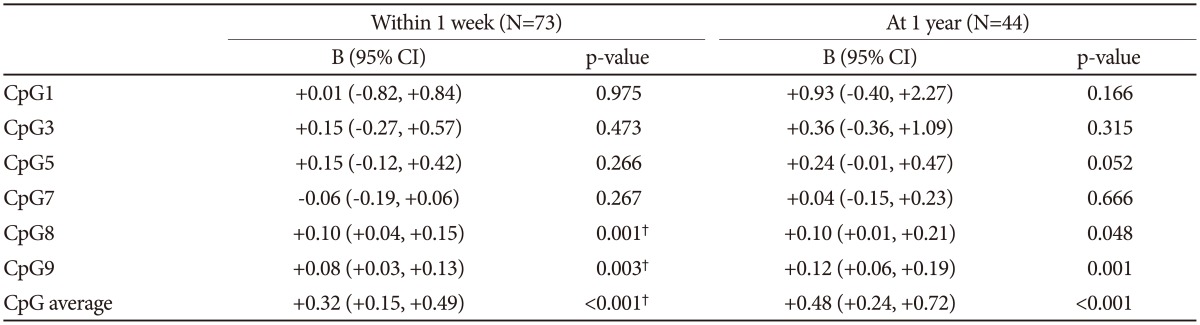
All analyses are adjusted for personal and family histories of depression. †statistically significant after Bonferroni's correction. BDNF: brain-derived neurotrophic factor
Interaction between BDNF promoter methylation status and genotype by depression status in patients with breast cancer
Supplementary Table 2 (in the online-only Data Supplement) presents the analysis of average BDNF promoter methylation and methylation at each CpG site by BDNF genotype. No significant association was observed between BDNF promoter methylation and genotype. The interactive effects of BDNF methylation percentages and genotype on depression status among patients with breast cancer were estimated in the adjusted multivariate logistic regression model, which is summarized in Supplementary Table 3 (in the online-only Data Supplement). No significant interactive effects were found for any combination of BDNF CpG site methylation percentage and genotype after applying the Bonferroni correction.
Results after excluding participants with a personal history of depression and antidepressants treatment
All analyses were repeated after excluding the participants with a personal history of depression (n=285 with one week and n=226 at one year). However, the results were not changed substantially (data not shown). Two of 309 participants were receiving antidepressants at baseline, and two of 241 participants were receiving them at follow up. Repeated analyses excluding these participants showed no substantial changes (data not shown).
DISCUSSION
The principal findings of this longitudinal study of a breast cancer cohort were that higher BDNF promoter methylation status was significantly associated with depression diagnosis at both 1 week and 1 year after breast surgery. Moreover, at both time points, higher BDNF promoter methylation status was more prominently associated with more severe depressive symptoms in those with depression. This association was independent of potential covariates such as personal or family history of depression and/or BDNF genotype. No significant methylation-genotype interactions were found.
Interpretations of our findings should be made in the context of several considerations concerning study design. First, the blood samples used to determine BDNF status were obtained within 1 week after breast surgery rather than at the time of cancer diagnosis. Thus, it was difficult to discern whether BDNF promoter methylation interacted with diagnosis in terms of its power to predict the development of sub-sequent depression. However, it has been reported that epigenetic status of particular genes such as DNA methylation is life-long.30 Second, our study did not include a control group of breast cancer patients who did not undergo surgery nor did we evaluate other post-surgery life stressors. Therefore, it was difficult to determine whether the depression was specifically precipitated by breast cancer or whether the DNA methylation differences were specifically related to breast cancer patients. However, analyses excluding those with a personal history of depression did not yield substantially different results.
Given these considerations, we can conclude that BDNF promoter methylation status was significantly associated with depression after breast cancer surgery A growing body of evidence suggests that epigenetic mechanisms, especially DNA methylation, play an important role in susceptibility to depression.22,31,32 Epigenetic factor provides an explanation of the risk factors,33,34 clinical features,32,35,36 and treatment responses associated with depression.37 However, few studies have examined epigenetic mechanisms involved in depression that is precipitated by medical conditions, except with regard to stroke23 and systemic lupus erythematosus.38
There are several possible explanations for the significant association between BDNF methylation status and depression in patients with breast cancer. First, consistent with previous findings,21 BDNF release may be decreased in individuals with higher methylation percentages. BDNF provides necessary trophic support and is critical in regulating structural and synaptic plasticity and in modulating neurotransmission.15,39 Thus, deficits in neural maintenance and reduced neural plasticity associated with decreased BDNF release may result in impaired ability to adapt to stressful situations, such as breast cancer diagnosis and treatment; this may, in turn, account for the higher risk of depression diagnosis and more severe symptoms. Our findings are consistent with those of a recent study reporting a significant association between BDNF promoter methylation status and post-stroke depression,40 which gives epidemiological support for this biological mechanism. Our findings are also consistent with reports of a significant association between the BDNF met allele, which is related to reduced activity-dependent secretion of BDNF,16 and depression among patients with breast cancer;10 they are also consistent with data on the prevalence of major depressive disorder and the treatment response associated with this condition.41,42 Second, BDNF appears to influence serotonin transmission. Recent evidence suggests that BDNF has a direct influence on serotonergic transmission in humans.43 Therefore, decreased BDNF due to higher methylation may result in decreased serotonergic function, which has been linked to depression diagnosis and its severity.
It is noteworthy that the association of higher BDNF methylation status with depression and its severity was significant at both 1 week and 1 year after breast surgery. This finding supports the assumption that genetic predispositions are important in the etiology of depression precipitated by medical conditions. Our finding is in keeping with recent studies on the relationship between methylation status, such as that of BDNF and SLC6A4, and post-stroke depression, which have consistently reported an association between higher methylation status and depression, although the strength of these associations differs slightly according to the time elapsed after stroke.23,40 However, it is not known whether the influence of BDNF methylation differs according to the time elapsed after breast surgery, and this issue may require further research. Taken together, these findings suggest that higher BDNF promoter methylation status may be a biological marker of depression in patients with breast cancer, at least at 1 week and 1 year after breast surgery.
Although the BDNF val66met polymorphism was significantly associated with depression at both 1 week and 1 year after breast surgery, we found no significant associations between methylation and genotype and no methylation-genotype interactions in our sample. This finding is consistent with our recent study on the relationship between BDNF methylation and post-stroke depression, which similarly found no interaction between BDNF methylation and its genotype.23 However, other previous research on the association between SLC6A4 methylation and depression found that SLC6A4 methylation status was dependent on its genotype and consequently had interactive effects on depression.40,44,45 Considering these results, further research on the relationship between BDNF methylation and genotype should be conducted in a broader range of populations.
Our study has several strengths in addition to its being the first to report on associations between BDNF methylation status and depression related to breast cancer. First, depression was formally identified using a structured diagnostic interview, and it was evaluated at similar time points (at 1 week and 1 year after breast surgery) in all participants. As the etiology of depression may differ according to the time elapsed after diagnosis of breast cancer,46 this particular study design enabled a more accurate investigation with fewer risks of errors arising from heterogeneous examination times. Second, a range of covariates were considered in the analyses, and the follow-up rate was reasonable, and participation in the follow-up examination was unrelated to the risk factors of interest. Finally, participants were consecutively enrolled from among all patients with a diagnosis of breast cancer who were undergoing breast surgery at the study hospital, which resulted in a lower likelihood of selection bias and increased the potential generalizability of the results.
The present study also has several limitations. First, due to resource constraints, the methylation status for only one CpG island of the BDNF gene could be investigated. The methylation status for this single island is only a part of the whole gene, and this may have biased associations toward the null, thus obscuring true group differences. However, such a measurement error would not account for the observed associations. Studies of other CpG islands for this gene, of other genes, and of genome-wide DNA methylation are clearly indicated. Second, the BDNF methylation levels were tested using peripheral blood rather than using brain tissue. As the BDNF promoter methylation profile may be tissue-specific, it represents only weak evidence for a correlation between peripheral methylation patterns and those seen in the CNS. Furthermore, different patterns of methylation can be observed across CNS regions.47 However, there is increasing evidence that epimutation may not be limited to the affected tissue but can also be detected in peripheral blood.48 Therefore, our results should be interpreted carefully because they may not provide a direct index of BDNF methylation in the CNS and may not represent synaptic plasticity or neuronal maintenance. Moreover, the functional status of BDNF, such as mRNA expression or BDNF level in the plasma and CNS were not investigated, which may have detracted from our analysis of the interaction between BDNF methylation status and expression Third, it has been reported that promoter methylation is affected by various drug regimens and by a history of abuse during childhood. In our study, data on drug regimen and history of early childhood adversities were not evaluated. However, as stated, we believe that this is the first study in this area and therefore provides an important basis for further, more extensive research.
In conclusion, breast cancer patients with higher BDNF promoter methylation status were more susceptible to depression at 1 week and at 1 year after breast surgery. Considering the higher morbidity associated with depression in breast cancer, it is possible that more careful evaluation and management are indicated for those with increased genetic vulnerability. A BDNF methylation test may be a useful tool for identifying those at high risk for depression related to breast cancer, as this approach is non-invasive and simple if more evident associations on the methylation status between CNS and blood will be found in the future studies. Additionally, given that DNA methylation status is potentially reversible by treatment with pharmacological agents,31 development of a new drug that regulates DNA promoter methylation may be helpful for improving the treatment of suicidal ideation in patients with depression.32,49 We believe that our study represents an important first step in elucidating the role of epigenetic mechanisms in the etiology of depression in breast cancer and that it serves as a foundation for future research.
Acknowledgments
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C0003) and was supported by a grant of Chonnam National University Hospital Biomedical Research Institute (CRI 14032-1). Prof. M-G Shin is part-funded by a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (No. 2013-1320070). The Ministry of Health and Welfare of Korea and Chonnam National University Hospital Biomedical Research Institute had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.4306/pi.2015.12.4.523.
Flow diagram for baseline recruitment and follow-up.
Scatter plots for the associations between average brain-derived neurotrophic factor (BDNF) methylation percentage and Montgomery-Asberg Depression Rating Scale (MADRS) scores at baseline (A) and at follow-up (B).
Sociodemographic and clinical characteristics by depression
BDNF promoter methylation percentages by val66met polymorphism
Multi-variate analyses of examining the interactive effects of BDNF methylation percentages (CpGs) and val66met polymorphism (P) on depression
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Bower J. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shim EJ, Shin YW, Jeon HJ, Hahm BJ. Distress and its correlates in Korean cancer patients: pilot use of the distress thermometer and the problem list. Psychooncology. 2008;17:548–555. doi: 10.1002/pon.1275. [DOI] [PubMed] [Google Scholar]
- 6.Ell K, Sanchez K, Vourlekis B, Lee PJ, Dwight-Johnson M, Lagomasino I, et al. Depression, correlates of depression, and receipt of depression care among low-income women with breast or gynecologic cancer. J Clin Oncol. 2005;23:3052–3060. doi: 10.1200/JCO.2005.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fann JR, Thomas-Rich AM, Katon WJ, Cowley D, Pepping M, McGregor BA, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30:112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger T, Forrester A, Markov D, Chism K, Kunkel EJ. Women at a dangerous intersection: diagnosis and treatment of depression and related disorders in patients with breast cancer. Psychiatr Clin North Am. 2010;33:409–422. doi: 10.1016/j.psc.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JM, Kim SW, Stewart R, Kim SY, Shin IS, Park MH, et al. Serotonergic and BDNF genes associated with depression 1 week and 1 year after mastectomy for breast cancer. Psychosom Med. 2012;74:8–15. doi: 10.1097/PSY.0b013e318241530c. [DOI] [PubMed] [Google Scholar]
- 11.Kim JM, Stewart R, Kim SY, Kang HJ, Jang JE, Kim SW, et al. A one year longitudinal study of cytokine genes and depression in breast cancer. J Affect Disord. 2013;148:57–65. doi: 10.1016/j.jad.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 12.Fingeret MC, Nipomnick SW, Crosby MA, Reece GP. Developing a theoretical framework to illustrate associations among patient satisfaction, body image and quality of life for women undergoing breast reconstruction. Cancer Treat Rev. 2013;39:673–681. doi: 10.1016/j.ctrv.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safarinejad MR, Shafiei N, Safarinejad S. Quality of life and sexual functioning in young women with early-stage breast cancer 1 year after lumpectomy. Psychooncology. 2013;22:1242–1248. doi: 10.1002/pon.3130. [DOI] [PubMed] [Google Scholar]
- 14.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic Sensitivity to the Environment: the Case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang EJ, Reichardt LF. Neutrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, et al. BDNF genotype potentially modifying the association between incident stroke and depression. Neurobiol Aging. 2008;29:789–792. doi: 10.1016/j.neurobiolaging.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Zhou JX, Li HC, Bai XJ, Chang BC, Li CJ, Sun P, et al. Functional Val66Met polymorphism of Brain derived neurotophic factor in type 2 diabetes with depression in Han Chinese subjects. Behav Brain Funct. 2013;9:34. doi: 10.1186/1744-9081-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozzini S, Gambelli P, Boiocchi C, Schirinzi S, Falcone R, Buzzi P, et al. Coronary artery disease and depression: possible role of brain-derived neurotrophic factor and serotonin transporter gene polymorphisms. Int J Mol Med. 2009;24:813–818. doi: 10.3892/ijmm_00000297. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg Z, Feil R, Constancia M, Fraga M, Junien C, Carel JC, et al. Child health, developmental plasticity, and epigenetic programming. Endocr Rev. 2011;32:159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, et al. DNA methylation related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 22.Fuchikami M, Morinobu S, Segawa M, Okamoto Y, Yamawaki S, Ozaki N, et al. DNA methylation profiles of the brain-derived neurotrophic factor (BDNF) gene as a potent diagnostic biomarker in major depression. PLoS One. 2011;6:e23881. doi: 10.1371/journal.pone.0023881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JM, Stewart R, Kang HJ, Kim SY, Kim SW, Shin IS, et al. A longitudinal study of BDNF promoter methylation and genotype with poststroke depression. J Affect Disord. 2013;149:93–99. doi: 10.1016/j.jad.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One. 2010;5:e12201. doi: 10.1371/journal.pone.0012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-VI and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 27.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 28.Lindesay J. The Guy's/Age Concern Survey: physical health and psychiatric disorder in an urban elderly community. Int J Geriatr Psychiatry. 1990;5:171–178. [Google Scholar]
- 29.American Joint Committee on Cancer (AJCC) AJCC Cancer Staging Handbook from the AJCC Cancer Staging Manual. 6th Edition. New York: Springer-Verlag; 2002. pp. 257–281. [Google Scholar]
- 30.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 31.Mill J, Petronis A. Molecular studies of major depressive disorder: the epigenetic perspective. Mol Psychiatry. 2007;12:799–814. doi: 10.1038/sj.mp.4001992. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder M, Krebs MO, Bleich S, Frieling H. Epigenetics and depression: current challenges and new therapeutic options. Curr Opin Psychiatry. 2010;23:588–592. doi: 10.1097/YCO.0b013e32833d16c1. [DOI] [PubMed] [Google Scholar]
- 33.Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zill P, Baghai TC, Schüle C, Born C, Früstück C, Büttner A, et al. DNA methylation analysis of the Angiotensin Converting Enzyme (ACE) gene in major depression. PLoS One. 2012;7:e40479. doi: 10.1371/journal.pone.0040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang HJ, Kim JM, Stewart R, Kim SY, Bae KY, Kim SW, et al. Association of SLC6A4 methylation with early adversity, characteristics and outcomes in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:23–28. doi: 10.1016/j.pnpbp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Goldberg J, Bremner JD, Vaccarino V. Association between promoter methylation of serotonin transpoter gene and depressive symptoms: a monozygotic twin study. Psychosom Med. 2013;75:523–529. doi: 10.1097/PSY.0b013e3182924cf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell TR, Smith RG, Hackinger S, Schalkwyk LC, Uher R, McGuffin P, et al. DNA methylation in interleukin-11 predicts clinical response to antidepressants in GENDEP. Transl Psychiatry. 2013;3:e300. doi: 10.1038/tp.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Cheng YQ, Chen B, Bai R, Li S, Xu XF, et al. Depression in systemic lupus erythematosus patients is associated with link-polymorphism but not methylation status of the 5HTT promoter region. Lupus. 2013;22:1001–1010. doi: 10.1177/0961203313498793. [DOI] [PubMed] [Google Scholar]
- 39.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 40.Kim JM, Stewart R, Kang HJ, Kim SW, Shin IS, Kim HR, et al. A longitudinal study of SLC6A4 DNA promoter methylation and poststroke depression. J Psychiatr Res. 2013;47:1222–1227. doi: 10.1016/j.jpsychires.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Niitsu T, Fabbri C, Bentini F, Serretti A. Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:183–194. doi: 10.1016/j.pnpbp.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Pei Y, Smith AK, Wang Y, Pan Y, Yang J, Chen Q, et al. The brain-derived neurotrophic-factor (BDNF) val66met polymorphism is associated with geriatric depression: a meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:560–566. doi: 10.1002/ajmg.b.32062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henningsson S, Borg J, Lundberg J, Bah J, Lindström M, Ryding E, et al. Genetic variation in brain-derived neurotrophic factor is associated with serotonin transporter but not serotonin-1A receptor availability in men. Biol Psychiatry. 2009;66:477–485. doi: 10.1016/j.biopsych.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Philibert RA, Madan A, Andersen A, Cadoret R, Packer H, Sandhu H. Serotonin transporter mRNA levels are associated with the methylation of an upstream CpG island. Am J Med Genet B Neuropsychiatr Genet. 2007;144:101–105. doi: 10.1002/ajmg.b.30414. [DOI] [PubMed] [Google Scholar]
- 45.Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, et al. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes Brain Behav. 2010;9:575–582. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat. 2008;110:9–17. doi: 10.1007/s10549-007-9706-5. [DOI] [PubMed] [Google Scholar]
- 47.Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, et al. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 2012;13:R43. doi: 10.1186/gb-2012-13-6-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pidsley R, Mill J. Epigenetic studies of psychosis: current findings, methodological approaches, and implications for postmortem research. Biol Psychiatry. 2011;69:146–156. doi: 10.1016/j.biopsych.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 49.Melas PA, Rogdaki M, Lennartsson A, Björk K, Qi H, Witasp A, et al. Antidepressant treatment is associated with epigenetic alterations in the promoter of P11 in a genetic model of depression. Int J Neuropsychopharmacol. 2012;15:669–679. doi: 10.1017/S1461145711000940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram for baseline recruitment and follow-up.
Scatter plots for the associations between average brain-derived neurotrophic factor (BDNF) methylation percentage and Montgomery-Asberg Depression Rating Scale (MADRS) scores at baseline (A) and at follow-up (B).
Sociodemographic and clinical characteristics by depression
BDNF promoter methylation percentages by val66met polymorphism
Multi-variate analyses of examining the interactive effects of BDNF methylation percentages (CpGs) and val66met polymorphism (P) on depression