Abstract
Objective
Electroconvulsive therapy (ECT) is a reasonable option for intractable depression or schizophrenia, but a mechanism of action has not been established. One credible hypothesis is related to neural plasticity. Three genes (Oct4, Sox2, c-Myc) involved in the induction of induced pluripotent stem (iPS) cells are Wnt-target genes, which constitute a key gene group involved in neural plasticity through the TCF family. Klf4 is the other gene among Yamanaka's four transcription factors, and increases in its expression are induced by stimulation of the canonical Wnt pathway.
Methods
We compared the peripheral blood gene expression of the four iPS genes (Oct4, Sox2, c-Myc, and Klf4) before and after modified ECT (specifically ECT with general anesthesia) of patients with intractable depression (n=6) or schizophrenia (n=6). Using Thymatron ten times the total bilateral electrical stimulation was evoked.
Results
Both assessments of the symptoms demonstrated significant improvement after mECT stimulation. Expression of all four genes was confirmed to increase after initial stimulation. The gene expression levels after treatment were significantly different from the initial gene expression in all twelve cases at the following treatment stages: at the 3rd mECT for Oct4; at the 6th and 10th mECT for Sox2; and at the 3rd, 6th and 10th mECT for c-Myc.
Conclusion
These significant differences were not present after correction for multiple testing; however, our data have the potential to explain the molecular mechanisms of mECT from a unique perspective. Further studie should be conducted to clarify the pathophysiological involvement of iPS-inducing genes in ECT.
Keywords: iPS cells, Electroconvulsive therapy, Schizophrenia, Depression, Oct4, Sox2
INTRODUCTION
Electroconvulsive treatment (ECT) is currently regarded to be a reasonable option for intractable psychiatric disorders. If performed correctly, ECT is one of the most effective treatments for drug-resistant depression1,2 and catatonic schizophrenia.3,4 However, the underlying molecular mechanism of ECT's therapeutic effects has not been fully elucidated, although some important findings have been reported.5,6 Briefly, in the context of animal models, electroconvulsive shock (ECS) induces the activation of N-methyl-D-asparate (NMDA),7,8 and AMPA-related (DL-α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid9) receptors, and hippocampal neurogenesis is subsequently increased.
Long-term potentiation (LTP10,11), with the feature of synaptic plasticity, is thought to form the basis of memory.12,13 One well-known hippocampal LTP circuits is NMDAR-dependent,14,15 but the mechanism of LTP remains controversial. A recent finding suggested that chronic stress provoked a deficit in LTP in the rat hippocampus, although electroconvulsive stimulation recovered this LTP.16
In the adult brain, as the functional activation of the NMDA receptor increases, beta-catenin (cadherin-associated protein, beta 1, 88 kDa, with the HUGO-approved official symbol of CTNNB1) forms a complex with lymphoid enhancer-binding factor-1/T cell factor (LEF1/TCF),17,18 subsequently inducing the transcription of genes related to neural plasticity. The genes associated with neural plasticity through the TCF-family are called Wnt-target genes.19 Approximately 100 genes are included among the Wnt-target genes,20,21,22 and three genes (Oct4 [POU5F1 POU class 5 homeobox 1 GeneID: 5460], Sox2 [SOX2 SRY (sex determining region Y)-box 2 GeneID: 6657], c-Myc [MYC v-myc avian myelocytomatosis viral oncogene homolog GeneID; 4609] genes) involved in the induction of iPS cells are included among the Wnt-target genes.23,24,25 Regarding the LIF gene (leukemia inhibitory factor GeneID: 3976), which is located upstream of Klf4, stimulation along the canonical Wnt pathway induced an increase in LIF expression.26 Therefore, it has been suspected that electric stimuli, such as ECT, promote the expression of LIF.
The aim of the current study was to reveal the mRNA expression alterations in Yamanaka's four transcription factors before and after mECT treatment.
METHODS
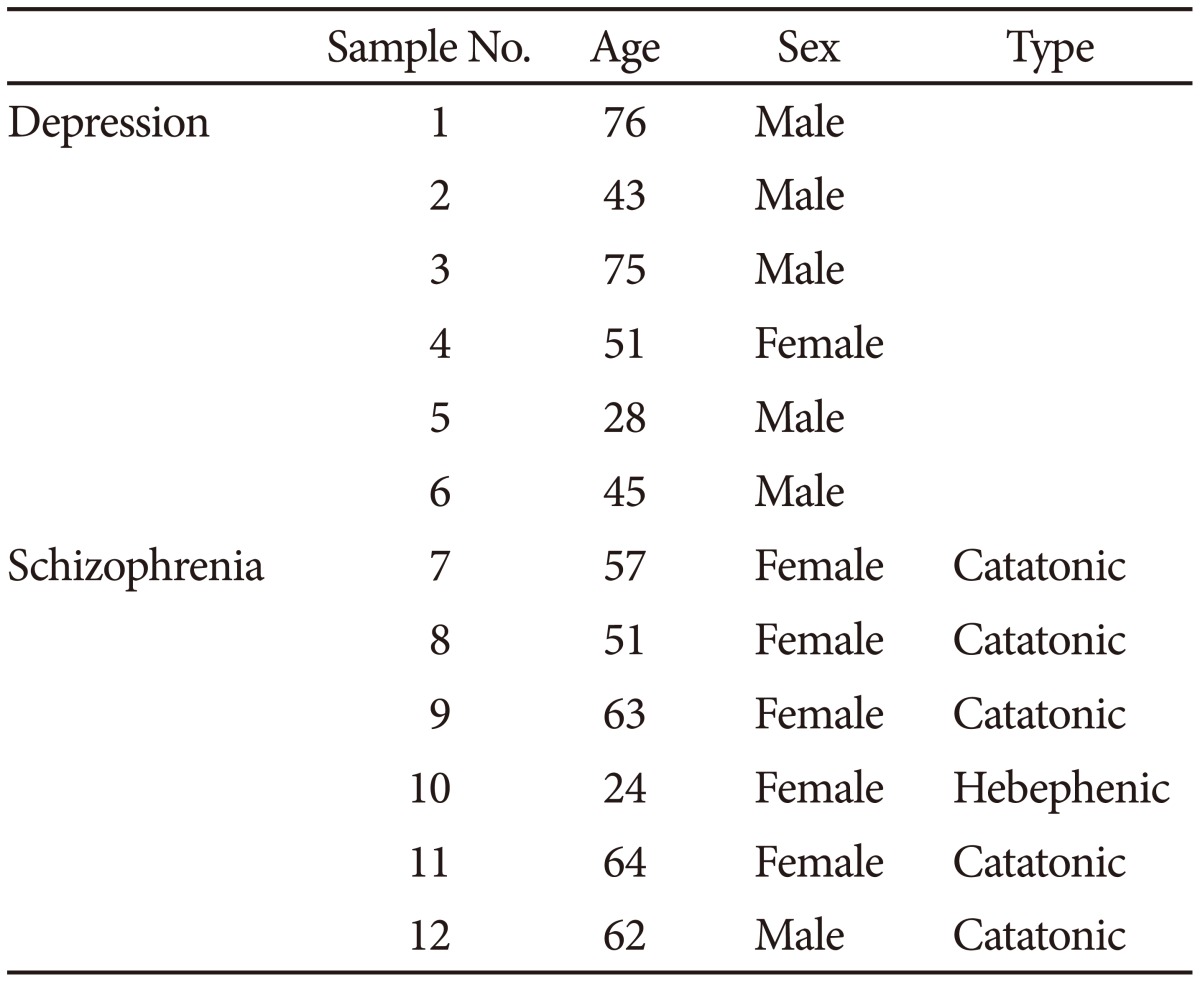
We administered modified electroconvulsive therapy (mECT), specifically electroconvulsive therapy with general anesthesia), at the Osaka Medical College Hospital, to patients diagnosed with either depression or schizophrenia based on the DSM-IV TR27 and with a prescription history of two or more types of antidepressants or antipsychotics at the maximal doses for more than four weeks and with insufficient response to treatment. Six depressed patients and six schizophrenic patients were recruited for further assessment. We included only patients with improved symptoms based on the PANSS28 for schizophrenia or the HAM-D29 for depression after the treatment. This study was approved by the ethics committee of the Osaka Medical College. Written informed consent for biological assessment was provided by all of the participants after a documentary and oral explanation. This work has been conducted according to the principles expressed in the Declaration of Helsinki, and we have not obtained the consent from the surrogates of the participants in the current study. Once the patients were suspected to have a disturbance of consciousness, the patients were omitted from the study. The demographic data for the sample, including the assessed symptoms, are provided in Table 1.
Table 1. The demographic data of the sample with depression and schizophrenia.
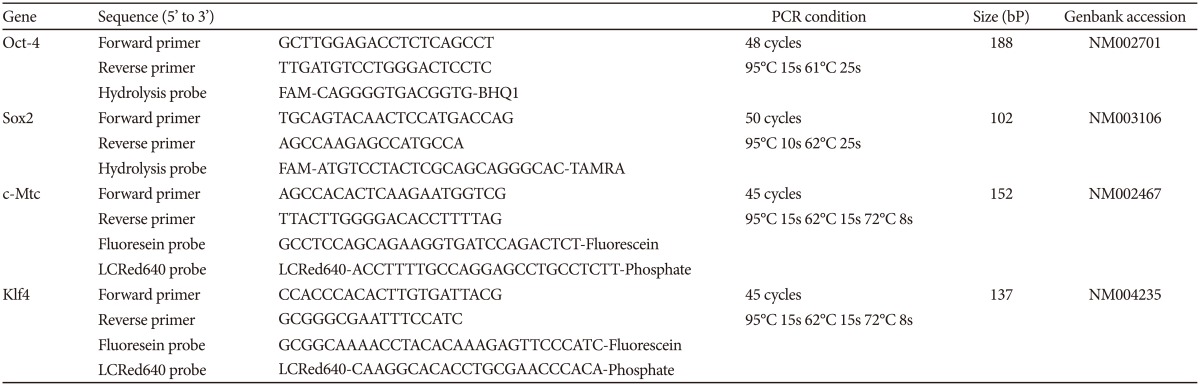
Electroconvulsive therapy was administered under general anesthesia ten times in total, at a frequency of twice per week. Using Thymatron® (Somatics LLC, Lake Bluff, IL, USA), the patients were given bilateral electrical stimulation with a pulse wave. We set the initial quantity of electricity according to the half-age method (quantity of electricity at half age30), and the stimulation dose was increased to 1.5 times if an effective convulsive seizure was not obtained. We used 1.0 mg/kg propofol intravenously and 0.8 mg/kg suxamethonium, which is a muscle relaxant, for general anesthesia. mECT was performed during hospitalization to minimize changes in sleep habits, diet habits, smoking habits, and medication use over the course of the study. Three milliliters of peripheral blood was extracted at approximately 10:00 AM on the day before the 1st ECT (day 0), on the day after the 3rd ECT (day 9), on the day after the 6th ECT (day 19), and on the day after the 10th ECT (day 33). The samples were immediately stored at -80℃ (-112℉). RNA was extracted using an mRNA Isolation Kit for Blood® (Roche Diagnostics, Tokyo, Japan), and complementary DNA was subsequently synthesized. For quantitative PCR, the gene expression of oct4, sox2, c-myc, klf4 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the last of which was used as a housekeeping gene, were analyzed using a Light Cycler® (Roche Diagnostics, Tokyo, Japan) with specific primers and probes (Table 2).
Table 2. Primer sequence and PCR condition of each gene for quantitative PCR.
PCR: polymerase chain reaction
Statistical comparison was performed using a paired t-test. The repeated measures ANOVA was applied for the overall analysis, although clear statistical significance was not achieved even on the post-hoc analysis. The average value of the expression level of the mRNA of each gene one day before the 1st ECT was regarded as 1, and significance was set at <0.05. All of the statistical analyses were performed with SPSS® software (IBM, Japan).
RESULTS
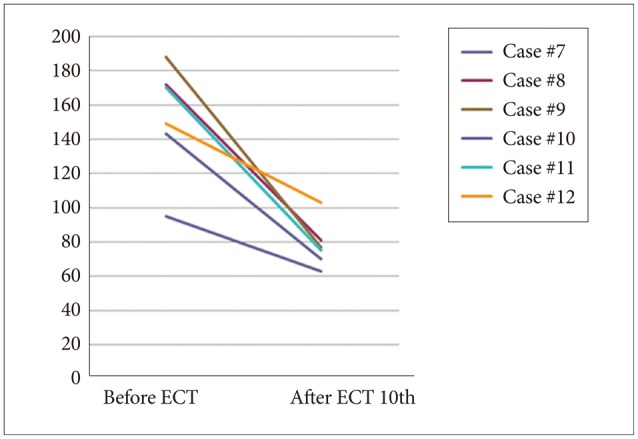
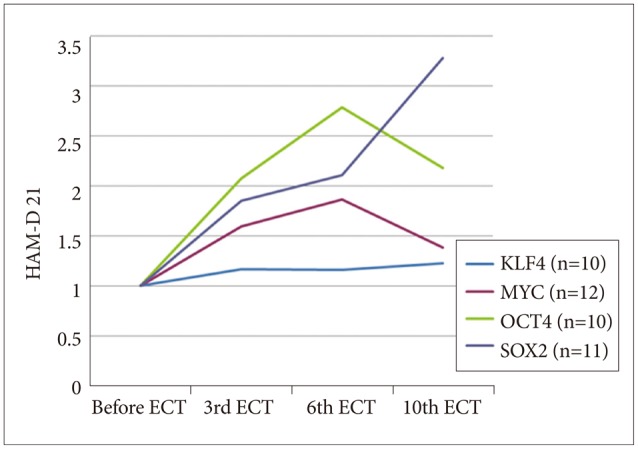
As shown in Figures 1 (HAM-D 21 for depression) and 2 (PANSS for schizophrenia), both severity scales for clinical symptoms indicated an improvement after mECT. The scales showed significant recovery for the patients with depression, particularly for the descriptor Depressed Mood and Feeling of Guilt, while Delusions and Conceptual Disorganization were relatively improved for the schizophrenia patients. No obvious adverse events were observed during the over the course of the study.
Figure 1. Severity scale for depression using HAM-D 21. HAM-D 21: Hamilton depression rating scale 21 items version, ECT: electroconvulsive therapy.
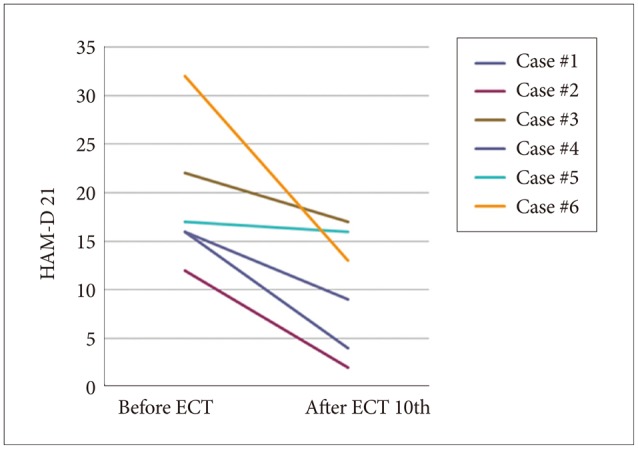
Figure 2. Severity scale for schizophrenia using PANSS. PANSS: Positive and Negative Syndrome Scale, ECT: electroconvulsive therapy.
Gene expression analysis of all of the samples revealed increases in the four tested genes over the course of the study (Figure 3), some of which were significant, as determined by uncorrected t-test (p<0.05, not corrected, after the 3rd mECT for Oct4, after the 6th and 10th mECT for Sox2, and after the 3rd, 6th and 10th mECT for c-Myc; p>0.05 after Bonferroni correction). Compared to the overall analysis of mECT, when the data for schizophrenia and depression were analyzed individually, only c-Myc gene expression was significantly different in the schizophrenic patients after mECT, as determined by uncorrected t-test (p<0.05, not corrected after the 3rd and 6th mECT; p>0.05 for multiple testing, as in the overall analysis).
Figure 3. Gene expression assay for four genes. HAM-D 21: Hamilton depression rating scale 21 items version, ECT: electroconvulsive therapy.
DISCUSSION
The current results clearly validate the clinical utility of mECT for the treatment of depression and schizophrenia, based on symptomatic assessment using the HAM-D and PANSS. Additionally, the comparison of the mRNA expression of Yamanaka's four transcription factors in the peripheral blood showed a tendency toward higher levels after treatment. Detailed assessment of the expression of each gene indicated gradual decreases in the expression of some of the genes (e.g., c-Myc and Klf4) over the course of mECT treatment. Klf4 cooperates with Oct3/4 for the maintenance of pluripotency,31 but not every gene work simultaneously. Therefore, the speed or timing of the expression peak during neurogenesis could be expected to differ for each of these four genes.
iPS cells show triploblastic pluripotency, similar to fertilized eggs or embryonic stem cells. Because the transcription of specific mRNA in mature cells is generally restricted by epigenetic events, such as DNA methylation in the early developing stages, the mRNA expression balance varies for each cell. Although every cell has the same DNA-sequence information in a single individual, different cells are produced because of the different transcriptional patterns in each cell type. Introducing Yamanaka's four transcription factors into mature cells reprograms the epigenetic restriction that occurs during the early stages of development, allowing iPS cells to acquire their pluripotency. Thus, these cells can again transcribe genes that have been repressed.
It is known that BDNF expression is increased by ECT and that an epigenetic mechanism is involved in this change. Ma et al. found an increase in BDNF in the hippocampal dentate gyrus of adult wild-type mice after ECS, but this change was not observed in Gadd45b knockout mice; Gadd45b encodes a DNA demethylase.32 Therefore, DNA demethylation is thought to be involved in the BDNF increase caused by ECT.
DNA methylation of the CpG island in a gene promoter region inhibits the binding of a transcription factor and thus prevents the gene's transcription. Yamanaka's four transcription factors promote DNA demethylation on the CpG island as a part of the reprogramming. Because the assumed mechanism of ECT is based on both neurogenesis and the increase in BDNF levels, we conclude that the observed ECT-stimulated increase in the expression of Yamanaka's four transcription factors, some of which are Wnt-target genes needed for neurogenesis, is associated with both neurogenesis and the increase in BDNF.
Our findings should be interpreted cautiously. The first limitation of this study is its small sample size. The diagnoses of schizophrenia and depression in the enrolled sample of patients were based strictly on the SCID, and strict timing of the drawing of blood was applied. Because of these restrictions, only 12 patients were analyzed. Therefore, the small sample size could have led to misleading results. The second limitation arose from the lack of an unmedicated sample group. Our sample consisted only of patients treated with a few medications (it is recommended that drugs be avoided during mECT treatment). However, some drugs were prescribed for the extraction of peripheral blood. These drugs might have affected mRNA expression. Therefore, medication-free samples should be analyzed in future studies. There have not been any analyses of how epigenetic changes in the cerebral cortex of the frontal brain and hippocampus affect mRNA expression in peripheral blood, and this lack of analyses should be considered a third limitation of this study. In depression, methylation of the BDNF gene has been suggested as a biomarker, indicating that Oct4, Sox2, c-Myc, and Klf4 could be useful as biomarkers for ECT treatment.
Although the current findings were from the analysis of mRNA derived from leukocyte, other previous groups have reported the direct analysis of leukocyte fraction changes according to the ECT. Chaturvedi et al.33 have reported the results, based on the hematological difference between pre-ECT and two hours after ECT stimulation, that the increased lymphocyte and granulocyte subsets, and the increased number of leukocyte soon after the ECT stimulation. In contrast, the decreased lymphocyte subset and the increased granulocyte subsets were detected at two-hours later from ECT stimulation. In addition, Kronfol et al.34 have reported the increment of lymphocyte natural killer cell activity (NKCA) one hour after the ECT stimulation. They have pointed out the possible mechanism of the trafficking of the accumulated NK cell at the blood vessel wall on the circulating NK cell because of the reason that the number of circulating NK cells is relatively small. The changing of number of leucocyte at the short period as early 1 to 2 hours after ECT stimulation is not mainly from the differential leucocyte but the trafficking of the accumulated NK cell on the blood vessel wall to the circulating NK cell.34 It is not considered that these early response is cause only by the changing of Yamanaka's four transcription factor. In addition, the gene expression of mRNA was corrected by the house keeping gene's expression quantity, therefore the increment of leukocyte is not simply contributed by the increased expression of Yamanaka's four transcription factor, however the lack of the observation for differential subsets of pre- and post-ECT is one of the considerable limitation of the current finding.
Another limitation should be noted regarding the method of statistical analysis. By adopting the Smirnov-Grubbs outlier test, we extracted 2 samples for Oct4 (sample #1 and #2), 1 sample for Sox2 (sample #7), and 2 samples for Klf4 (samples #3 and #5). There was no individual sample that did not duplicate the outlier across the gene. The Kolmogorov-Smirnov test for normality of the distribution did not reveal non-normality of our sample. We adopted the t-test to analyze the significant differences in mRNA expression between one day prior to the initial mECT session and the other days of observation (at the 3rd, 6th and 10th mECT). Significance differences were obtained when comparing the initial mRNA expression levels with the 6th mECT for Oct4 [t(9)=-1.95, p=0.04], the 10th mECT for Sox2 [t(10)=-2.14, p=0.03], and the 3rd, 6th and 10th mECT for c-Myc [t (11)=-2.22, p=0.02 on 3rd, t (11)=-2.16, p=0.03 on 6th, t (11)=-1.87, p=0.04 on 10th]. However, the differences were not significant when a two-tailed t-test was applied Another concern was the lack of Bonferroni correction in multiple testing. As described in the Results section, all of the significance differences, as indicated by the p-values from the one-tailed t-tests, disappeared on correction (number of tests: 3). The most attractive finding of the current study was that the mRNA expression of all of the selected genes increased after ECT during the observed period.
There is evidence of the correlation of cytokine or BDNF in between blood and brain, although no evidence on Yamanaka's four transcription factor has been reported till date. Yang et al.35 have reported the up-regulation of Sox2, Nanog, Oct4, and Lin28 at the culture of anti-tumor T cell when IL-12 plus IL-7 or IL-21 were added into the culture fluid for three days. Because of ECT, the expression changing of brain cytokine was induced. Due to the correlation of cytokine expression between blood and brain, it is suspected that the cytokine in the peripheral blood up-regulated the Yamanaka's four transcription factor in the peripheral blood. In the future, more research especially for the validation of the expression of cultural cell after ECT stimulation is warranted.
This study found that ECT reversed epigenetic changes that occur during development and resulted in clinical effects. Future progress could reveal how the Oct4, Sox2, c-Myc, and Klf4 genes are involved in the mechanism of ECT.
References
- 1.Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357:1939–1945. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- 2.Kellner CH, Greenberg RM, Murrough JW, Bryson EO, Briggs MC, Pasculli RM. ECT in treatment-resistant depression. Am J Psychiatry. 2012;169:1238–1244. doi: 10.1176/appi.ajp.2012.12050648. [DOI] [PubMed] [Google Scholar]
- 3.Zervas IM, Theleritis C, Soldatos CR. Using ECT in schizophrenia: a review from a clinical perspective. World J Biol Psychiatry. 2012;13:96–105. doi: 10.3109/15622975.2011.564653. [DOI] [PubMed] [Google Scholar]
- 4.Pompili M, Lester D, Dominici G, Longo L, Marconi G, Forte A, et al. Indications for electroconvulsive treatment in schizophrenia: a systematic review. Schizophr Res. 2013;146:1–9. doi: 10.1016/j.schres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Bolwig TG. How does electroconvulsive therapy work? Theories on its mechanism. Can J Psychiatry. 2011;56:13–18. doi: 10.1177/070674371105600104. [DOI] [PubMed] [Google Scholar]
- 6.Fochtmann LJ. Genetic approaches to the neurobiology of electroconvulsive therapy. J ECT. 1998;14:206–219. [PubMed] [Google Scholar]
- 7.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 8.Andrade C, Singh NM, Thyagarajan S, Nagaraja N, Sanjay Kumar Rao N, Suresh Chandra J. Possible glutamatergic and lipid signalling mechanisms in ECT-induced retrograde amnesia: experimental evidence for involvement of COX-2, and review of literature. J Psychiatr Res. 2008;42:837–850. doi: 10.1016/j.jpsychires.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Naylor P, Stewart CA, Wright SR, Pearson RC, Reid IC. Repeated ECS induces GluR1 mRNA but not NMDAR1A-G mRNA in the rat hippocampus. Brain Res Mol Brain Res. 1996;35:349–353. doi: 10.1016/0169-328x(95)00264-s. [DOI] [PubMed] [Google Scholar]
- 10.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen P. A prelude to long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:613–615. doi: 10.1098/rstb.2002.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 13.Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 14.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 15.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Liu L, Liu YY, Luo J, Lin JY, Li X, et al. Effects of electroconvulsive stimulation on long-term potentiation and synaptophysin in the hippocampus of rats with depressive behavior. J ECT. 2012;28:111–117. doi: 10.1097/YCT.0b013e31824a47ca. [DOI] [PubMed] [Google Scholar]
- 17.Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 18.Vleminckx K, Kemler R, Hecht A. The C-terminal transactivation domain of beta-catenin is necessary and sufficient for signaling by the LEF-1/beta-catenin complex in Xenopus laevis. Mech Dev. 1999;81:65–74. doi: 10.1016/s0925-4773(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 21.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The wnt homepage. [Accessed August 31, 2015]. Available at: http://web.stanford.edu/group/nusselab/cgi-bin/wnt/
- 23.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118–122. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision (DSM-IV-TR) Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 28.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 29.Hedlund JL, Viewig BW. HRSD-21: The Hamilton Rating Scale for Depression. J Oper Psychiatry. 1979;10:149–165. [Google Scholar]
- 30.Petrides G, Fink M. The "half-age" stimulation strategy for ECT dosing. Convuls Ther. 1996;12:138–146. [PubMed] [Google Scholar]
- 31.Nakatake Y, Fukui N, Iwamatsu Y, Masui S, Takahashi K, Yagi R, et al. Klf4 cooperates with Oct3/4 and Sox2 to activate the Lefty1 core promoter in embryonic stem cells. Mol Cell Biol. 2006;26:7772–7782. doi: 10.1128/MCB.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaturvedi S, Chadda RK, Rusia U, Jain N. Effect of electroconvulsive therapy on hematological parameters. Psychiatry Res. 2001;104:265–268. doi: 10.1016/s0165-1781(01)00303-1. [DOI] [PubMed] [Google Scholar]
- 34.Kronfol Z, Nair MP, Weinberg V, Young EA, Aziz M. Acute effects of electroconvulsive therapy on lymphocyte natural killer cell activity in patients with major depression. J Affect Disord. 2002;71:211–215. doi: 10.1016/s0165-0327(01)00399-8. [DOI] [PubMed] [Google Scholar]
- 35.Yang S, Ji Y, Gattinoni L, Zhang L, Yu Z, Restifo NP, et al. Modulating the differentiation status of ex vivo-cultured anti-tumor T cells using cytokine cocktails. Cancer Immunol Immunother. 2013;62:727–736. doi: 10.1007/s00262-012-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


