Abstract
A 45-year-old man presented with dyspnea and hemoptysis during exercise. A chest computed tomography (CT) revealed multifocal diffuse patchy ground glass opacity and interlobular septal thickening in both the lungs. Permeability pulmonary edema or pulmonary hemorrhage was suspected. Serologic studies for autoimmune disorders and vasculitis were negative. There was no laboratory evidence of coagulopathy, other hematopoietic disease or infectious disease. Considering correlation with exercise, we diagnosed exercise-induced pulmonary hemorrhage (EIPH) or exercise-induced pulmonary edema (EIPE). The patient was managed with antifibrinolytics, antibiotics, and antitussive agent. After a week, follow-up chest CT revealed completely resolved pulmonary hemorrhage. About 2 months after the first event, he visited again with dyspnea and hemoptysis during running. In the present study, we report a case of recurrent pulmonary hemorrhage after exercise.
Keywords: Hemoptysis, Dyspnea, Hemorrhage
Introduction
Exercise-induced pulmonary hemorrhage (EIPH) or exercise-induced pulmonary edema (EIPE) have frequently been documented in racehorses. Exercise elevates pulmonary capillary pressures and can affect the integrity of the blood-gas barrier1. With extreme exertion, these changes result in increased permeability and cause bleeding into the lung1.
EIPH/EIPE have also been described with hard aerobic exercise in humans. The first report of hemoptysis in human athletes was published in 1979 after two marathon runners developed bloodstained sputum, dyspnea, and pulmonary edema during a race2. Since then, EIPH/EIPE have been reported often in marathon runners, triathletes, cyclists, and swimmers. Luks et al.3 described the case of a 38-year-old participant in the Bicycle Race Across America who developed pulmonary edema while riding. Lund et al.4 published three cases of well-trained men undergoing navy basic underwater training who experienced acute onset of swimming-induced pulmonary edema during 2-mile ocean swim. Most such cases have been described in extreme exertion.
We report a case of recurrent pulmonary hemorrhage after exercise in a healthy man, which begs the question: how can we prevent EIPH/EIPE during exercise?
Case Report
A 45-year-old male was referred with sudden symptoms of Medical Research Council (MRC) grade 4 dyspnea and hemoptysis after running 3 km. The volume of hemoptysis was about 30 mL of fresh blood with whitish secretion and bubbles. The patient had no specific past medical history except dyslipidemia. He was a commander and on indoor work ordinarily. The patient had no specific family history of medical disease. The patient was a 20 pack-years smoker.
The patient had bibasal crackles on auscultation. His initial oxygen saturation was 97% on 2 L of oxygen via nasal cannula. There was no laboratory evidence of coagulopathy or other hematopoietic disease. There was no laboratory evidence of infectious disease (white blood cell count, 7,200/µL; segment neutrophil, 64.8%; erythrocyte sedimentation rate, 5 mm/hr; C-reactive protein, 0.3 mg/dL; procalcitonin, 0.062 ng/mL). Rheumatoid factor, cold agglutinin, C3, C4, and CH50 were within normal range. Anti-glomerular basement membrane antibody, anti-myeloperoxidase antibody, anti-proteinase 3 antibody, anti-double stranded DNA antibody, and antinuclear antibody were negative. Serologic studies for human immunodeficiency virus also were negative. Pulmonary function tests were within normal range except high diffusing capacity of carbon monoxide/alveolar volume (134%).
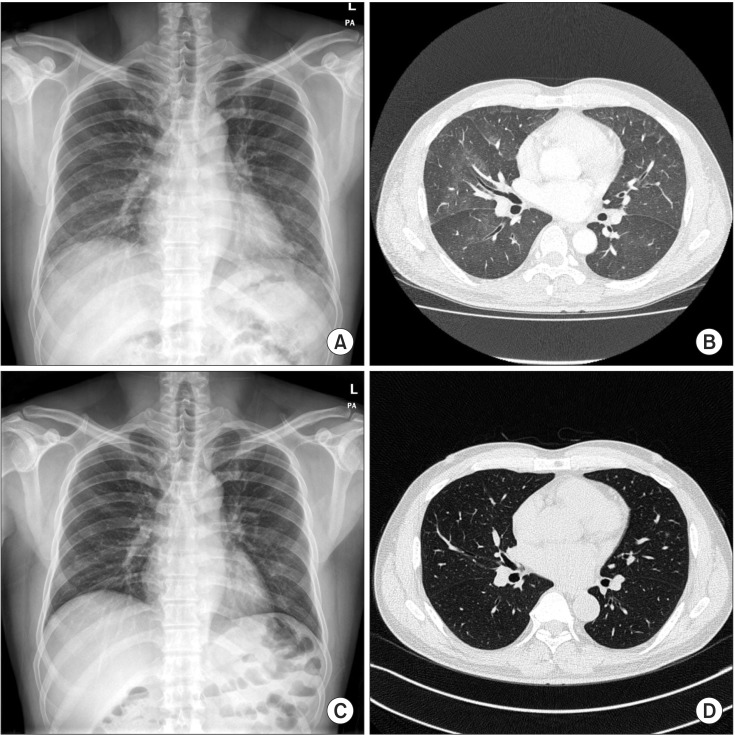
A chest radiograph showed ground glass opacity in both lower lung fields (Figure 1A). A chest computed tomography (CT) demonstrated multifocal diffuse, patchy ground glass opacity and interlobular septal thickening on both lungs (Figure 1B). Permeability pulmonary edema or pulmonary hemorrhage were compatible on chest CT scan.
Figure 1. (A) A chest radiograph obtained on the day of admission showed ground glass opacity in both the lower lung fields. (B) A chest computed tomography obtained on the day of admission demonstrates multifocal diffuse, patchy ground glass opacity and interlobular septal thickening in both the lungs and reveals permeability pulmonary edema or hemorrhage. (C) Five days after avoiding exercise, the chest radiograph shows resolving state of diffuse haziness in both the lungs. (D) Two weeks after avoiding exercise, a follow-up chest computed tomography was checked and reveals completely resolved multifocal diffuse, patchy ground glass opacity and interlobular septal thickening in both the lungs.
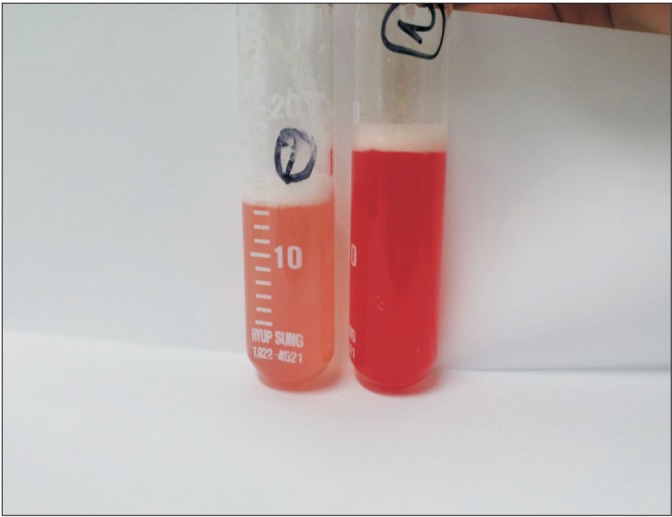
A flexible bronchoscopy was performed and small amount of blood was seen in right main bronchus, right middle lobe, right lower lobe, and left lung. Broncho-alveolar lavage (BAL) revealed diffuse alveolar hemorrhage and infection was ruled out (grossly pinkish color, red blood cell [RBC], 6,912; white blood cell, 238; polymorphonuclear cells, 6%; mononuclear cells, 83%; lymphocyte, 10%). The second aliquot of BAL fluid was more fresh blood than the first aliquot. It indicated alveolar hemorrhage (Figure 2). Transbronchial lung biopsy could not be performed because of severe cough. Cytologic evaluation and culture ruled out malignancy and infectious process. A trans-thoracic echocardiography showed no structural or functional basis for pulmonary venous hypertension, left ventricular dysfunction and valvular abnormality.
Figure 2. The first and second aliquots of broncho-alveolar lavage fluids became increasingly hemorrhagic on sequential sampling and revealed pulmonary hemorrhage.
The patient was managed with supplemental oxygen, antifibrinolytics, antibiotics, and antitussive agent. No more hemoptysis and dyspnea were seen during hospitalization several days later. Five days after avoiding exercise, a chest radiograph showed resolving state of diffuse haziness in both lungs (Figure 1C). The patient was discharged on hospital day 5 and then followed up in the outpatient department. One week after discharge, a follow-up chest CT scan revealed completely resolved multifocal diffuse, patchy ground glass opacity, and interlobular septal thickening in both lungs (Figure 1D).
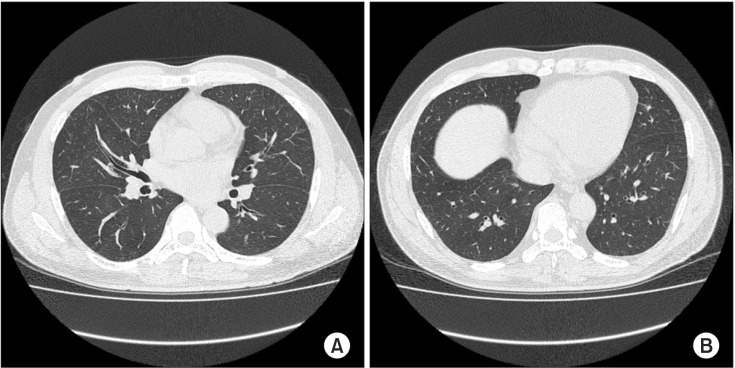
The patient was regularly followed-up in the outpatient department without exercise. About 2 months after discharge, the patient revisited with dyspnea and hemoptysis during running 10 days ago. Ground glass opacity was developed newly in the entire lung on chest CT scan (Figure 3). An exercise provocation test was performed and the result was negative and no hemoptysis was seen during the exam. We ruled out exercise-induced asthma and diagnosed EIPH/EIPE. After resting and avoiding exercise, dyspnea and hemoptysis were improved. The patient did not visit our hospital again.
Figure 3. (A, B) A chest computed tomography obtained on the second event of hemop tysis demonstrates presence of new ground glass opacity in the entire lung.
Discussion
EIPH/EIPE occurs frequently in horses. Almost all thoroughbreds show bleeding in the lower respiratory tract after a race5. Despite the high prevalence of EIPH/EIPE in a variety of species including horses, there is little understanding of its pathogenesis and prevention. The horse generates enormous cardiac outputs and ventilatory volumes during exercise6. Extremely positive intravascular and negative extravascular pressures are acquired within the pleural cavity6. These pressures summate at the fragile blood-gas barrier and lead to capillary stress failure and extravasation of RBCs into the alveoli6.
Recent studies suggest that the pathogenesis of EIPH/EIPE is more complex than simple capillary wall tearing7. In the hardly exercising horse, the transmural alveolar capillary pressure is about 42 mm Hg8. These pressures may not be sufficient to induce rupture of pulmonary capillaries. Thus, other factors besides pulmonary arterial hypertension may contribute to EIPH/EIPE in horses7. Recent histological studies identified extensive remodeling of small pulmonary veins in EIPH/EIPE-affected horse lungs9. Strenuous exercise results in high pulmonary vascular pressure and high vascular pressure results in pulmonary vein wall remodeling10. The venous lesion is represented by expansion of adventitial collagen, intimal fibrosis and medial smooth muscle hypertrophy9. Those lesions lead to diminished vein lumen diameter9. During hard exercise, venous occlusion induces regionally increased pulmonary capillary pressure, capillary rupture and bleeding10. Hull et al.11 reported a case of exercise-induced hemoptysis with significant focal stenosis of the left common pulmonary vein, which could be a complication of pulmonary venous ablation therapy for atrial fibrillation. Pulmonary venous stenosis can also induce increased pulmonary capillary pressure and capillary rupture. Therefore, pressure-induced stress failure of capillaries can arise from both physiological (e.g., exercise-induced capillary hypertension) and pathological (e.g., mitral stenosis and pulmonary veno-occlusive disease) causes12.
Consequently, there are studies of methods to reduce the pressure difference across the pulmonary capillary membrane in order to reduce EIPH by reducing the intravascular pressure or increasing the intrathoracic and alveolar pressure13. The treatment for horses with EIPH/EIPE is administration of furosemide before intense exercise13. Furosemide reduces pulmonary arterial pressure and pulmonary capillary wedge pressure14. Furosemide dose-dependent decreases were observed in exercising horses14. Therefore, furosemide may reduce the occurrence of EIPH/EIPE.
In the pathogenesis of EIPH/EIPE, the role of small airway inflammation and bronchoconstriction is unclear14. Horses with EIPH/EIPE are often treated with drugs to decrease lower airway inflammation and relieve bronchoconstriction13. The efficacy of beta-adrenergic bronchodilators for treatment of EIPH is still unclear in humans13. Ipratropium, an inhaled parasympatholytic drug, helped prevent EIPH/EIPE in two horses13. Corticosteroids are often administered to reduce pulmonary inflammation, but have no demonstrated efficacy in preventing EIPH14. There is a lack of information demonstrating the effectiveness of a medication or management in the treatment and prevention of EIPH/EIPE in humans. Further investigation is therefore needed.
There was no hemoptysis during the exercise provocation test in our case. The pressure of the pulmonary capillaries might be lower than threshold pressure for capillary breaks. The previous case report showed that EIPH in humans may occur without any evidence on clinical presentation (e.g., symptoms, findings on physical examination, radiographic abnormalities)1. In that case, only the collected fluid by bronchoscopy provides evidence of EIPH1. The incidence of EIPH may be greater than suspected. Although there was no evidence of EIPH in our patient after the first hospitalization, we might have been unable to detect the presentations of bleeding.
There was no evidence of structural abnormality in the lung parenchyma that would induce diffuse alveolar hemorrhage. Moreover, our patient recovered in a few days without sequelae. Considering correlation with exercise, we diagnosed EIPH.
Epp et al.15 reported EIPH during submaximal exercise in five thoroughbred horses that previously experienced EIPH. That report suggested that extravascular factors (e.g., elevated extravascular pressure, relatively large tidal volume during submaximal exercise, upper airway obstruction) are important in the etiology of EIPH15. We could not investigate extravascular factors during exercise, and we did not identify why the patient suffered from EIPH at relatively low exercise intensity.
There are some limitations of our report. First, because we could not perform a biopsy, possible histologic problems on lung parenchyma could not be ruled out. Second, further radiologic evaluation (i.e., magnetic resonance imaging or computed tomography angiography) was not performed so structural problems in the pulmonary vasculature could not be examined. Lung magnetic resonance angiography could be an especially good method for non-invasive acquisition of high-quality three-dimensional anatomical images without radiation11. If pulmonary vascular structure was evaluated, we might find the possible causes of hemoptysis. Third, because we did not check hemosiderin-laden macrophages on cytologic examination, we confirmed alveolar hemorrhage indirectly using sequential aliquots of BAL fluid.
This specific case demonstrates EIPH in a Korean male. EIPH is not well investigated and few cases have been reported in Korea. Therefore, further investigations that may define the incidence and characteristics of EIPH in our country are needed.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Ghio AJ, Ghio C, Bassett M. Exercise-induced pulmonary hemorrhage after running a marathon. Lung. 2006;184:331–333. doi: 10.1007/s00408-006-0023-2. [DOI] [PubMed] [Google Scholar]
- 2.McKechnie JK, Leary WP, Noakes TD, Kallmeyer JC, MacSearraigh ET, Olivier LR. Acute pulmonary oedema in two athletes during a 90-km running race. S Afr Med J. 1979;56:261–265. [PubMed] [Google Scholar]
- 3.Luks AM, Robertson HT, Swenson ER. An ultracyclist with pulmonary edema during the Bicycle Race Across America. Med Sci Sports Exerc. 2007;39:8–12. doi: 10.1249/01.mss.0000235885.79110.79. [DOI] [PubMed] [Google Scholar]
- 4.Lund KL, Mahon RT, Tanen DA, Bakhda S. Swimming-induced pulmonary edema. Ann Emerg Med. 2003;41:251–256. doi: 10.1067/mem.2003.69. [DOI] [PubMed] [Google Scholar]
- 5.West JB, Mathieu-Costello O, Jones JH, Birks EK, Logemann RB, Pascoe JR, et al. Stress failure of pulmonary capillaries in racehorses with exercise-induced pulmonary hemorrhage. J Appl Physiol (1985) 1993;75:1097–1109. doi: 10.1152/jappl.1993.75.3.1097. [DOI] [PubMed] [Google Scholar]
- 6.Kindig CA, McDonough P, Fenton G, Poole DC, Erickson HH. Efficacy of nasal strip and furosemide in mitigating EIPH in Thoroughbred horses. J Appl Physiol (1985) 2001;91:1396–1400. doi: 10.1152/jappl.2001.91.3.1396. [DOI] [PubMed] [Google Scholar]
- 7.Williams KJ, Robinson NE, Defeijter-Rupp H, Millerick-May M, Stack A, Hauptman J, et al. Distribution of venous remodeling in exercise-induced pulmonary hemorrhage of horses follows reported blood flow distribution in the equine lung. J Appl Physiol (1985) 2013;114:869–878. doi: 10.1152/japplphysiol.01170.2012. [DOI] [PubMed] [Google Scholar]
- 8.Sinha AK, Gleed RD, Hakim TS, Dobson A, Shannon KJ. Pulmonary capillary pressure during exercise in horses. J Appl Physiol (1985) 1996;80:1792–1798. doi: 10.1152/jappl.1996.80.5.1792. [DOI] [PubMed] [Google Scholar]
- 9.Williams KJ, Derksen FJ, de Feijter-Rupp H, Pannirselvam RR, Steel CM, Robinson NE. Regional pulmonary veno-occlusion: a newly identified lesion of equine exercise-induced pulmonary hemorrhage. Vet Pathol. 2008;45:316–326. doi: 10.1354/vp.45-3-316. [DOI] [PubMed] [Google Scholar]
- 10.Derksen F, Williams K, Stack A. Exercise-induced pulmonary hemorrhage in horses: the role of pulmonary veins. Compend Contin Educ Vet. 2011;33:E6. [PubMed] [Google Scholar]
- 11.Hull JH, Menzies-Gow A, Nicholson AG, Mohiaddin RH, Maher TM. Exercise-induced haemoptysis: a thoroughbred cause? Thorax. 2013;68:599–600. doi: 10.1136/thoraxjnl-2012-202209. [DOI] [PubMed] [Google Scholar]
- 12.West JB. Invited review: pulmonary capillary stress failure. J Appl Physiol (1985) 2000;89:2483–2489. doi: 10.1152/jappl.2000.89.6.2483. [DOI] [PubMed] [Google Scholar]
- 13.Hinchcliff KW. Exercise-induced pulmonary hemorrhage. In: Pagan JD, editor. Advanced in equine nutrition. Vol. IV. Nottingham: Nottingham University Press; 2009. pp. 367–377. [Google Scholar]
- 14.Manohar M, Hutchens E, Coney E. Frusemide attenuates the exercise-induced rise in pulmonary capillary blood pressure in horses. Equine Vet J. 1994;26:51–54. doi: 10.1111/j.2042-3306.1994.tb04331.x. [DOI] [PubMed] [Google Scholar]
- 15.Epp TS, McDonough P, Padilla DJ, Gentile JM, Edwards KL, Erickson HH, et al. Exercise-induced pulmonary haemorrhage during submaximal exercise. Equine Vet J Suppl. 2006;(36):502–507. doi: 10.1111/j.2042-3306.2006.tb05595.x. [DOI] [PubMed] [Google Scholar]