Abstract
Amyloidosis is defined as the presence of extra-cellular deposits of an insoluble fibrillar protein, amyloid. The pulmonary involvement of amyloidosis is usually classified as tracheobronchial, parenchymal nodular, or diffuse alveolar septal. A single nodular lesion can mimic various conditions, including malignancy, pulmonary tuberculosis, and fungal infection. To date, only one case of nodular pulmonary amyloidosis has been reported in Korea, a case involving multiple nodular lesions. Here, we report and discuss the case of a patient having single nodular amyloidosis.
Keywords: Amyloidosis
Introduction
Amyloidosis is a condition involving extra-cellular deposits of an insoluble fibrillar protein called amyloid1,2. It is classified as either primary or secondary based on the presence of concomitant diseases, and as limited or systemic based on the extent of organ invasion. Secondary limited pulmonary amyloidosis is extremely rare.
Cases of pulmonary invasion are categorized as tracheobronchial, pulmonary parenchymal nodular, or diffuse alveolar septal. The tracheobronchial type is the most common, and usually involves multiple lesions. The pulmonary parenchymal nodular type can easily be misidentified a slung carcinoma, and both single and multiple lesions are possible3. It has been reported that the pulmonary parenchymal nodular type occurs at higher frequency than the other types. However, to date only one case has been reported in Korea, a case involving multiple nodular lesion. Herein, we report a case of primary single nodular pulmonary amyloidosis recently encountered in Korea.
Case Report
A 54-year-old female was admitted to our hospital with subacute cough for the last month. The patient had a history of multilobar (right middle lobe and right upper lobe) pneumonia over the past 2 years, and had been admitted to a nearby hospital with the chief complaint of a cough that had worsened over the previous month. She usually had a dry cough after a cold, which did not last for more than 2 months. Following a diagnosis of acute eosinophilic bronchitis and focal pneumonia, the patient was administered antibiotics. However, her pneumonia did not improve and she was transferred to our hospital for further examination.
She had no disease and no operation history. She was nonsmoker and social drinker. She had no family history and her occupation was teacher.
1. Physical examination
Blood pressure was 110/60 mm Hg, pulse rate was 60 beats per minute, respiration rate was 20 per minute, and body temperature was 36.8℃. General findings indicated no pain, but chest auscultation revealed minor rales in the right middle lobe. Heart auscultation revealed a normal beat without cardiac murmur. No edema was observed in the abdomen or extremities, and no lymph node enlargement was apparent.
2. Laboratory findings
The results of an arterial blood gas examination performed at the time of admission were PH 7.43, PaCO2 33 mm Hg, PaO2 78 mm Hg, and HCO3- 22 mEq/L. A peripheral blood examination revealed a hemoglobin level of 13.1 g/dL, hematocrit 44%, a white blood cell count of 8,600/mm3, and a platelet count of 250,000/mm3.
The results of biochemical analysis were a total protein level of 6.8 g/dL, albumin 4.1 g/dL, aspirate aminotransferase 38 IU/L, alkaline phosphatase 200 mg/dL, erythrocyte sedimentation rate 30 m/hr, blood urea nitrogen 12.9 mg/dL, and creatinine 0.9 mg/dL. Electrolytes and blood glucose levels were normal, and urine examination revealed specific gravity of 1.025, pH 6.0, no proteinuria, and a red blood cell count of 30-49 per high-power field. Subsequent examination revealed no worsening of hematuria. Empirical antibiotic treatment, acid-fast bacillus (AFB) sputum smear, and sputum eosinophil tests were performed, in order to differentiate community-acquired pneumonia and pulmonary tuberculosis (TB). Sputum bacteria examination did not yield specific findings, and very few sputum eosinophils were observed. The concentration of the tumor marker carcinoembryonic antigen detected was 1.3 ng/mL(with-in normal range), and an interferon-gamma release assay was positive. However, the AFB smear and TB polymerase chain reaction were negative, and AFB culture yielded no-growth. Tests for rheumatic factors and antinuclear antibody yielded no specific findings, and the patient did not complain of rheumatic-disease-related symptoms. Electrocardiography and echocardiography yielded normal results, and pulmonary function examination revealed a forced vital capacity (FVC) of 2.83 L (90% of reference), forced expiratory volume in the first second (FEV1) 2.27 L (93% of reference), FEV1/FVC 92%, and diffusion capacity adjusted by the alveolar volume 97%, indicating mild obstructive ventilation disturbance.
3. Imaging findings
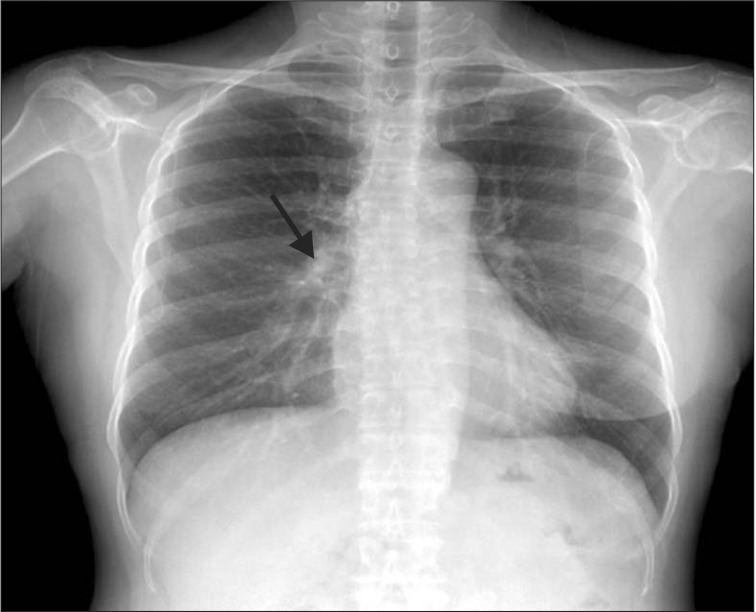
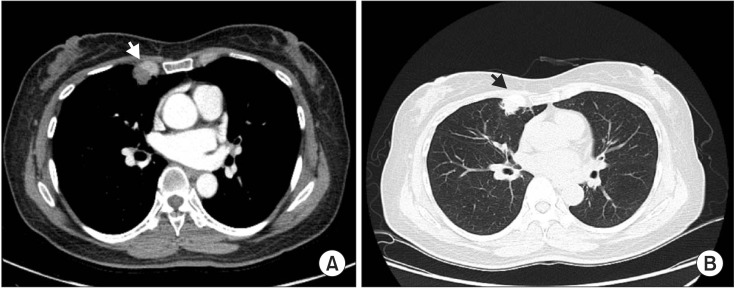
Simple chest radiography revealed nodular lesions around the pulmonary hilum of the right upper lobe (Figure 1). Chest computed tomography (CT) revealed a 1.8-cm speculated nodule around the inferior margin of the right upper lobe, and the differential diagnosis of focal pneumonia or lung cancer was required (Figure 2). A bronchoscopy revealed no abnormalities in the bronchi. Kidney ultrasonography and a gastroscopy that were performed to confirm the amyloidosis lesions did not yield specific findings; so video assisted thoracic surgery (VATS)-assisted pulmonary wedge resection was conducted for early differentiation of lung cancer. No surgical complications were encountered.
Figure 1. Simple chest radiography showing a nodular lesion in the right upper lobe inferior margin near the hilum.
Figure 2. (A, B) Chest computed tomography showing an approximately 1.8-cm subpleural nodular consolidation with some spiculation in the inferior margin of the right upper lobe (arrowheads).
4. Histology findings
Histology indicated a thickened interstitium due to eosinophilic amorphous material deposits, with some giant cells and histiocyte infiltration (Figure 3). Congo red staining for amyloidosis was positive, and under a polarized microscope the nodule exhibited a unique apple-green birefringence (Figure 4).
Figure 3. Histopathological examination of the nodule showing eosinophilic, amorphous material deposits (H&E stain, ×100).
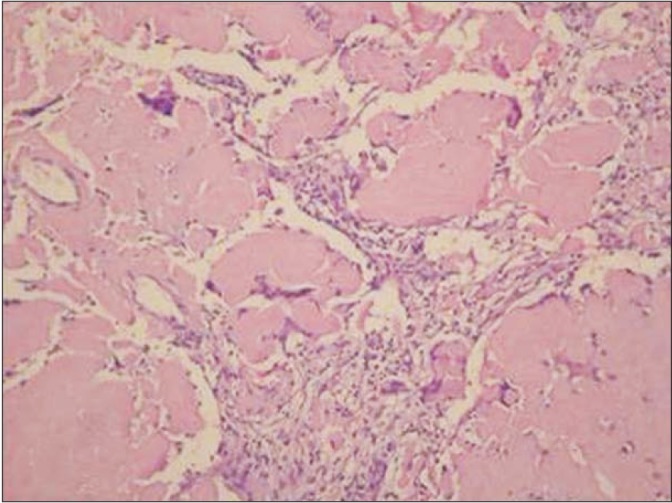
Figure 4. The nodule shows an apple-green birefringence, that is regarded as diagnostic (Congo red staining, as seen under a polarized microscope, ×100).

5. Additional examinations
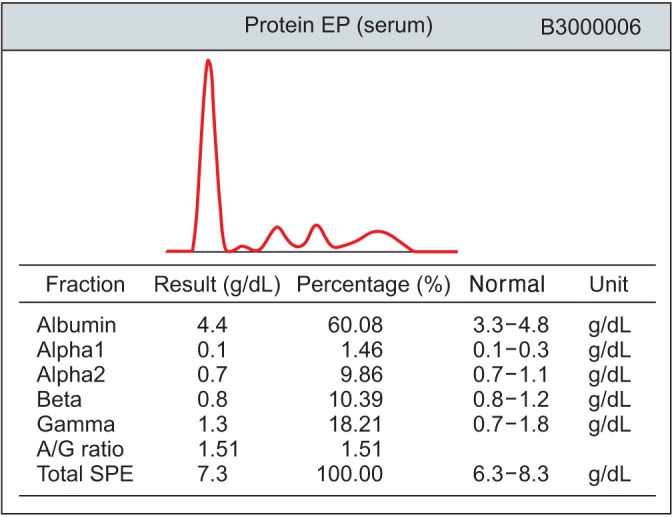
Additional examinations for amyloidosis, blood and urine immunoelectrophoresis, and immunofixation were performed. IgG, IgM, IgA, IgE, and IgD concentrations were normal, as were the immunofixation results (Figure 5). A bone marrow biopsy was not performed, in accordance with the patient's request.
Figure 5. Normal serum protein electrophoresis (EP) results. SPE: serum protein electrophoresis.
Discussion
Amyloidosis is characterized by extra-cellular deposits of an insoluble fibrillar protein (amyloid) with a corrugated plate structure. Upon reacting with Congo red, the protein exhibits an apple-green birefringence under a polarized microscope4. Amyloid deposits can appear in various locations including the kidney, nerves, blood vessels, liver, spleen, gastrointestinal tract, heart, and respiratory system.
Amyloidosis was generally categorized as "primary" and "secondary" in the past. As studies on amyloidosis have progressed however, cases with kappa and lambda light chain deposits are now referred to as amyloid light chain (AL) amyloidosis, and those with plasma amyloid P deposits due to chronic infectious disease are called amyloid A (AA) amyloidosis. AA amyloidosis mainly occurs in patients with rheumatic and autoimmune diseases. In the present case, the results of blood tests for rheumatic factors were normal, and there were no specific findings relating to disease history. AL amyloidosis is occasionally related to multiple myeloma and Waldenström macroglobulinemia, and asymptomatic monoclonal gammopathy can be detected via protein electrophoresis. Among the patients with AL amyloidosis who presented at the Mayo Clinic from 1960 to 1994, 0.4% developed multiple myeloma2. In a study of 4,319 patients with multiple myeloma conducted from 1990 to 2008, 1.1% had AL amyloidosis5,6.
Amyloidosis localized to the respiratory system is very rare, and is classified as parenchymal nodular, tracheobronchial, or diffuse alveolar septal according to the type of pulmonary invasion involved2. The Mayo Clinic reported 17 cases of pulmonary amyloidosis based on data from 1908 to 1993, and of them, seven were of the nodular type, four were of the tracheobronchial type, and none were of the alveolar septal type2. In a report by Hui et al.7, 28 of 48 cases were of the nodular type, 14 were tracheobronchial type, and six were alveolar septal type. In the collective international literature, cases of nodular pulmonary amyloidosis are more common than the other types.
The tracheobronchial type can accompany a variety of symptoms, including dyspnea, hemoptysis, and cough, but it can also be asymptomatic in some cases. On the other hand, the pulmonary parenchymal nodular type is mostly asymptomatic, and therefore it is usually detected via procedures performed for other reasons, such as pulmonary radiographic scanning. The diffuse alveolar septal type exhibits progressive pulmonary fibrosis patterns, and most patients experience respiratory failure leading to death2. On the other hand, the pulmonary parenchymal nodular and tracheobronchial types have a favorable prognosis including long-term survival, and are seldom associated with systemic amyloidosis. However, they may be associated with worsening of dyspnea and pneumonia, depending on the location of the obstruction2.
Once single nodular lesions are observed, it is necessary to differentiate the condition from other diseases such as malignant nodules and pulmonary TB. Some studies suggest that positron emission tomography-computed tomography (PET-CT), considered for malignancy differentiation, can show significant fluorine-18-2-fluoro-2-deoxy-d-glucose activity even in positive nodules associated with pulmonary parenchymal amyloidosis8. Because we consider the benign nature first, then do PET-CT according to biopsy result. But a bronchoscopy revealed no lesions within the bronchi in the case presented herein, therefore VATS-assisted pulmonary wedge resection was performed for diagnostic confirmation and to assist in the formulation of therapeutic approaches. Although pulmonary wedge resection was performed in the present case for malignancy differentiation, the medical history and imaging investigations were not clearly indicative of amyloidosis as a potential diagnosis.
Pulmonary amyloidosis is relatively rare in Korea. Individual cases of diffuse alveolar septal pulmonary amyloidosis identified via transtracheal lung biopsy were reported in 1990, 1998, and 20143,9,10. Tracheobronchial cases were confirmed and reported via transtracheal lung biopsy in 1993 and 200211,12. A case of the pulmonary parenchymal nodular type was confirmed via a pulmonary fine needle biopsy in 200013, and multiple lesions were detected in that case. To date, single nodular amyloidosis has not been reported in Korea.
Generally, treatment of the different types of amyloidosis varies with the cause of fibril production. As examples, therapy is aimed at the underlying infection or inflammatory disorder in secondary amyloidosis, and at the underlying plasma cell dyscrasia in primary amyloidosis. The preferred therapies of secondary amyloidosis are colchicine (particularly in familial Mediterranean fever), and anti-proinflammatory cytokines such as interleukin 1β and tumor necrosis factor α (in rheumatic and hereditary auto-inflammatory disorders)14. The treatment of AL amyloidosis is similar to that for multiple myeloma, and may include melphalan, and occasionally hematopoietic cell transplantation15. In cases of pulmonary nodular amyloidosis however, resection of lesions should be considered, and the patient should be followed up regardless of whether the condition recurs or not. The size of nodular amyloidosis has 3 cm in average, the right lung was involved 2.5 times as often as the left, especially the right lower lobe. The prognosis of the nodular type of pulmonary amyloidosis remains unclear, due to a lack of reports; however, it is better than that of septal and tracheobronchial types. The nodular type usually does not lead to death and is not associated with systemic amyloidosis7. In the case reported herein, the patient refused a bone marrow biopsy and had no rheumatoid disease or familial autoimmune disease history. After lung surgery, she exhibited no symptoms or complications. She has subsequently visited every 1 or 2 months, at which times she has been checked via simple X-rays, which to date have shown no significant changes. She is scheduled for imaging tests such as 2D-echocardiogram, chest and abdominal CT and renal ultrasound to screen for the invasion of other organs within 6 months.
Nodular pulmonary amyloidosis is not frequently reported in Korea. Additional studies are required to investigate whether this is due to a low diagnostic rate, or other factors such as race. This case-report has the limitation that bone marrow and amyloid kappa and lambda light chain examinations were not performed. However, biopsies are highly recommended for pulmonary solitary nodular lesions, and further studies on this rare condition are necessary. CT follow-up examinations tend to be preferred for lesions with positive nodules, and biopsies are mostly performed after changes in lesion size and number. This may function to reduce the diagnostic rate of single nodules. Unfortunately, much information on pulmonary nodular amyloidosis is lacking, such as that relating to prognosis, recurrence rate, and definitive diagnostic course. Thus, further studies of amyloidosis are necessary in Korea, and the authors considered the reporting of this case to be a valuable contribution to the collective knowledge base.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, et al. Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2012;19:167–170. doi: 10.3109/13506129.2012.734345. [DOI] [PubMed] [Google Scholar]
- 2.Utz JP, Swensen SJ, Gertz MA. Pulmonary amyloidosis. The Mayo Clinic experience from 1980 to 1993. Ann Intern Med. 1996;124:407–413. doi: 10.7326/0003-4819-124-4-199602150-00004. [DOI] [PubMed] [Google Scholar]
- 3.Shin B, Ko J, Lee SS, Lim KS, Han JH, Chung MP, et al. A case of pulmonary amyloidosis mimicking lymphangitic lung carcinomatosis. Korean J Med. 2014;86:339–342. [Google Scholar]
- 4.Glenner GG. Amyloid deposits and amyloidosis: the beta-fibrilloses (first of two parts) N Engl J Med. 1980;302:1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Gertz MA, Kyle RA. Primary systemic amyloidosis with delayed progression to multiple myeloma. Cancer. 1998;82:1501–1505. [PubMed] [Google Scholar]
- 6.Madan S, Dispenzieri A, Lacy MQ, Buadi F, Hayman SR, Zeldenrust SR, et al. Clinical features and treatment response of light chain (AL) amyloidosis diagnosed in patients with previous diagnosis of multiple myeloma. Mayo Clin Proc. 2010;85:232–238. doi: 10.4065/mcp.2009.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui AN, Koss MN, Hochholzer L, Wehunt WD. Amyloidosis presenting in the lower respiratory tract. Clinicopathologic, radiologic, immunohistochemical, and histochemical studies on 48 cases. Arch Pathol Lab Med. 1986;110:212–218. [PubMed] [Google Scholar]
- 8.Kung J, Zhuang H, Yu JQ, Duarte PS, Alavi A. Intense fluorodeoxyglucose activity in pulmonary amyloid lesions on positron emission tomography. Clin Nucl Med. 2003;28:975–976. doi: 10.1097/01.rlu.0000099807.66221.64. [DOI] [PubMed] [Google Scholar]
- 9.Kim CH, Kim S, Kwon OJ, Han SK, Lee JS, Kim KY. Pulmonary diffuse alveolar septal amyloidosis: diagnosed by transbronchial lung biopsy. Korean J Intern Med. 1990;5:63–68. doi: 10.3904/kjim.1990.5.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HS, Kim HJ, Kho YM, Seo JY, Chung MP, Kwon OJ, et al. A case of pulmonary diffuse alveolar amyloidosis localized in the lung. Korean J Med. 1998;55:956–959. [Google Scholar]
- 11.Kwon SW, Kim YK, Jung KH, Kim DS, Jeon WK, Suh YL. A case of tracheo-bronchial amyloidosis. Korean J Med. 1993;45:690–695. [Google Scholar]
- 12.Kwak YG, Kim HJ, Lee CH, Kim SY, Cho JH, Kwak SM, et al. A case of primary localized tracheobronchial amyloidosis. Tuberc Respir Dis. 2002;52:174–178. [Google Scholar]
- 13.Jung SK, Oh J, Roh YW, Kong HS, Park KY, Park JW, et al. A case of primary diffuse nodular pulmonary amyloidosis localized in the lung. Tuberc Respir Dis. 2000;49:365–371. [Google Scholar]
- 14.Gottenberg JE, Merle-Vincent F, Bentaberry F, Allanore Y, Berenbaum F, Fautrel B, et al. Anti-tumor necrosis factor alpha therapy in fifteen patients with AA amyloidosis secondary to inflammatory arthritides: a followup report of tolerability and efficacy. Arthritis Rheum. 2003;48:2019–2024. doi: 10.1002/art.11163. [DOI] [PubMed] [Google Scholar]
- 15.Palladini G, Perfetti V, Obici L, Caccialanza R, Semino A, Adami F, et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103:2936–2938. doi: 10.1182/blood-2003-08-2788. [DOI] [PubMed] [Google Scholar]