Abstract
Objective
The aim of this study was to evaluate the impact of surgical waiting time on clinical outcome in early stage cervical cancer.
Methods
The cohort consisted of 441 patients diagnosed with stages IA2-IB1cervical cancer who underwent radical hysterectomy and pelvic node dissection. The patients were divided into two groups based on surgical waiting time. The associations between waiting time and other potential prognostic factors with clinical outcome were evaluated.
Results
The median surgical waiting time was 43 days. Deep stromal invasion (hazard ratio [HR], 2.5; 95% confidence interval [CI], 1.4 to 4.6; p=0.003) and lymph node metastasis (HR, 2.9; 95% CI, 1.3 to 6.7; p=0.026) were identified as independent prognostic factors for recurrence-free survival while no prognostic significance of surgical waiting time was found (p=0.677). On multivariate analysis of overall survival (OS), only deep stromal invasion (HR, 2.6; 95% CI, 1.3 to 5.0; p=0.009) and lymph node metastasis (HR, 3.6; 95% CI, 1.5 to 8.6; p=0.009) were identified as independent prognostic factors for OS. Although OS showed no significant difference between short (≤8 weeks) and long (>8 weeks) waiting times, multivariate analysis of OS with time-varying effects revealed that a waiting time longer than 8 weeks was associated with poorer long-term survival (after 5 years; HR, 3.4; 95% CI, 1.3 to 9.2; p=0.021).
Conclusion
A longer surgical waiting time was associated with diminished long-term OS of early stage cervical cancer patients.
Keywords: Prognosis, Radical Hysterectomy, Uterine Cervical Neoplasms, Waiting Time
INTRODUCTION
Cervical cancer is the fourth most common cancer in women with an estimated 527,600 new cases and 265,700 deaths from cervical cancer worldwide in 2012. It is the second most commonly diagnosed cancer and third leading cause of cancer death among females in developing countries [1]. The large geographic variation in cervical cancer rates reflects differences in the availability of screening for detection and removal of precancerous lesions as well as human papillomavirus infection prevalence [1,2]. In Thailand, cervical cancer is the second most common cancer in women after breast cancer, and it is an important public health problem. In 2008, there were 6,453 new cases of cervical cancer [3]. However, the incidence of cervical cancer in Thailand is decreasing. The significant factor underlying this decrease is the dual-tract cervical screening program using both a Pap smear and a visual inspection with acetic acid and cryotherapy treatment [3,4]. This program helps identify cervical cancer at the earliest stages [4].
Radical hysterectomy with bilateral lymph node dissection is the mainstay surgical treatment for early stage cervical cancer [5]. Consequently, the time from diagnosis of cervical cancer until the time that it is resected can influence the overall outcome. In general, long waiting times are associated with poor access to services and poor quality of care [6]. The effect of surgical delay on survival has been studied in a variety of cancers including breast cancer, renal cell carcinoma, pancreatic cancer, stomach cancer, uterine cancer, and head and neck cancer. However, the evidence for this relationship remains inconclusive [6,7,8,9,10].
Currently, no guidelines exist regarding the appropriate interval from diagnosis to surgery for early stage cervical cancer. Recommendations for timely referral and treatment have been suggested, but no clinical data yet confirm a specific time frame. Currently, the Canadian Society of Surgical Oncology and the United Kingdom Health Service recommend no more than 4 weeks of waiting time from diagnosis to surgery for any malignancy [7].
To the best our knowledge, only two studies have addressed the effect of waiting time for cervical cancer surgery [11,12]. Umezu et al. [11] evaluated the impact of waiting time in 117 early stage cervical cancer patients. They reported that the waiting time between operation and initial visit did not adversely affect the outcome in cervical cancer [11]. Perri et al. [12] studied 321 cervical cancer patients and also found that longer waiting times from diagnosis to treatment (surgery, chemoradiotherapy, or neoadjuvant) were not associated with worse survival. However, both studies were based on retrospective data from a single institution with a limited number of patients. One of these studies included patients who were heterogeneous in terms of tumor stage and treatment modalities [12]. Therefore, to address this issue, we evaluated the effect of surgical waiting time on clinical outcomes in a large cohort of early stage cervical cancer patients at Songklanagarind Hospital, the major tertiary care institution in southern Thailand.
MATERIALS AND METHODS
We obtained approval from the Ethics Committee for Research Involving Human Subjects of the Prince of Songkla University Faculty of Medicine before the study. From January 1996 to September 2012, 474 patients with cervical cancer were enrolled. These patients had International Federation of Gynecology and Obstetrics (FIGO) stages IA2 or IB1 and underwent a radical hysterectomy with pelvic lymph node dissection at Songklanagarind Hospital. Patients who had received chemotherapy before undergoing radical hysterectomy were excluded. Of these 474 patients, 43 patients were excluded due to incomplete data leaving 441 patients in this study.
All pertinent clinical data from the medical records (age, tumor size, stage, histology, lymph-vascular space invasion (LVSI), deep stromal invasion (DSI), lymph node status, parametrial involvement, marginal involvement, adjuvant therapy, and clinical outcome) were retrospectively reviewed. Clinical stage and histological classification were based on the criteria established by the revised FIGO 2009 and World Health Organization. Adjuvant therapy after surgery was administered according to surgical risk factors. Follow-up after treatment was every 3 months in the first year, every 4 months in the second year, every 6 months in the 3rd to 5th years, and annually thereafter.
The waiting time was calculated from the date of diagnosis (date of entry for clinical staging at our hospital) to the date of operation. The primary endpoints were recurrence-free survival (RFS) and overall survival (OS). The RFS was calculated from the date of operation to the date of recurrence, and OS from the date of operation to the date of death or last follow-up, whichever occurred earlier. Patients who were lost to follow-up or did not experience an event during follow-up were considered to be censored at the latest date at which their status was known. Surgical waiting times were analyzed as categorical variables by grouping fewer than or equal to 4 weeks versus longer than 4 weeks and also grouping fewer than or equal to 8 weeks versus longer than 8 weeks. Univariate analysis of RFS and OS was performed using the Kaplan-Meier method. Multivariate Cox proportional-hazards regression identified variables independently associated with RFS and OS, and models refined by a process of backward elimination of variables did not significantly contribute to the fit as indicated by the change in log likelihood of successive models. A p<0.05 was considered to be statistically significant. To satisfy the assumption of proportional hazards, waiting times ≤8 weeks versus >8 weeks were fitted as a time-varying effect variable in the OS model. The data were analyzed using STATA ver. 10 (Stata Co., College Station, TX, USA).
RESULTS
There were 441 patients identified. The mean age of these patients was 45.9 years with a range of 26 to 78 years. Histopathological diagnosis included 266 (60.3%) squamous cell carcinoma, 140 (31.8%) adenocarcinoma, 25 (5.7%) adenosquamous carcinoma, and 10 (2.3%) other cell types. The median surgical waiting time was 43 days (interquartile range, 29 to 65 days). Overall, 64.4% underwent surgery within 8 weeks and 35.6% after 8 weeks. Table 1 shows the clinicopathological characteristics of the 441 patients.
Table 1. The clinicopathological characteristics of the early stage cervical cancer patients.
| Characteristic | No. (%) |
|---|---|
| Age (yr) | |
| <35 | 40 (9.1) |
| 35-60 | 369 (83.7) |
| >60 | 32 (7.2) |
| Tumor size (cm) | |
| ≤2 | 305 (69.2) |
| >2 | 136 (30.8) |
| FIGO stage | |
| IA2 | 45 (10.2) |
| IB1 | 395 (89.8) |
| Histopathology | |
| Squamous cell carcinoma | 266 (60.3) |
| Adenocarcinoma | 140 (31.8) |
| Adenosquamous carcinoma | 25 (5.7) |
| Other | 10 (2.3) |
| Lymph vascular space invasion | |
| Yes | 109 (24.7) |
| No | 332 (75.3) |
| Deep stromal invasion | |
| Yes | 99 (22.5) |
| No | 342 (77.5) |
| Lymph node metastasis | |
| Yes | 18 (4.1) |
| No | 423 (95.9) |
| Parametrial involvement | |
| Yes | 23 (5.2) |
| No | 418 (94.8) |
| Resection margin involvement | |
| Yes | 19 (4.3) |
| No | 422 (95.7) |
| Adjuvant therapy | |
| No | 356 (80.7) |
| Radiation | 57 (12.9) |
| Chemotherapy | 4 (0.9) |
| Concurrent chemoradiation | 24 (5.4) |
| Surgical waiting time (wk) | |
| ≤4 | 105 (23.8) |
| >4 | 336 (76.2) |
| Surgical waiting time (wk) | |
| ≤8 | 284 (64.4) |
| >8 | 157 (35.6) |
FIGO, International Federation of Gynecology and Obstetrics.
The median follow-up time was 51.05 months (range, 0.26 to 239.3 months). At analysis, 51 of the 441 patients had disease recurrence. The 5-year RFS was 87.7% (95% confidence interval [CI], 83.6 to 90.8). When the cohort was subdivided into waiting times of equal to or less, or longer than 4 and 8 weeks, the 5-year RFS was 83.6% (95% CI, 74 to 89.8) and 86.8% (95% CI, 81.5 to 90.6) for patients whose surgical waiting time was equal to or fewer than 4 and 8 weeks, respectively, and 89.1% (95% CI, 84.3 to 92.5) and 89.6% (95% CI, 82.1 to 94.1) for patients whose waiting time was more than 4 and 8 weeks, respectively (p=0.230 and p=0.551, respectively) (Fig. 1). Of the various clinicopathological prognostic factors under study (Table 2), DSI (p=0.001), lymph node status (p=0.001), parametrial involvement (p=0.001), resection margin involvement (p=0.023), and adjuvant therapy (p=0.041) had a lower 5-year RFS. There was no evidence for a relationship with patients' age, tumor size, histologic type, LVSI, or surgical waiting time. Table 3 shows the results of multivariate analysis for RFS according to the cut-off point of surgical waiting time (4 and 8 weeks). The DSI and lymph node metastasis were both identified as independent prognostic factors for RFS. Waiting time (4 and 8 weeks) was not significant.
Fig. 1. (A) Recurrence-free survival (RFS) among cervical patients assigned to waiting times of fewer than or equal to and more than 4 weeks. (B) RFS among cervical patients assigned to waiting times of fewer than or equal to, and more than 8 weeks.
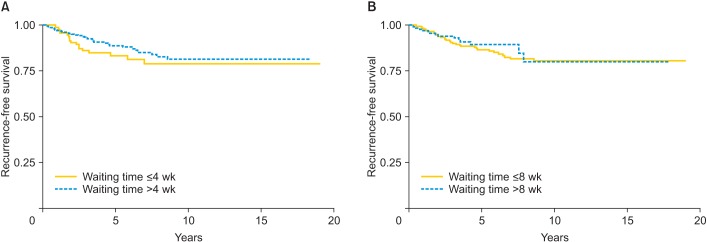
Table 2. Univariate analysis of clinicopathological prognostic factors and 5-year recurrence-free survival.
| Characteristic | 5-Year RFS | 95% CI | p-value |
|---|---|---|---|
| Age (yr) | 0.387 | ||
| <35 | 81.5 | 62.8-91.4 | |
| 35-60 | 87.9 | 83.4-91.3 | |
| >60 | 92.9 | 74.4-98.2 | |
| Tumor size (cm) | 0.056 | ||
| ≤2 | 89.4 | 84.5-92.9 | |
| >2 | 83.9 | 75.5-89.5 | |
| Histopathology | 0.072 | ||
| Squamous cell carcinoma | 88.1 | 82.6-91.9 | |
| Adenocarcinoma | 90.4 | 83.2-94.6 | |
| Adenosquamous carcinoma | 77.1 | 47.9-91.3 | |
| Lymph vascular space invasion | 0.123 | ||
| Yes | 81.9 | 70.6-89.2 | |
| No | 89.4 | 85.0-92.6 | |
| Deep stromal invasion | <0.001 | ||
| Yes | 76.1 | 63.3-84.9 | |
| No | 90.8 | 86.5-93.7 | |
| Lymph node metastasis | <0.001 | ||
| Yes | 68.8 | 40.5-85.6 | |
| No | 88.5 | 84.4-91.6 | |
| Parametrial involvement | <0.001 | ||
| Yes | 63.5 | 37.0-81.3 | |
| No | 89.1 | 85.0-92.1 | |
| Resection margin involvement | 0.023 | ||
| Yes | 69.1 | 39.8-86.3 | |
| No | 88.6 | 84.5-91.7 | |
| Adjuvant therapy | 0.041 | ||
| No | 89.7 | 85.3-92.8 | |
| Radiation | 78.3 | 64.0-87.4 | |
| Chemotherapy | 100.0 | - | |
| Concurrent chemoradiation | 60.1 | 8.1-90.2 | |
| Surgical waiting time (wk) | 0.230 | ||
| ≤4 | 83.6 | 74.0-89.8 | |
| >4 | 89.1 | 84.3-92.5 | |
| Surgical waiting time (wk) | 0.551 | ||
| ≤8 | 86.8 | 81.5-90.6 | |
| >8 | 89.6 | 82.1-94.1 |
CI, confidence interval; RFS, recurrence-free survival.
Table 3. Multivariate analysis of clinicopathological prognostic factors and recurrence-free survival according to the cut-off point of surgical waiting time (4 and 8 weeks).
| Characteristic | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| 4 Weeks | |||
| Surgical waiting time (wk) | 0.353 | ||
| ≤4 | 1 | - | |
| >4 | 0.8 | 0.4-1.4 | |
| Deep stromal invasion | 0.003 | ||
| Yes | 1 | - | |
| No | 2.5 | 1.4-4.5 | |
| Lymph node metastasis | 0.029 | ||
| Yes | 1 | - | |
| No | 2.8 | 1.2-6.5 | |
| 8 Weeks | |||
| Surgical waiting time (wk) | 0.677 | ||
| ≤8 | 1 | - | |
| >8 | 0.9 | 0.5-1.6 | |
| Deep stromal invasion | 0.003 | ||
| Yes | 1 | - | |
| No | 2.5 | 1.4-4.6 | |
| Lymph node metastasis | 0.026 | ||
| Yes | 1 | - | |
| No | 2.9 | 1.3-6.7 |
CI, confidence interval.
The overall 5-year OS was 92.4% (95% CI, 88.8 to 94.9). When the cohort was subdivided into waiting times of equal to or less, or longer than 4 and 8 weeks, the 5-year OS was 89.2% (95% CI, 80.0 to 94.3) and 90.7% (95% CI, 85.9 to 94.0) for patients whose surgical waiting time was equal to or fewer than 4 and 8 weeks, respectively, and 93.6% (95% CI, 89.4 to 96.2) and 96% (95% CI, 88.9 to 98.6) for patients whose waiting time was more than 4 and 8 weeks, respectively (p=0.209 and p=0.973, respectively) (Fig. 2). In univariate Kaplan-Meier analysis, the OS was significantly worse in younger patients (p=0.041), larger tumor size (p=0.044), DSI (p=0.001), lymph node metastasis (p=0.001), parametrial involvement (p=0.01), and adjuvant therapy (p=0.028) as shown in Table 4. Table 5 shows the results of multivariate analysis for OS according to the cut-off point of surgical waiting time (4 and 8 weeks). The DSI and lymph node metastasis were identified as independent prognostic factors for OS. Waiting time (whether cut-off as 4 or 8 weeks) was not found to be significant. Although, the OS profile did not differ significantly between patients whose surgical waiting time was equal to or fewer than 8 weeks and patients those waiting time of more than 8 weeks (90.7% [95% CI, 85.9 to 94.0] and 96% [95% CI, 88.9 to 98.6], respectively; p=0.973), as shown in Fig. 2B, longer surgical waiting times may have a negative impact on long-term OS. This was confirmed in the time-varying effect Cox model in which waiting times longer than 8 weeks were associated with a worse OS after 5 years (hazard ratio [HR], 3.4; 95% CI, 1.3 to 9.2; p=0.021).
Fig. 2. (A) Overall survival (OS) among cervical patients assigned to waiting times of fewer than or equal to, and more than 4 weeks. (B) OS among cervical patients assigned to waiting times of fewer than or equal to, and more than 8 weeks.
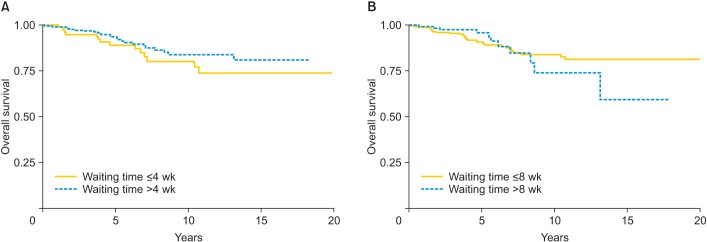
Table 4. Univariate analysis of clinicopathological prognostic factors and 5-year overall survival.
| Characteristic | 5-Year OS | 95% CI | p-value |
|---|---|---|---|
| Age (yr) | 0.041 | ||
| <35 | 86.3 | 66.7-94.8 | |
| 35-60 | 93.1 | 89.0-95.6 | |
| >60 | 92.6 | 73.5-98.1 | |
| Tumor size (cm) | 0.044 | ||
| ≤2 | 93.6 | 89.1-96.3 | |
| >2 | 89.7 | 82.0-94.2 | |
| Histopathology | 0.341 | ||
| Squamous cell carcinoma | 92.2 | 87.2-95.3 | |
| Adenocarcinoma | 93.9 | 86.3-97.4 | |
| Adenosquamous carcinoma | 95.2 | 70.7-99.3 | |
| Lymph vascular space invasion | 0.527 | ||
| Yes | 90.3 | 81.4-95.1 | |
| No | 93.2 | 89.0-95.9 | |
| Deep stromal invasion | <0.001 | ||
| Yes | 89.6 | 80.0-94.8 | |
| No | 93.4 | 89.3-96.0 | |
| Lymph node metastasis | <0.001 | ||
| Yes | 64.6 | 33.7-83.9 | |
| No | 93.8 | 90.3-96.0 | |
| Parametrial involvement | <0.001 | ||
| Yes | 77.4 | 54.0-89.9 | |
| No | 93.3 | 89.6-95.8 | |
| Resection margin involvement | 0.161 | ||
| Yes | 83.0 | 55.9-94.2 | |
| No | 93.0 | 89.2-95.4 | |
| Adjuvant therapy | 0.028 | ||
| No | 94.0 | 90.2-96.4 | |
| Radiation | 82.9 | 68.3-91.2 | |
| Chemotherapy | 100.0 | - | |
| Concurrent chemoradiation | 95.2 | 70.7-99.3 | |
| Surgical waiting time (wk) | 0.209 | ||
| ≤4 | 89.2 | 80.0-94.3 | |
| >4 | 93.6 | 89.4-96.2 | |
| Surgical waiting time (wk) | 0.973 | ||
| ≤8 | 90.7 | 85.9-94.0 | |
| >8 | 96.0 | 88.9-98.6 |
CI, confidence interval; OS, overall survival.
Table 5. Multivariate analysis of clinicopathological prognostic factors and overall survival according to the cut-off point of surgical waiting time (4 and 8 weeks).
| Characteristic | HR | 95% CI | p-value |
|---|---|---|---|
| 4 Weeks | |||
| Surgical waiting time (wk) | 0.312 | ||
| ≤4 | 1 | - | |
| >4 | 0.7 | 0.4-1.3 | |
| Deep stromal invasion | 0.015 | ||
| Yes | 1 | - | |
| No | 2.4 | 1.2-4.8 | |
| Lymph node metastasis | 0.009 | ||
| Yes | 1 | - | |
| No | 3.6 | 1.5-8.6 | |
| 8 Weeks | |||
| Surgical waiting time (wk)* | |||
| In first 5 years | 0.097 | ||
| ≤8 | 1 | - | |
| >8 | 0.4 | 0.1-1.3 | |
| After 5 years | 0.021 | ||
| ≤8 | 1 | - | |
| >8 | 3.4 | 1.3-9.2 | |
| Deep stromal invasion | 0.009 | ||
| Yes | 1 | - | |
| No | 2.6 | 1.3-5.0 | |
| Lymph node metastasis | 0.009 | ||
| Yes | 1 | - | |
| No | 3.6 | 1.5-8.6 |
CI, confidence interval; HR, hazard ratio.
*Time-varying effect Cox model.
DISCUSSION
The principle of treatment is similar in any malignancy, that the doctor should treat the patients as soon as possible for the best outcome. A delay in treatment might be attributable to many factors including availability of pretreatment evaluation (such as imaging), limited treatment resources (such as availability of an operating room, availability of radiation facilities), limited human resources (such as surgical oncologist), and the ability of an uninsured patient to obtain funding [6,9,11,12]. In countries with a high incidence of cervical cancer and limited resources such as Thailand where surgical delay is a major health problem, the question to ask is not whether surgical delay affects patient outcome, but rather how much delay is acceptable before the clinical outcome is unacceptably affected.
Until now, there have been no published randomized controlled trials focusing on the impact of waiting times on clinical outcomes. Despite the general recommendation that the waiting time from diagnosis to surgery should not exceed 4 weeks, our study found that in our institution, the median surgical waiting time was 43 days, and only 24% of patients underwent surgery within 4 weeks. These results match those of an earlier study [11]. Our study found that a longer waiting time was not associated with RFS similar to other studies [11,12,13]. We also found that no evidence that a longer waiting time affects OS in the first 5 years, which was similar to the previous findings [11,12]. However, a study by E et al. [13] that assessed 195 cervical cancer patients treated with radiotherapy between 1990 and 2001 found correlations between prolonged waiting time for radiotherapy and a negative impact on OS as well as disease-specific survival in these patients.
These contrasting results are probably because all cervical cancer patients in our study and the Umezu et al. [11] study had early stage disease in which prognosis is generally good after radical hysterectomy. In contrast, E et al. [13] had nearly 85% of the patients with locally advanced staging, in which the prognosis is generally poor. In addition, the number of patients in our study and earlier studies [11,12] with similar findings may not have been sufficient to detect the small effect of waiting time on clinical outcomes in early stage disease. In their breast cancer study, McLaughlin et al. [14] found that prolonged waiting times had no effect on OS among early stage breast cancer patients; however, this did lead to significantly worse OS among late stage breast cancer patients. However a study by Brazda et al. [15] which assessed 1,337 women with breast cancer found no effect of waiting time on survival when controlling for stage, and this finding was supported by a meta-analysis of high quality studies of breast cancer [16]. Thus, further study with larger sample sizes including long term follow-up or meta-analysis of the impact of surgical waiting time as an independent prognostic factor on clinical outcome in early stage cervical cancer would be worthwhile to address this issue.
Although, our study could not detect an effect of waiting time from diagnosis to surgery on RFS and OS in the first 5 years following surgery, the most obvious finding to emerge from the analysis was that longer waiting times affected OS after 5 years. We believe that this is the first work to address the impact of waiting times longer than 8 weeks for surgery on the long-term clinical outcome of early stage cervical cancer patients. Our findings support the recommendations of the American College of Chest Physicians and the British Thoracic Society that the waiting time from diagnosis to surgery should not exceed 8 weeks [17,18].
Among the various clinicopathological variables of early stage cervical cancer that have been reported as prognostic factors, i.e., tumor size, histopathology, LVSI, DSI parametrial involvement and lymph node metastasis [5,19,20,21], we found only DSI and lymph node metastasis to be significant adverse indicators for RFS and OS.
This is the largest cohort study with long-term follow-up to date in which surgical waiting time is assessed for its potential prognostic effects on clinical outcome in early stage cervical cancer. However, our study has some limitations. This was a retrospective study, which has the same inherent bias found in any retrospective study. Although fewer than half of the patients (42%) had follow-up times longer than 5 years, the time-varying effect Cox model indicated that a waiting time longer than 8 weeks was a significant predictor of poorer long-term OS (HR, 3.4; p=0.021). Information on the cause of death in our study was not available; thus, no cancer deaths contributed to the survival times. Finally, the reason for treatment delay was unknown in these cases.
Despite its limitations, our study suggests that surgical waiting time from date of entry for clinical staging to the date of operation does not adversely affect the RFS and OS in the first 5 years post-surgery, but did lead to significantly worse OS after 5 years. We also found that DSI and lymph node metastasis were independent prognostic indicators for RFS and OS. Our findings have a number of important implications for future practice. They indicate that early stage cervical cancer patients should undergo radical hysterectomy within 8 weeks of diagnosis.
ACKNOWLEDGMENTS
We thank Dr. Alan Geater, Epidemiology Unit, Prince of Songkla University Faculty of Medicine for assistance with statistical analysis and valuable comments. This research is supported by the Prince of Songkla University Faculty of Medicine.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49:3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Kruhaprema T, Attasara P, Sriplung H, Wiangnon S, Sangrajrang S. Cancer in Thailand vol VII, 2007-2009. Bangkok: Bangkok Medical Publisher; 2013. [Google Scholar]
- 4.Wilailak S. Epidemiologic report of gynecologic cancer in Thailand. J Gynecol Oncol. 2009;20:81–83. doi: 10.3802/jgo.2016.27.e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chittithaworn S, Hanprasertpong J, Tungsinmunkong K, Geater A. Association between prognostic factors and disease-free survival of cervical cancer stage IB1 patients undergoing radical hysterectomy. Asian Pac J Cancer Prev. 2007;8:530–534. [PubMed] [Google Scholar]
- 6.Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012;23:2731–2737. doi: 10.1093/annonc/mds101. [DOI] [PubMed] [Google Scholar]
- 7.Stec AA, Coons BJ, Chang SS, Cookson MS, Herrell SD, Smith JA, Jr, et al. Waiting time from initial urological consultation to nephrectomy for renal cell carcinoma: does it affect survival? J Urol. 2008;179:2152–2157. doi: 10.1016/j.juro.2008.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raptis DA, Fessas C, Belasyse-Smith P, Kurzawinski TR. Clinical presentation and waiting time targets do not affect prognosis in patients with pancreatic cancer. Surgeon. 2010;8:239–246. doi: 10.1016/j.surge.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Elit LM, O'Leary EM, Pond GR, Seow HY. Impact of wait times on survival for women with uterine cancer. J Clin Oncol. 2014;32:27–33. doi: 10.1200/JCO.2013.51.3671. [DOI] [PubMed] [Google Scholar]
- 10.van Harten MC, Hoebers FJ, Kross KW, van Werkhoven ED, van den Brekel MW, van Dijk BA. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol. 2015;51:272–278. doi: 10.1016/j.oraloncology.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Umezu T, Shibata K, Kajiyama H, Yamamoto E, Mizuno M, Kikkawa F. Prognostic factors in stage IA-IIA cervical cancer patients treated surgically: does the waiting time to the operation affect survival? Arch Gynecol Obstet. 2012;285:493–497. doi: 10.1007/s00404-011-1966-y. [DOI] [PubMed] [Google Scholar]
- 12.Perri T, Issakov G, Ben-Baruch G, Felder S, Beiner ME, Helpman L, et al. Effect of treatment delay on survival in patients with cervical cancer: a historical cohort study. Int J Gynecol Cancer. 2014;24:1326–1332. doi: 10.1097/IGC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 13.E C, Dahrouge S, Samant R, Mirzaei A, Price J. Radical radiotherapy for cervix cancer: the effect of waiting time on outcome. Int J Radiat Oncol Biol Phys. 2005;61:1071–1077. doi: 10.1016/j.ijrobp.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012;30:4493–4500. doi: 10.1200/JCO.2012.39.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brazda A, Estroff J, Euhus D, Leitch AM, Huth J, Andrews V, et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol. 2010;17(Suppl 3):291–296. doi: 10.1245/s10434-010-1250-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, King W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3–16. doi: 10.1016/j.radonc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 17.The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. Thorax. 1998;53(Suppl 1):S1–S8. doi: 10.1136/thx.53.suppl_1.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberts WM, Bepler G, Hazelton T, Ruckdeschel JC, Williams JH American College of Chest Physicians. Lung cancer: practice organization. Chest. 2003;123(1 Suppl):332S–337S. doi: 10.1378/chest.123.1_suppl.332s. [DOI] [PubMed] [Google Scholar]
- 19.Takeda N, Sakuragi N, Takeda M, Okamoto K, Kuwabara M, Negishi H, et al. Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol Scand. 2002;81:1144–1151. doi: 10.1034/j.1600-0412.2002.811208.x. [DOI] [PubMed] [Google Scholar]
- 20.Phoophitphong T, Hanprasertpong J, Dechsukhum C, Geater A. Correlation of angiogenesis and recurrence-free survival of early stage cervical cancer patients undergoing radical hysterectomy with pelvic lymph node dissection. J Obstet Gynaecol Res. 2007;33:840–848. doi: 10.1111/j.1447-0756.2007.00666.x. [DOI] [PubMed] [Google Scholar]
- 21.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes after radical hysterectomy according to tumor size divided by 2-cm interval in patients with early cervical cancer. Ann Oncol. 2011;22:59–67. doi: 10.1093/annonc/mdq321. [DOI] [PubMed] [Google Scholar]


