Abstract
Objective
The aim of this study was to investigate outcomes in uterine cancer patients undergoing pulmonary metastasectomy and prognostic factors associated with survival after the procedure.
Methods
A retrospective study was performed in 29 uterine cancer patients who underwent surgical resection of pulmonary metastatic lesions at Samsung Medical Center between June 1995 and December 2011.
Results
Histopathology showed carcinoma in 17 patients (58.6%) and sarcoma in 12 patients (41.4%). Of the 29 patients, 17 (58.6%) had less than three pulmonary metastatic lesions. Eight (27.6%) had symptoms related to lung metastasis. The 5-year survival rate after pulmonary metastasectomy for the entire cohort was 48.2%. On univariate and multivariate analysis, the presence of pulmonary symptoms and more than three lesions of metastasis were associated with poor survival after pulmonary metastasectomy.
Conclusion
Pulmonary metastasectomy for uterine cancer is an acceptable treatment in selected patients. Patients with more than three pulmonary metastatic lesions and pulmonary symptoms related to lung metastasis could expect to have worse prognosis after pulmonary metastasectomy.
Keywords: Metastasectomy, Prognosis, Retrospective Studies, Survival Rate, Uterine Neoplasms
INTRODUCTION
Endometrial cancer is the most common malignancy of the female genital tract [1]. Most cases of endometrial cancer show good prognosis, but in approximately 25% of cases appear as extrauterine disease [2]. In distant metastasis, endometrial cancer commonly spreads through pelvic and paraaortic lymph nodes or pelvic viscera including adnexae. Incidence of hematogenous metastasis is low in endometrial cancer. Pulmonary metastasis represent a common site of extrapelvic spread of disease but incidence is only 2.3%-4.6% [3,4]. Few studies have been introduced relating the pattern and treatment of pulmonary metastasis.
In cases with other solid tumors, nearly 30% of patients experience pulmonary metastases [5]. Pulmonary metastasis is generally considered a systemic disease and may require systemic chemotherapy; however, it is thought that selected patients with pulmonary metastasis can benefit from a surgical approach. Although there have been no randomized controlled trials of pulmonary metastasectomy, the therapeutic value of surgical resection has been noted by a number of studies in terms of survival benefit in a variety of cancers (colorectal, renal cell, hepatocellular, breast, head and neck) [6,7,8,9,10,11].
Pulmonary metastasectomy was first introduced in the setting of uterine cancer metastasis in 1930 by Torek [12] and resection of metastatic lung lesions has been adopted as the treatment of choice in selected patients. There was a study that reported the safety and effectiveness of pulmonary metastasectomy in 23 cases of endometrial cancer [13]. Currently, The Clinical Practice Guidelines in Oncology developed by the National Comprehensive Cancer Network (NCCN) recommend surgical resection for possibly removable regional uterine cancer metastasis. However, more evidence is needed to support surgical resection as the primary treatment for pulmonary metastases.
The objective of this study was to assess outcomes of uterine cancer patients undergoing pulmonary metastasectomy. We speculated that it is important to find the variables affecting the survival in determining the treatment method. We also sought to determine prognostic factors associated with survival after pulmonary metastasectomy.
MATERIALS AND METHODS
1. Patients and methods
With Institutional Review Board approval (IRB no. 2015-06-104), we reviewed medical records to identify uterine cancer patients diagnosed with pulmonary metastases who underwent curative resection via thoracotomy or video-assisted thoracic surgery (VATS) between June 1994 and December 2011. At our institution, the following selection criteria were used to identify candidates for pulmonary metastasectomy: (1) controlled primary tumor, (2) no extrapulmonary lesions at the time of metastasectomy, (3) pulmonary lesions amenable to surgical resection based on chest computed tomography (CT) or positron emission tomography (PET)-CT scan, (4) clinical status and pulmonary function compatible with planned operation, and (5) more effective treatment options unavailable. All patients underwent a chest CT or PET-CT prior to operation, and the resectability of pulmonary metastatic lesions was discussed with thoracic surgeons. All procedures were performed by thoracic surgeons. Pulmonary biopsies for the confirmation of metastasis were excluded.
Demographic, clinicopathologic, surgical, and survival data were retrospectively collected from medical records. Potential prognostic variables included in this study were age at pulmonary metastasectomy, initial stage (following the 2009 International Federation of Gynecology and Obstetrics [FIGO] staging system), symptoms related to lung metastasis, laterality, number and largest size of metastatic foci, disease-free interval (DFI), post-metastasectomy chemotherapy and recurrence after metastasectomy. The number and largest size of pulmonary lesions were documented based on final pathologic reports. Symptoms related metastases were limited to the symptoms of the respiratory systems in medical record at the time of metastatic diagnosis. For investigation of performance status, we used Eastern Cooperative Oncology Group (ECOG) performance status. DFI was defined as the time from diagnosis of the primary tumor to the detection of pulmonary metastases. Survival from initial metastasectomy was defined as the interval between pulmonary metastasectomy and death or last follow-up.
2. Statistical analysis
Survival curves were generated according to the Kaplan-Meier method. To evaluate the prognostic significance of the variables, the log-rank test and Cox proportional hazards model were used for univariate and multivariate analysis, respectively. All statistical analyses were performed using IBM SPSS ver. 21.0 (IBM Co., Armonk, NY, USA). A p<0.05 was considered statistically significant and all p-values were two-sided.
RESULTS
During the study period, a total of 57 uterine cancer patients with pulmonary metastasis were identified. Of them, 23 patients who did not undergo surgery and 5 who underwent pulmonary biopsy for the diagnosis of metastasis were excluded. Finally, 29 patients were included in the study. The median follow-up time was 49 months (range, 18 to 152 months).
1. Baseline characteristics
Demographics of patients with pulmonary metastasis are shown in Table 1. The median age at diagnosis of the primary tumor was 53 years (range, 34 to 72 years). Twelve patients (41.4%) had stage I, two (6.9%) stage II, six (20.7%) stage III, and nine (31.0%) stage IV disease according to the 2009 FIGO staging system. Histopathologic findings were endometrial adenocarcinoma in 15 patients (41.4%) and leiomyosarcoma in 8 patients (27.6%). All patients underwent primary surgical treatment. A staging operation, including hysterectomy, bilateral salpingoophorectomy and lymphadenectomy and/or omentectomy, was performed in 19 patients (65.6%). Five patients (17.2%) had only a hysterectomy and five (17.2%) had a hysterectomy with bilateral salpingoophorectomy. For adjuvant therapy after primary treatment, thirteen patients (44.8%) did not receive any adjuvant chemotherapy, nine (31.0%) received radiation therapy, one (3.4%) received concurrent chemoradiotherapy, and six (20.7%) had chemotherapy.
Table 1. Demographics of 29 patients with pulmonary metastasis of uterine cancer.
| Demographic | No. (%) |
|---|---|
| Age at initial diagnosis (yr), median (range) | 53 (34-72) |
| Body mass index (kg/m2), median (range) | 24.2 (17.3-29.2) |
| Menopause | |
| Yes | 16 (55.2) |
| No | 13 (44.8) |
| 2009 FIGO stage | |
| I | 12 (41.4) |
| II | 2 (6.9) |
| III | 6 (20.7) |
| IV | 9 (31.0) |
| Histology | |
| Endometrial adenocarcinoma | 15 (51.7) |
| Papillary serous adenocarcinoma | 2 (6.9) |
| Leiomyosarcoma | 8 (27.6) |
| Others | 4 (13.8) |
| Primary surgical treatment | |
| Hysterectomy | 5 (17.2) |
| Hysterectomy+bilateral salpingoophorectomy | 5 (17.2) |
| Staging operation* | 19 (65.6) |
| Postoperative adjuvant treatment after primary treatment | |
| None | 13 (44.8) |
| Radiotherapy | 9 (31.0) |
| Chemotherapy | 6 (20.7) |
| Concurrent chemo radiotherapy | 1 (3.4) |
FIGO, International Federation of Gynecology and Obstetrics.
*Staging operations included hysterectomy, bilateral salpingoophorectomy and lymphadenectomy and/or omentectomy.
2. Characteristics of pulmonary metastases
The characteristics of pulmonary metastases are shown in Table 2. The median age at pulmonary metastasectomy was 54 years (range, 35 to 74 years). Most patients had no symptoms suggestive of lung lesions (21/29, 72.4%). Eight patients showed symptoms related to lung lesions including cough (4), dyspnea with cough (3) and hemoptysis (1). Three patients (10.3%) had lung metastasis at the time of primary tumor diagnosis and one of them underwent pulmonary metastasectomy concomitant with surgical resection of the primary tumor. Of the 26 remaining patients who subsequently developed pulmonary metastasis, 23 patients (23/26, 88.4%) showed initial disease recurrence in the lung. Seventeen patients (58.6%) had unilateral lung lesions as determined by chest CT or PET-CT. According to the final pathology, 17 patients (58.6%) had less than three metastatic lesions and 18 patients (62.1%) patients had lesions larger than 1cm. The median DFI was 23.4 months (range, 3.0 to 112.3 months). When the patients were subdivided by DFI, 21 patients (72.4%) had greater than 12 months of DFI. The majority of patients (69%) received post-metastasectomy chemotherapy.
Table 2. Characteristics of patients with pulmonary metastasectomy (n=29).
| Characteristic | No. (%) |
|---|---|
| Age at metastasectomy (yr), median (range) | 54 (35-74) |
| DFI (mo), median (range) | 23.4 (3-112.3) |
| No. of metastatic lesion, median (range) | 3 (1-6) |
| Largest size of metastatic lesion (mm), median (range) | 15 (5-67) |
| Hospital stay (day), median (range) | 8 (3-27) |
| No. of operations performed in each patient | |
| Single | 22 (53.7) |
| Multiple | 19 (46.3) |
| ECOG performance status | |
| 0 | 10 (34.5) |
| 1 | 14 (48.3) |
| 2 | 5 (17.2) |
| Symptoms related to lung metastasis | |
| No symptoms | 21 (72.4) |
| Cough | 4 (13.8) |
| Dyspnea and cough | 3 (16.7) |
| Hemoptysis | 1 (5.9) |
| Disease status | |
| Lung metastasis at primary diagnosis | 3 (10.3) |
| Pulmonary metastasis after primary treatment | 26 (89.7) |
| Laterality | |
| Unilateral | 17 (58.6) |
| Bilateral | 12 (41.4) |
| Recurrence after metastasectomy | |
| Recurrence | 7 (24.1) |
| No recurrence | 22 (75.9) |
| Post metastasectomy adjuvant treatment | |
| Adjuvant chemotherapy | 20 (69.0) |
| No adjuvant treatment | 9 (31.0) |
DFI, disease-free interval; ECOG, Eastern Cooperative Oncology Group.
A total of 41 surgical procedures for the resection of pulmonary lesions were performed in these 29 patients via thoracotomy (4/41, 9.8%) and VATS (37/41, 90.2%); 22 patients had a single pulmonary operation, 7 patients had multiple operations (4 patients had two, 2 patients had three, and 1 patient had five) (Table 3). The patient who underwent five pulmonary metastasectomies was diagnosed with primary leiomyosarcoma and showed no evidence of lung lesions after the fifth operation. The 41 total pulmonary resections consisted of 32 wedge resections (78.0%), 6 lobectomies with mediastinal lymphadenectomy (14.6%), 1 lobectomy (2.4%), 1 lobectomy with partial pericardiectomy (2.4%), and 1 segmentectomy (2.4%). The pathologic assessment revealed metastasis from uterine cancer in 39 (95.1%) and primary lung cancer in 2 cases (4.9%). There was no mortality within the first 30 days after operation. One patient developed a hemothorax postoperatively.
Table 3. Characteristics of 41 surgical procedures for metastasectomy.
| Characteristic | No. (%) |
|---|---|
| Type of operation | |
| Video-assisted thoracic surgery | 4 (9.8) |
| Thoracotomy | 37 (90.2) |
| Operative time (min), median (range) | 90 (30-370) |
| Extent of metastasectomy | |
| Wedge resection | 32 (78.0) |
| Lobectomy with mediastinal lymphadenectomy | 6 (14.6) |
| Lobectomy with partial cardiectomy | 1 (2.4) |
| Lobectomy | 1 (2.4) |
| Segmentectomy | 1 (2.4) |
| Pathologic finding | |
| Metastasis from uterine cancer | 39 (95.1) |
| Primary lung cancer | 2 (4.9) |
3. Analysis of survival and prognostic factors
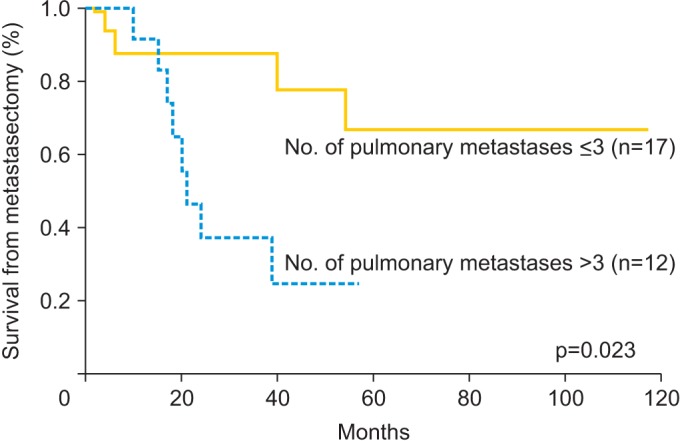
Univariate and multivariate analyses were performed to identify relationships between survival and prognostic variables. Table 4 shows the 5-year survival rate and results of the univariate and multivariate analyses according to the potential prognostic variables. The 5-year survival rate was 48.2%. The median overall survival of the study population was 26 months (range, 2 to 117 months) and 12 patients (41.3%) were dead at the time of censor. Significant prognostic variables for survival from metastasectomy on univariate analysis were symptoms related to lung metastasis (hazard ratio [HR], 4.013; 95% confidence interval [CI], 1.272 to 12.655; p=0.011) and number of pulmonary metastatic lesions (>3, HR, 3.822; 95% CI, 1.119 to 13.059; p=0.023). On multivariate analysis, which included statistically significant variables from the univariate analysis, pulmonary symptoms (HR, 4.011; 95% CI, 1.219 to 13.200; p=0.022) and more than three metastatic lesions (HR, 3.885; 95% CI, 1.076 to 14.029; p=0.038) were significant prognostic factors associated with survival following metastasectomy. Figs. 1, 2 show survival curves from the time of metastasectomy influenced by significant prognostic factors, pulmonary symptoms and number of metastatic lesions.
Table 4. Univariate and multivariate analysis of survival from initial metastasectomy (n=29).
| Variable | No. | Five-year survival rate (%) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| Age at metastasectomy (yr) | ||||||
| ≤54 | 14 | 50.5 | 1 | |||
| >54 | 15 | 45.0 | 1.341 (0.429-4.192) | 0.613 | ||
| 2009 FIGO stage | ||||||
| I-II | 14 | 61.1 | 1 | |||
| III-IV | 15 | 40.0 | 2.482 (0.669-9.216) | 0.174 | ||
| Histology | ||||||
| Carcinoma | 17 | 57.3 | 1 | |||
| Sarcoma | 12 | 40.0 | 1.444 (0.456-4.569) | 0.530 | ||
| Symptoms | ||||||
| No | 21 | 61.6 | 1 | 1 | ||
| Yes | 8 | 15.6 | 4.013 (1.272-12.655) | 0.011 | 4.011 (1.129-13.200) | 0.022 |
| Laterality | ||||||
| Unilateral | 17 | 57.1 | 1 | |||
| Bilateral | 12 | 30.3 | 1.966 (0.620-6.234) | 0.243 | ||
| No. of pulmonary metastatic lesions | ||||||
| ≤3 | 17 | 66.7 | 1 | 1 | ||
| >3 | 12 | 24.7 | 3.822 (1.119-13.059) | 0.023 | 3.885 (1.076-14.029) | 0.038 |
| Largest size (cm) | ||||||
| ≤1 | 8 | 51.4 | 1 | |||
| >1 | 21 | 48.6 | 1.560 (0.420-5.794) | 0.346 | ||
| DFI (mo) | ||||||
| ≤12 | 8 | 28.6 | 1 | |||
| >12 | 21 | 55.1 | 0.393 (0.123-1.255) | 0.103 | ||
| Post metastasectomy chemotherapy | ||||||
| Yes | 20 | 53.3 | 1 | |||
| No | 9 | 37.5 | 2.134 (0.675-6.751) | 0.186 | ||
| Recurrence after metastasectomy | ||||||
| Yes | 7 | 46.5 | 1 | |||
| No | 22 | 53.6 | 0.833 (0.223-3.113) | 0.785 | ||
The log-rank test was used for univariate analysis. Cox's proportional hazard regression test was used for multivariate analysis.
CI, confidence interval; DFI, disease-free interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio.
Fig. 1. Kaplan-Meier curves for survival from metastasectomy based on the symptoms related to lung metastasis.
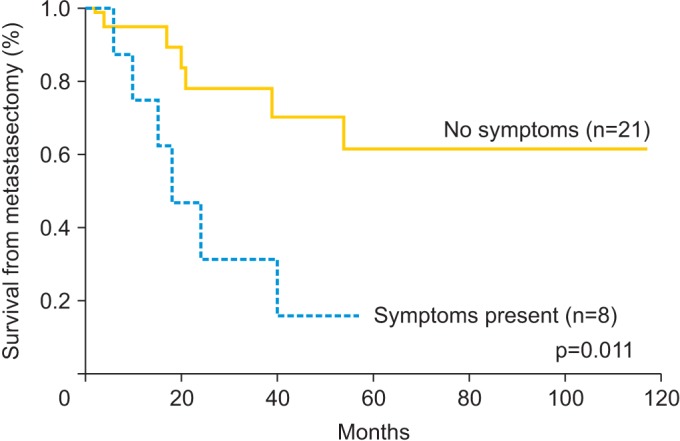
Fig. 2. Kaplan-Meier curves for survival from metastasectomy according to the number of pulmonary lesions.
DISCUSSION
Studies of various cancers including uterine cancer have shown that surgical methods in the treatment of pulmonary metastasis may contribute to survival benefit [6,7,8,9,10,11,13]. Other studies have reported a 5-year survival rate ranging from 32.9%-46.8% after pulmonary metastasectomy of gynecologic cancers [13,14,15,16,17]. Similar to these results, our cohort had a 5-year survival after metastasectomy of 48.2%. We could draw inference from our results that a surgical approach is a feasible and acceptable treatment in selected patients with resectable pulmonary metastasis of uterine cancer.
In general, distant metastasis (extra-abdominal metastasis) is considered a poor prognostic sign [18]. This is because complete resection is difficult in many cases of extra-abdominal metastasis. If complete resection is possible, a surgical approach could improve the prognosis as has been shown in previous studies of gynecologic cancers [19,20]. However, surgery may not be appropriate in all patients. Analysis of prognostic factors is needed in the selection of patients for metastasectomy.
In patients' demographics, 41.4% of the patients were at stage I, 6.9% at stage II, 20.7% at stage III, and 31.0% at stage IV. Number of stage I patients is greater than patients of other stages shown. Although advanced stage is associated with recurrence, incidence of metastasis and recurrences was not always associated with initial stage [21,22]. Considering the largest proportion of stage I disease at the time of initial diagnosis of endometrial cancer [23], we can speculate that greater stage I patients than other stages in our study population might the reflect of the initial proportion, rather than many recurrences at early stage.
In our study, the presence of symptoms related to pulmonary metastasis and the number of lung lesions were significant prognostic factors associated with survival after metastasectomy. In other studies related to pulmonary metastasis, DFI was found to be the most significant prognostic factor [3,15,22,24,25,26], in addition to the number of pulmonary lesions [24,26,27,28]. Our study also showed that patients with a DFI less than 12 months had a lower 5-year survival rate than patients with more than 12 months of DFI (28.6 vs. 55.1; p=0.103), however the difference was not statistically significant. Both univariate and multivariate analyses showed that a DFI less than 12 months was a negative prognostic factor, which was not statistically significant. The lack of significance may have been due to the small sample size, and a large scale study is needed for more accurate analysis.
Our study has limitations associated with its retrospective nature and small sample size. The absence of a comparison group is also drawback in assessing the feasibility of pulmonary metastasectomy in this study. Initially we aimed to identify factors that affect survival of uterine cancer patients undergoing pulmonary metastasectomy. Despite difficulties with given numerous confounders and biases, some valid conclusions can be drawn from this limited data.
We demonstrated that the presence of pulmonary symptoms and the number of pulmonary metastatic lesions are independent predictors of survival in patients undergoing pulmonary metastasectomy. Pulmonary metastasectomy is a feasible and acceptable treatment in selected patients with pulmonary metastasis of uterine cancer. Patients with more than three pulmonary metastatic lesions and pulmonary symptoms related to lung metastasis could expect to have worse prognosis after pulmonary metastasectomy.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8 Suppl):2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Ballon SC, Berman ML, Donaldson RC, Growdon WA, Lagasse LD. Pulmonary metastases of endometrial carcinoma. Gynecol Oncol. 1979;7:56–65. doi: 10.1016/0090-8258(79)90081-7. [DOI] [PubMed] [Google Scholar]
- 4.Bouros D, Papadakis K, Siafakas N, Fuller AF., Jr Patterns of pulmonary metastasis from uterine cancer. Oncology. 1996;53:360–363. doi: 10.1159/000227588. [DOI] [PubMed] [Google Scholar]
- 5.Downey RJ. Surgical treatment of pulmonary metastases. Surg Oncol Clin N Am. 1999;8:341. [PubMed] [Google Scholar]
- 6.Dabestani S, Marconi L, Hofmann F, Stewart F, Lam TB, Canfield SE, et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 2014;15:e549–e561. doi: 10.1016/S1470-2045(14)70235-9. [DOI] [PubMed] [Google Scholar]
- 7.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84:324–338. doi: 10.1016/j.athoracsur.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 8.Tomimaru Y, Sasaki Y, Yamada T, Eguchi H, Takami K, Ohigashi H, et al. The significance of surgical resection for pulmonary metastasis from hepatocellular carcinoma. Am J Surg. 2006;192:46–51. doi: 10.1016/j.amjsurg.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Friedel G, Pastorino U, Ginsberg RJ, Goldstraw P, Johnston M, Pass H, et al. Results of lung metastasectomy from breast cancer: prognostic criteria on the basis of 467 cases of the International Registry of Lung Metastases. Eur J Cardiothorac Surg. 2002;22:335–344. doi: 10.1016/s1010-7940(02)00331-7. [DOI] [PubMed] [Google Scholar]
- 10.Yano T, Shoji F, Maehara Y. Current status of pulmonary metastasectomy from primary epithelial tumors. Surg Today. 2009;39:91–97. doi: 10.1007/s00595-008-3820-9. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki T, Hasegawa Y, Hanai N, Ozawa T, Hirakawa H, Suzuki A, et al. Survival impact of pulmonary metastasectomy for patients with head and neck cancer. Head Neck. 2013;35:1745–1751. doi: 10.1002/hed.23232. [DOI] [PubMed] [Google Scholar]
- 12.Torek F. Removal of metastatic carcinoma of the lung and mediastinum: suggestions as to technic. Arch Surg. 1930;21:1416–1424. [Google Scholar]
- 13.Clavero JM, Deschamps C, Cassivi SD, Allen MS, Nichols FC, 3rd, Barrette BA, et al. Gynecologic cancers: factors affecting survival after pulmonary metastasectomy. Ann Thorac Surg. 2006;81:2004–2007. doi: 10.1016/j.athoracsur.2006.01.068. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Yoshikawa H, Shiromizu K, Saito T, Kuzuya K, Tsunematsu R, et al. Pulmonary metastasectomy for uterine cervical cancer: a multivariate analysis. Ann Thorac Surg. 2004;77:1179–1182. doi: 10.1016/j.athoracsur.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Fuller AF, Jr, Scannell JG, Wilkins EW., Jr Pulmonary resection for metastases from gynecologic cancers: Massachusetts General Hospital experience, 1943-1982. Gynecol Oncol. 1985;22:174–180. doi: 10.1016/0090-8258(85)90024-1. [DOI] [PubMed] [Google Scholar]
- 16.Levenback C, Rubin SC, McCormack PM, Hoskins WJ, Atkinson EN, Lewis JL., Jr Resection of pulmonary metastases from uterine sarcomas. Gynecol Oncol. 1992;45:202–205. doi: 10.1016/0090-8258(92)90286-r. [DOI] [PubMed] [Google Scholar]
- 17.Shiromizu K, Kasamatsu T, Takahashi M, Kikuchi A, Yoshinari T, Matsuzawa M. A clinicopathological study of postoperative pulmonary metastasis of uterine cervical carcinomas. J Obstet Gynaecol Res. 1999;25:245–249. doi: 10.1111/j.1447-0756.1999.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 18.Ayhan A, Taskiran C, Celik C, Yuce K, Kucukali T. The influence of cytoreductive surgery on survival and morbidity in stage IVB endometrial cancer. Int J Gynecol Cancer. 2002;12:448–453. doi: 10.1046/j.1525-1438.2002.t01-1-01133.x. [DOI] [PubMed] [Google Scholar]
- 19.Ueda Y, Enomoto T, Miyatake T, Egawa-Takata T, Ugaki H, Yoshino K, et al. Endometrial carcinoma with extra-abdominal metastasis: improved prognosis following cytoreductive surgery. Ann Surg Oncol. 2010;17:1111–1117. doi: 10.1245/s10434-009-0892-8. [DOI] [PubMed] [Google Scholar]
- 20.Eto T, Saito T, Kasamatsu T, Nakanishi T, Yokota H, Satoh T, et al. Clinicopathological prognostic factors and the role of cytoreduction in surgical stage IVb endometrial cancer: a retrospective multi-institutional analysis of 248 patients in Japan. Gynecol Oncol. 2012;127:338–344. doi: 10.1016/j.ygyno.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Dowdy SC, Mariani A, Bakkum JN, Cliby WA, Keeney GL, Podratz KC. Treatment of pulmonary recurrences in patients with endometrial cancer. Gynecol Oncol. 2007;107:242–247. doi: 10.1016/j.ygyno.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Otsuka I, Ono I, Akamatsu H, Sunamori M, Aso T. Pulmonary metastasis from endometrial carcinoma. Int J Gynecol Cancer. 2002;12:208–213. doi: 10.1046/j.1525-1438.2002.01095.x. [DOI] [PubMed] [Google Scholar]
- 23.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 24.Anraku M, Yokoi K, Nakagawa K, Fujisawa T, Nakajima J, Akiyama H, et al. Pulmonary metastases from uterine malignancies: results of surgical resection in 133 patients. J Thorac Cardiovasc Surg. 2004;127:1107–1112. doi: 10.1016/j.jtcvs.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Todd TR. The surgical treatment of pulmonary metastases. Chest. 1997;112(4 Suppl):287S–290S. doi: 10.1378/chest.112.4_supplement.287s. [DOI] [PubMed] [Google Scholar]
- 26.Takita H, Edgerton F, Karakousis C, Douglass HO, Jr, Vincent RG, Beckley S. Surgical management of metastases to the lung. Surg Gynecol Obstet. 1981;152:191–194. [PubMed] [Google Scholar]
- 27.Zink S, Kayser G, Gabius HJ, Kayser K. Survival, disease-free interval, and associated tumor features in patients with colon/rectal carcinomas and their resected intra-pulmonary metastases. Eur J Cardiothorac Surg. 2001;19:908–913. doi: 10.1016/s1010-7940(01)00724-2. [DOI] [PubMed] [Google Scholar]
- 28.Girard P, Spaggiari L, Baldeyrou P, Le Chevalier T, Le Cesne A, Escudier B, et al. Should the number of pulmonary metastases influence the surgical decision. Eur J Cardiothorac Surg. 1997;12:385–391. doi: 10.1016/s1010-7940(97)00203-0. [DOI] [PubMed] [Google Scholar]


