Abstract
Objective
The aim of this study was to investigate the clinical effects of sartorius tendon transposition versus sartorius transposition during bilateral inguinal lymphadenectomy of radical vulvectomy.
Methods
A total of 58 vulvar cancer patients who had surgery from May 2007 to October 2013, in which 30 patients received sartorius transposition and 28 patients received sartorius tendon transposition. All patients were matched by age, body mass index, stage, histology, and grade. Intraoperative variables and postoperative complications, recurrence, progression-free survival (PFS), and overall survival (OS) and postoperative life quality were compared and analyzed.
Results
No significant differences were found at median surgical times and amounts of bleeding (p=0.316 and p=0.249, respectively), neither at the incidences of groin cellulitis and lymphocele (p=0.673 and p=0.473, respectively), but the recovery times of the inguinal wounds were shorter (p=0.026) and the incidences of wound break and chronic lymphedema were significantly decreased in the tendon transposition group (p=0.012 and p=0.022, respectively). Postoperative quality of life in tendon transposition group was significantly improved as indicated by the EORTC QLQ-C30 questionnaire. Recurrences were similar (p=0.346) and no significant differences were found at PFS and OS (p=0.990 and p=0.683, respectively).
Conclusion
Compared to sartorius transposition, sartorius tendon transposition during inguinal lymphadenectomy led to improved patient recovery, reduced postoperative complications, and improved life quality without compromising the outcomes.
Keywords: Bilateral Inguinal Lymphadenectomy, Radical Vulvectomy, Sartorius Transposition, Vulvar Carcinoma
INTRODUCTION
Vulvar cancer is uncommon, accounts for approximately 3% to 5% of gynecologic cancers, and has an incidence of 0.6% of all cancers in women [1], and this disease classically affects older women with an average age of onset of 65 to 70 years. Radical vulvectomy with en bloc resection of the inguinofemoral lymphadenectomy was the standard treatment for invasive cancer patients, but the postoperative morbidity of this procedure was high, and prolonged hospitalization was common [2,3]. In 1960, Way [4] introduced the technique of sartorius transposition following inguinofemoral lymphadenectomy to protect the femoral vessels in the event of wound breakdown and to reduce the risk of secondary hemorrhage. This procedure has been shown to reduce infections in vascular grafts in the groin. However, Judson et al. [5] in 2004 performed a prospective, randomized study and failed to show any decrease in the incidences of wound cellulitis, wound breakdown, lymphedema, or rehospitalization in the sartorius transposition group. Without doubt, sartorius transposition procedure can cause unnecessary damage to the skeleton muscle, which can result in ischemia and incision necrosis following the operation, and seriously affect the quality of life (QOL) of the patients. In order to improve the operation process and reduce the postoperative complications, we conducted a matched case-control study using the sartorius tendon transposition method compared with those of conventional sartorius transposition method following inguinofemoral lymphadenectomy in vulvar carcinoma patients to assess the outcomes.
MATERIALS AND METHODS
1. Patients and procedures
Over the period of 5 years from May 2007 to October 2013, 62 patients had been histologically diagnosed with invasive carcinoma of the vulva requiring bilateral inguinal-femoral lymphadenectomy at the Department of Gynecologic Oncology of the Affiliated Tumor Hospital of Zhengzhou University School of Medicine. The inclusion criteria were: (1) age 20 to 75 years old; (2) with definite histological diagnosis; (3) normal liver and renal function; (4) acceptable cardiovascular pulmonary and other major organ functions. Exclusion criteria including: (1) age <20 or >75 years; (2) any lung, liver, or cardiovascular pulmonary and other major organ dysfunctions; (3) massive lymph node invasion with femoral-vessels attack. A random grouping software RandA ver. 1.0 (Random Grouping System for Medical Research, Beijing, China) was used to have the patients randomly grouped in order to minimize the selection bias. Patients were matched with regard to age, body mass index (BMI), and clinical stage. The perioperative results included the operative time, amount of estimated blood loss, surgery complications, and the length of postoperative hospital stay. The Institutional Ethics Review Board of the Tumor Hospital of Zhengzhou University reviewed and approved this study and all participants were well informed and provided written informed consent (number: 15CT069).
2. Surgical technique
Radical vulvectomy combined with bilateral inguinal lymphadenectomy was employed in all the cases. During this procedure, the adipose and lymphatic tissues, including the superficial and deep inguinofemoral nodes, were removed, and the saphenous vein was spared. Of all the patients, sartorius transposition following inguinal-femoral lymphadenectomy (sartorius transposition group) was employed in 30 patients; in this procedure, the sartorius muscle is cut off at its attachment point, freed for approximately 5 cm and was then translocated medially over the femoral artery and vein and fixed to the inguinal ligament and adductor longus muscle with interrupted 1-0 polyglycolic acid sutures.
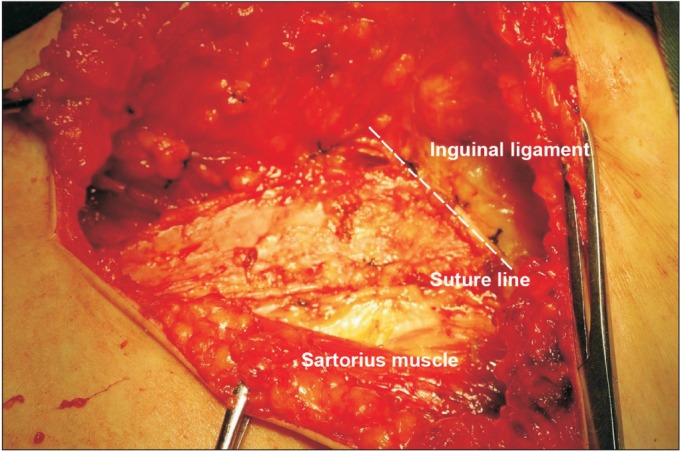
Sartorius tendon transposition following inguinal-femoral lymphadenectomy was employed in 28 patients (tendon transposition group). During this procedure, after inguinalfemoral lymphadenectomy was completed, the membrane of the sartorius tendon was cut from the sartorius muscle surface at its attachment points, freed for approximately 5 cm (Fig. 1), transposed and was then translocated medially over the femoral artery and vein and fixed to the inguinal ligament (Fig. 2).
Fig. 1. The membrane of the sartorius tendon was cut from the surface of the sartorius at its attachment points and freed for approximately 5 cm.
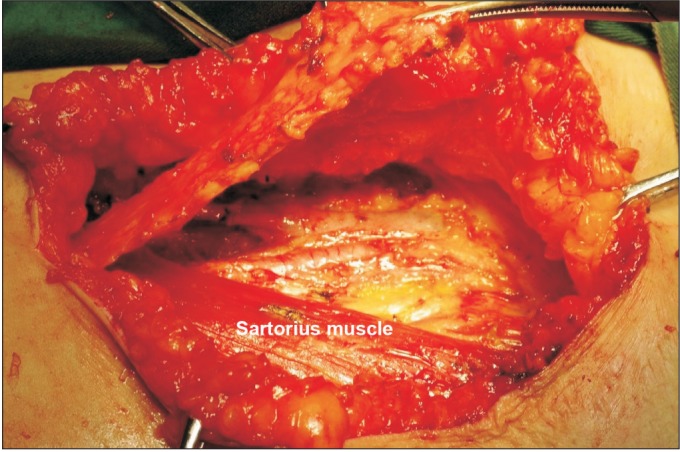
Fig. 2. The membrane of the sartorius tendon was transposed and sutured to the inguinal ligament.
At the end of surgery, all patients at both group received postoperative negative pressure Redon drainage in the groins at both side, and the drainages were removed when the production was less than 30 mL per day. Surgical wounds were checked every 3 days and the dressing got changed. After the patients got the stitches out, they were courage to start exercise, which takes about 10 to 12 days.
3. Follow-up
Follow-ups were conducted by telephone and though outpatient rechecks. At our institution, the patients receive recommends to return for clinical visits every 3 months for the first 2 years of follow-up and subsequently every 4 to 6 months until 5 years. Four had incomplete follow-up and were not included. The mean follow-up for the patients in this study was 55.6 months (range, 18 to 89 months), and the follow-ups concluded in October of 2013. Wound breakdown was defined if the wound was mechanically disrupted over at least 25% of the incision, and groin cellulitis if the severity of the infection required drainage and antibiotic therapy. Lymphedema was defined by Miller's clinical evaluation and persisting for greater than 3 months postoperatively [6]. The evaluations of postoperative QOL were conducted by using the Chinese version of QLQ-C30 ver. 3.0 (EORTC, Brussels, Belgium). Progression-free survival (PFS) was defined as the time from diagnosis to disease progression or the time of the first failure (loco regional or distant), and overall survival (OS) was defined as the time from the initial diagnosis until death from any cause.
4. Statistical analyses
The chi-square test and independent sample t-test for proportions were used to analyze differences in the distributions of the different variables between groups. Kaplan-Meier estimates of the OSs and PFSs and comparisons between the survival curves of each were performed using log-rank tests. All analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). All reported p-values are two-sided, and p<0.05 was taken to indicate statistical significance.
RESULTS
In terms of age, BMI, International Federation of Gynecology and Obstetrics (FIGO) staging, histology and grade, the patients in the sartorius transposition and tendon transposition group were comparable. The median age and BMI were 57.5 and 57.0 years, 24.5 and 23.3 kg/m2 at the sartorius transposition group and tendon transposition group (p=0.889 and p=0.320). No significant differences were found at FIGO stage histology grade between the two group (p=0.932, p=0.701, and p=0.493, respectively). The data for both groups are shown in Table 1.
Table 1. Clinical and pathologic characteristics.
| Characteristic | Sartorius transposition group (n=30) | Tendon transposition group (n=28) | p-value |
|---|---|---|---|
| Age (yr) | 57.5 | 57.0 | 0.889 |
| Body mass index (kg/m2) | 24.5 | 23.3 | 0.320 |
| FIGO stage | 0.932 | ||
| I | 12 (40.0) | 11 (39.3) | |
| II | 9 (30.0) | 8 (28.6) | |
| III | 6 (20.0) | 5 (17.9) | |
| IV | 1 (3.3) | 1 (3.6) | |
| Unknown | 2 (6.7) | 3 (10.7) | |
| Histology | 0.701 | ||
| Squamous carcinoma | 27 (90) | 24 (85.7) | |
| Non-squamous carcinoma | 3 (10) | 4 (14.3) | |
| Grade | 0.493 | ||
| 1 | 11 (36.7) | 11 (39.3) | |
| 2 | 11 (36.7) | 13 (46.4) | |
| 3 | 8 (26.7) | 4 (14.3) |
Values are presented as number (%).
FIGO, International Federation of Gynecology and Obstetrics.
1. Operative time, intraoperative blood loss and complications
No intraoperative bleeding and other complications occurred in either group, and none of the patients received intraoperative or postoperative blood transfusions. The median surgical time for the sartorius transposition group was 132±18 minutes (range, 107 to 185 minutes), and this time in the tendon transposition group was 139±20 minutes (range, 109 to 178 minutes); these times were not significantly different between the groups (p=0.316). The amount of bleeding in the sartorius transposition group was 50 to 180 mL, and the average bleeding volume was 128±37 mL. The amount of bleeding in the tendon transposition group was 40 to 170 mL and the average bleeding volume was 115±40 mL. No significant difference was found for either measure (p=0.249).
2. Wound healing, complications, and follow-up of quality of life
In the sartorius transposition group of 30 patients, the shortest healing time was 21 days, the longest time was 31 days, and the median length of hospitalization was 18.1±6 days. In the tendon transposition group, the shortest wound healing time was 10 days, the longest time was 26 days, and the median hospital stay was 14.81±4.8 days. The postoperative healing time in the tendon transposition group was significantly reduced compared to that of the sartorius transposition group (p=0.026), and the average hospital stay was also significantly reduced.
In the sartorius transposition group, breakdown of the groin wounds occurred in nine patients (30.0%), cellulitis occurred in four patients (13.3%), and postoperative follow-up revealed lymphoceles in six patients (20.0%), chronic lymphedema in 10 patients (33.3%). Sixteen cases exhibited good recovery with no chronic lymphedema, sensory abnormalities or limitations in lower limb activity. In the tendon transposition group, wound breakdown was observed in one patient (3.6%) and cellulitis occurred in two patients (7.1%). Three patients (10.7%) developed lymphoceles, chronic lymphedema occurred in two patients (7.1%). The rates of chronic lower extremity lymphedema and lower extremity pain were decreased in this group.
The incidence of groin cellulitis in tendon transposition group decreased compared to that of the sartorius transposition group; however, this difference was not statistically significant (p=0.673). Similar results were found for the incidences of lymphocele (p=0.473) (Table 2). However, the incidence of wound break and chronic lymphedema were significantly decreased in the tendon transposition group (p=0.012 and p=0.022, respectively).
Table 2. Incidences of complications in sartorius transposition and tendon transposition group.
| Variable | Sartorius transposition group (n=30) | Tendon transposition group (n=28) | p-value |
|---|---|---|---|
| Groin cellulitis | 4 (13.3) | 2 (7.1) | 0.673 |
| Wound break | 9 (30.0) | 1 (3.6) | 0.012 |
| Lymphocele | 6 (20.0) | 3 (10.7) | 0.473 |
| Chronic lymphedema | 10 (33.3) | 2 (7.1) | 0.022 |
Values are presented as number (%).
Statistically significant differences were found in global health status, physical functioning, emotional functioning, and pain as measured by the EORTC QLQ-C30 questionnaire, but no differences were found between the groups in QOL for role functioning, cognitive functioning, social functioning, and other symptom scales through which were reported more often among patients who underwent sartorius transposition group (Table 3). More patients in the tendon transposition group (13/28, 46.4%) resumed intercourse than sartorius transposition group (7/30, 23.3%) though no differences was found (p=0.097).
Table 3. Results of the QLQ-C30 questionnaire: sartorius transposition (n=30) versus tendon transposition group (n=28).
| Variable | Sartorius transposition group (n=30) | Tendon transposition group (n=28) | p-value |
|---|---|---|---|
| Functioning scale | |||
| Global health status | 43.3±26.7 | 72.9± 18.1 | 0.018 |
| Physical functioning | 72.8±17.3 | 88.0±11.3 | 0.044 |
| Role functioning | 88.7±11.5 | 86.6±13.1 | 0.546 |
| Emotional functioning | 84.8±13.9 | 91.7±9.2 | 0.020 |
| Cognitive functioning | 87.9±11.6 | 88.4±11.7 | 0.892 |
| Social functioning | 88.3±12.3 | 91.1±10.1 | 0.562 |
| Symptom scale | |||
| Fatigue | 16.2±6.1 | 18.2±5.8.4 | 0.761 |
| Nausea and vomiting | 4.0±1.9 | 3.6±1.5 | 0.177 |
| Pain | 15.3±5.5 | 13.7±2.1 | 0.004 |
| Dyspnea | 13.3±16.6 | 11.9±16.3 | 0.514 |
| Insomnia | 25.6±23.2 | 22.6±24.1 | 0.361 |
| Appetite loss | 20.0±18.8 | 16.7±17.0 | 0.598 |
| Constipation | 25.6±25.8 | 23.8±25.4 | 0.646 |
| Diarrhea | 13.3±20.7 | 15.5±23.1 | 0.449 |
| Financial difficulty | 34.8±24.3 | 34.5±26.4 | 0.727 |
Values are presented as mean±SD.
3. Recurrence
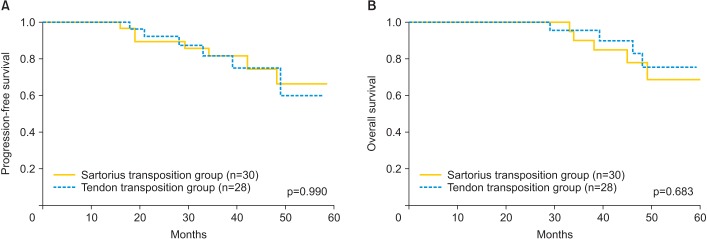
During the postoperative follow-up, seven patients in the sartorius transposition group experienced recurrences that included two cases of vulvar recurrence, three cases of inguinal region lymph node recurrence, and two cases of distant metastasis. There were six recurrences in the tendon transposition-treated group that included three case of inguinal region lymph node recurrence, one case of pelvic recurrence, and two cases of distant metastasis. The postoperative recurrence rates of the sartorius transposition and tendon transposition groups were 23.3% and 21.4%. The median PFS at sartorius transposition group and tendon transposition group were 66.0% and 60.0% (Fig. 3A). Corresponding figures for OS were 68.7% and 75.4% (Fig. 3B). The log-rank test on PFS and OS did not show significant difference between two groups (p=0.990 and p=0.683, respectively).
Fig. 3. Kaplan-Meier survival curves for the (A) progression-free survival (PFS) and (B) overall survival (OS) of the patients. No significance differences in PFS (p=0.990) or OS (p=0.683) were found.
DISCUSSION
Taussig (1940) [7] and Way (1948) [4] presented a standard operation for cancer of the vulva (i.e., radical vulvar resection and inguinal lymph node dissection), and the 5-year survival rate of the patients who underwent this procedure was approximately 70%. Since then, this radical surgery became the most common treatment for vulvar carcinoma patients. However, this procedure is often accompanied with physical and psychological adverse effects that seriously affect the patient's QOL, and the high rate of postoperative groin wound morbidity that is observed reflects the continued need for improvements in the surgical technique for inguinal-femoral lymphadenectomy and the postoperative care of these patients [8,9]. In an attempt to reduce severe complications, changes to surgical therapy have been continuously reported [10,11,12,13,14].
The use of muscular flaps to improve the healing time of wounds is commonplace in plastic and reconstructive surgery, especially the well-vascularized tissue for chronically infected regions. As a treatment for vulvar carcinoma, sartorius transposition was first introduced by Way [4] in 1960. This procedure was originally introduced to protect the femoral blood vessels in cases of wound breakdown. In 1997, Paley et al. [15] reported that groin wound complications, such as wound breakdown and cellulitis, are significant reduced by the use of the sartorius transposition method following inguinal-femoral lymphadenectomy. Based on their findings, these authors inferred that the morbidity that follows groin dissection likely arises from anatomical or surgical factors rather than microbial factors. However, Judson et al. [5] subsequently performed a prospective, randomized study and found no statistically significant differences in the incidences of wound cellulitis, wound breakdown, lymphedema, or rehospitalization in the sartorius transposition group. This author found that this method does not reduce postoperative wound morbidity [5]. This procedure undoubtedly requires the freeing of the upper portion of the sartorius and the suturing of this portion to the inguinal ligament to cover the surface of the femoral vessels for protection, which will certainly cause unnecessary damage [16]. The functions of the sartorius include flexing the hip and knee joints, and aiding in the external rotation and abduction of the hip, and these functions are affected to a certain extent. Furthermore, during this process, the lateral femoral cutaneous nerve might be cut off, which might cause persistent pain and sensory disturbances in the femoral anterolateral area. All of these factors increase tissue edema following the operation, which leads to poor wound healing and promotes edema and ischemia in the tissues of the inguinal operation area and results in flap incision necrosis reduced QOL for the patients following such operations [17,18].
In the present study, rather than cutting the sartorius itself, we only tore the tendon off of the surface of the sartorius and assessed the efficacy of replacing the sartorius transposition method in terms of reducing groin wound complications in vulvar carcinoma patients who underwent inguinal-femoral lymphadenectomy. Compared with the traditional operation method [19,20], this modification not only obviated the need to cut off skeleton muscle but also protected the femoral vascular and femoral nerve. The postoperative healing time and the average hospital stay in the tendon transposition group were significantly reduced. Furthermore, this new procedure was well tolerated and greatly reduced the overall complication rate. Despite the similarities of the patient populations and the surgical factors, significant differences were found at wound break and chronic lymphedema, which decreased from 30.0% to 3.6% and 33.3% to 7.1% respectively. The incidences of lymphocele and groin cellulitis decreased from 13.3% to 7.1% and 20.0% to 10.7% respectively due to the use of this new method although on significance were noticed.
Concerning the postoperative life quality in our study, the postoperative QOL of the in tendon transposition group was significantly improved compared to that of the control group as indicated by the postoperative follow-up EORTC QLQ-C30 questionnaire, which included the overall QOL, physical functioning, emotional functioning, and pain. This is consistent to Gunther et al. [21] report that women who had wide local excision with radical vulvectomy instead of underwent radical vulvectomy have a superior QOL with regard to global health status and physical, role, emotional and cognitive functioning. Sexual activity, in addition, was a major concern for most QOL studies in relation to vulvar cancer, and factors associated with sexual dysfunction after vulvar surgery include age, depression and anxiety, and type of surgery. Gunther et al. [21] found that there is a correlation between the extent of the operation and the presence of problems in desire, orgasm, and resolution phase. In our finding, as indicated by ratio in the tendon transposition group, more patients resumed intercourse than sartorius transposition group.
The benefits observed in the tendon transposition group demonstrate that this technique is an effective means of reducing complications in patients who undergo vulvectomy and inguinofemoral lymphadenectomy. However, the present study cannot determine it is the muscle and/or nerve preservation that made the difference and due to its non-randomized nature and the size of the groups of patients is small because of low prevalence of vulvar cancer; future prospective, randomized studies with larger samples are needed. Another limitation is that several gynecologic oncologists working in our unit during the studied period performed groin lymphadenectomies, which likely resulted in variations in surgical practice.
ACKNOWLEDGMENTS
We thank the participating patients and their families who contributed to and were recruited in this study. Grant sponsor(s): this research was supported by Projects from Department of Science & Technology of Henan Province and Health Department of Henan Province (Grant number: 142012310434 and Y2090084).
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Woelber L, Kock L, Gieseking F, Petersen C, Trillsch F, Choschzick M, et al. Clinical management of primary vulvar cancer. Eur J Cancer. 2011;47:2315–2321. doi: 10.1016/j.ejca.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 2.van der Steen S, de Nieuwenhof HP, Massuger L, Bulten J, de Hullu JA. New FIGO staging system of vulvar cancer indeed provides a better reflection of prognosis. Gynecol Oncol. 2010;119:520–525. doi: 10.1016/j.ygyno.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 3.Soliman AA, Heubner M, Kimmig R, Wimberger P. Morbidity of inguinofemoral lymphadenectomy in vulval cancer. ScientificWorldJournal. 2012;2012:341253. doi: 10.1100/2012/341253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Way S. Carcinoma of the vulva. Am J Obstet Gynecol. 1960;79:692–697. doi: 10.1016/0002-9378(60)90626-8. [DOI] [PubMed] [Google Scholar]
- 5.Judson PL, Jonson AL, Paley PJ, Bliss RL, Murray KP, Downs LS, Jr, et al. A prospective, randomized study analyzing sartorius transposition following inguinal-femoral lymphadenectomy. Gynecol Oncol. 2004;95:226–230. doi: 10.1016/j.ygyno.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Miller AJ, Bruna J, Beninson J. A universally applicable clinical classification of lymphedema. Angiology. 1999;50:189–192. doi: 10.1177/000331979905000302. [DOI] [PubMed] [Google Scholar]
- 7.Taussig FJ. Cancer of the vulva: an analysis of 155 cases (1911-1940) Am J Obstet Gynecol. 1940;40:764–779. [Google Scholar]
- 8.Wills A, Obermair A. A review of complications associated with the surgical treatment of vulvar cancer. Gynecol Oncol. 2013;131:467–479. doi: 10.1016/j.ygyno.2013.07.082. [DOI] [PubMed] [Google Scholar]
- 9.Gaarenstroom KN, Kenter GG, Trimbos JB, Agous I, Amant F, Peters AA, et al. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int J Gynecol Cancer. 2003;13:522–527. doi: 10.1046/j.1525-1438.2003.13304.x. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhou H, Wang LJ, Lu XM, Rao QX, Lu HW, et al. A modified triple incision technique for women with locally advanced vulvar cancer: a description of the technique and outcomes. Eur J Obstet Gynecol Reprod Biol. 2012;164:185–190. doi: 10.1016/j.ejogrb.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 11.DiSaia PJ, Creasman WT, Rich WM. An alternate approach to early cancer of the vulva. Am J Obstet Gynecol. 1979;133:825–832. doi: 10.1016/0002-9378(79)90119-4. [DOI] [PubMed] [Google Scholar]
- 12.Bell JG, Lea JS, Reid GC. Complete groin lymphadenectomy with preservation of the fascia lata in the treatment of vulvar carcinoma. Gynecol Oncol. 2000;77:314–318. doi: 10.1006/gyno.2000.5790. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Sheng X, Niu J, Li H, Li D, Tang L, et al. Sparing of saphenous vein during inguinal lymphadenectomy for vulval malignancies. Gynecol Oncol. 2007;105:722–726. doi: 10.1016/j.ygyno.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Manci N, Marchetti C, Esposito F, De Falco C, Bellati F, Giorgini M, et al. Inguinofemoral lymphadenectomy: randomized trial comparing inguinal skin access above or below the inguinal ligament. Ann Surg Oncol. 2009;16:721–728. doi: 10.1245/s10434-008-0216-4. [DOI] [PubMed] [Google Scholar]
- 15.Paley PJ, Johnson PR, Adcock LL, Cosin JA, Chen MD, Fowler JM, et al. The effect of sartorius transposition on wound morbidity following inguinal-femoral lymphadenectomy. Gynecol Oncol. 1997;64:237–241. doi: 10.1006/gyno.1996.4557. [DOI] [PubMed] [Google Scholar]
- 16.Walker KF, Day H, Abu J, Nunns D, Williamson K, Duncan T. Do surgical techniques used in groin lymphadenectomy for vulval cancer affect morbidity rates? Int J Gynecol Cancer. 2011;21:1495–1499. doi: 10.1097/IGC.0b013e318228f314. [DOI] [PubMed] [Google Scholar]
- 17.Senn B, Mueller MD, Cignacco EL, Eicher M. Period prevalence and risk factors for postoperative short-term wound complications in vulvar cancer: a cross-sectional study. Int J Gynecol Cancer. 2010;20:646–654. doi: 10.1111/IGC.0b013e3181d92723. [DOI] [PubMed] [Google Scholar]
- 18.Baiocchi G, Rocha RM. Vulvar cancer surgery. Curr Opin Obstet Gynecol. 2014;26:9–17. doi: 10.1097/GCO.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 19.Rouzier R, Haddad B, Dubernard G, Dubois P, Paniel BJ. Inguinofemoral dissection for carcinoma of the vulva: effect of modifications of extent and technique on morbidity and survival. J Am Coll Surg. 2003;196:442–450. doi: 10.1016/S1072-7515(02)01895-1. [DOI] [PubMed] [Google Scholar]
- 20.Senn B, Eicher M, Mueller MD, Hornung R, Fink D, Baessler K, et al. A patient-reported outcome measure to identify occurrence and distress of post-surgery symptoms of WOMen with vulvAr Neoplasia (WOMAN-PRO): a cross sectional study. Gynecol Oncol. 2013;129:234–240. doi: 10.1016/j.ygyno.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 21.Gunther V, Malchow B, Schubert M, Andresen L, Jochens A, Jonat W, et al. Impact of radical operative treatment on the quality of life in women with vulvar cancer: a retrospective study. Eur J Surg Oncol. 2014;40:875–882. doi: 10.1016/j.ejso.2014.03.027. [DOI] [PubMed] [Google Scholar]


