ABSTRACT
A 2014 multistate listeriosis outbreak was linked to consumption of caramel-coated apples, an unexpected and previously unreported vehicle for Listeria monocytogenes. This outbreak was unanticipated because both the pH of apples (<4.0) and the water activity of the caramel coating (<0.80) are too low to support Listeria growth. In this study, Granny Smith apples were inoculated with approximately 4 log10 CFU of L. monocytogenes (a cocktail of serotype 4b strains associated with the outbreak) on each apple’s skin, stem, and calyx. Half of the apples had sticks inserted into the core, while the remaining apples were left intact. Apples were dipped into hot caramel and stored at either 7°C or 25°C for up to 11 or 28 days, respectively. Data revealed that apples with inserted sticks supported significantly more L. monocytogenes growth than apples without sticks under both storage conditions. Within 3 days at 25°C, L. monocytogenes populations increased >3 log10 in apples with sticks, whereas only a 1-log10 increase was observed even after 1 week for caramel-coated apples without sticks. When stored at 7°C, apples with sticks exhibited an approximately 1.5-log10 increase in L. monocytogenes levels at 28 days, whereas no growth was observed in apples without sticks. We infer that insertion of a stick into the apple accelerates the transfer of juice from the interior of the apple to its surface, creating a microenvironment at the apple-caramel interface where L. monocytogenes can rapidly grow to levels sufficient to cause disease when stored at room temperature.
IMPORTANCE
Neither caramel nor apples are a food where the pathogenic bacterium Listeria monocytogenes should grow, as caramel does not contain enough free water and apples are too acidic. Caramel-coated apples, however, were recently linked to a deadly outbreak of listeriosis. We hypothesized that inserting a stick into the apple releases juice to the interface between the apple and caramel, providing a more hospitable environment than either component alone. To test this hypothesis, apples were inoculated with L. monocytogenes prior to caramel dipping. Some apples had sticks inserted into them before dipping, while others did not. No growth of L. monocytogenes occurred on refrigerated caramel apples without sticks, whereas slow growth was observed on refrigerated caramel apples with sticks. In contrast, significant pathogen growth was observed within 3 days at room temperature on caramel apples with sticks inserted. Food producers should consider interfaces between components within foods as potential niches for pathogen growth.
OBSERVATION
The 2014 caramel apple listeriosis outbreak infected 35 people across the United States and one additional person in Canada; seven deaths were reported, with listeriosis directly causing three of the deaths (1, 2). The outbreak took producers, public health officials, and food safety experts by surprise: caramel-coated apples are not a food on which Listeria monocytogenes should grow. First, the pH of apples is too low (usually <4.0) to support growth of L. monocytogenes (3). Second, the caramel coating used on apples both is hot (~95°C) and has low water activity, usually <0.80 (4), and most L. monocytogenes strains require water activity (aw) of at least 0.93 for growth (5). Although Listeria spp. are common in the produce fields (6), there are no surveys that suggest that L. monocytogenes is a pathogen routinely associated with apples (7). Additionally, intact apples have not been implicated previously in foodborne disease outbreaks (8), with one exception due to an unknown etiological agent (9).
The epidemiological association with caramel apples was strong, as 28 of the 31 persons interviewed reported eating them (2). Three additional patients sickened with the outbreak strains did not remember eating caramel apples but did recall eating whole or sliced green apples from an unknown source (1). At least three different caramel apple manufacturers were involved in the outbreak, although the apples were sourced from a single common apple producer. Listeria monocytogenes isolates from environmental samples collected from that apple producer’s facility matched isolates from persons sickened in the outbreak, as determined by using whole-genome sequencing (2). These findings strongly suggested the L. monocytogenes originated on the apples but left unanswered how the pathogen multiplied on caramel-coated apples.
L. monocytogenes is thought to have an infectious dose of about 105 to 107 CFU in high-risk individuals (10, 11). As noted above, the pathogen is common in the environment, including in soils, pastures, and decaying vegetation, and can colonize food processing plants as well. Strains that cause foodborne disease tend to be particularly adept at biofilm formation (12), making them especially difficult to eliminate in the environment once established. Importantly, L. monocytogenes has the ability to multiply at refrigeration temperatures.
We hypothesized that the caramel layer on the apple traps moisture next to the surface, creating a microenvironment on the surface of the apple that facilitates growth of L. monocytogenes cells that are already present on the apple surface (Fig. 1A). Insertion of the stick may expedite juice migrating to the surface of the apple, increasing the water activity in or just below the caramel layer. Although caramel-coated apples are typically distributed under refrigeration conditions, they may be unrefrigerated for 2 to 4 weeks by retailers or consumers. Storage at nonrefrigeration temperatures can accelerate both moisture migration and microbial growth.
FIG 1 .
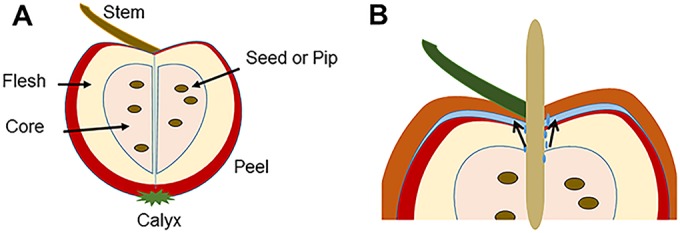
Key parts of the apple (A) and the caramel-apple interface microenvironment (B).
Listerial growth on caramel-coated apples.
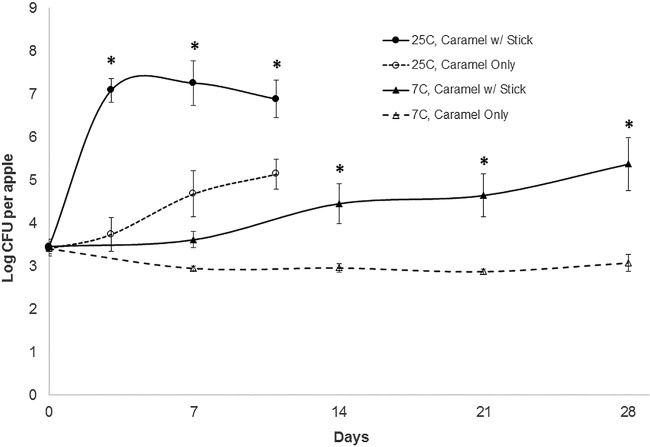
To test our hypothesis, three separate caramel apple growth trials were conducted, with three apples tested for each set of conditions and time point in each trial (a total of 144 apples assayed in the study). The results reported are the means and standard errors of enumeration data across all trials. We prepared a cocktail of four L. monocytogenes strains associated with the outbreak (all serotype 4b and described further in “Listeria monocytogenes inoculum preparation” below). Apples (as purchased, without any additional sanitation procedures or removal of wax) were inoculated on the skin, stem, and calyx regions (Fig. 1A) with an average of 4.2 ± 0.7 log10 CFU per apple. A wooden stick was inserted through the stem of half of the apples. The other apples did not receive a stick. Dipping the apples into the hot caramel (95°C) resulted in an immediate reduction of ~0.8 to 1.2 log10 L. monocytogenes per apple. Coated apples were allowed to cool and then stored at 25°C or 7°C. On caramel apples with sticks, the mean populations of L. monocytogenes increased an average 3.6 log10 CFU by day 3 when apples were stored at room temperature (25°C) and remained at least 3.4 log10 CFU above baseline for the duration of the study (Fig. 2). In contrast, listerial growth was delayed on caramel apples without sticks, with populations increasing an average 0.3, 1.5, and 2.1 log10 CFU above baseline by days 3, 7, and 11, respectively. Levels of L. monocytogenes growth on caramel-coated apples without sticks were statistically significantly different from those on apples without sticks (P < 0.05).
FIG 2 .
Changes in populations of L. monocytogenes in inoculated caramel-coated apples, with and without stick penetration, stored at 7 and 25°C for up to 28 days. Data are means and standard errors from three separate trials, with three apples per variable at each time interval (n = 9); a total of 144 apples were assayed for the data presented. Asterisks indicate values that are statistically significantly different (P < 0.05) from corresponding values for apples without sticks. After 3 days at 25°C, L. monocytogenes levels were statistically significantly different from baseline levels (P < 0.05) in caramel apples with a stick. In contrast, for caramel apples without sticks, L. monocytogenes levels did not become statistically significantly different from baseline levels until 11 days at 25°C. At 7°C, L. monocytogenes levels in apples with sticks did not become statistically significantly different from baseline until 28 days. In caramel apples without sticks at 7°C, no change in L. monocytogenes levels was observed at any time point compared to baseline.
Reducing the storage temperature to 7°C slowed L. monocytogenes growth on caramel apples, especially in the absence of sticks. No L. monocytogenes growth was observed on caramel apples without sticks during 4 weeks of storage at 7°C (Fig. 2). When caramel apples were penetrated with sticks and stored at 7°C, no growth was detected at 1 week, but populations increased 1.0, 1.2, and 1.9 log10 CFU per apple above baseline at 2, 3, and 4 weeks, respectively (Fig. 2). No L. monocytogenes growth (~0.4-log reduction) was observed on inoculated, uncoated apples stored at 7°C for 21 days (data not shown).
These data are consistent with the hypothesis that L. monocytogenes can grow in the microenvironment between the apple surface and caramel coating of contaminated caramel-coated apples that are stored at room temperature. We hypothesize that transpiration of moisture across the cuticle occurs during long-term storage of apples and that the moisture is trapped under the caramel coating, increasing the localized aw even in the absence of a stick. L. monocytogenes growth was greater in apples into which a stick was inserted. Juice from the apple is expressed when the stick initially penetrates the apple core, and liquid may continue to migrate to the surface along the region where the stick was inserted (Fig. 1B) during storage. This increased amount of liquid could further raise the aw under the caramel coating. The low pH of the juice is likely neutralized by the caramel during equilibration, resulting in conditions conducive to growth of L. monocytogenes.
Although we did not yet test whether L. monocytogenes grows on the surface of uncoated apples following stick insertion, the apple juice transported to the apple surface would evaporate quickly. This would restore a low aw to the surface that would be unsuitable for bacterial growth. The use of wax coating on the apple reduces dehydration of the apple during storage. Wax (e.g., carnauba-shellac wax) itself does not have antimicrobial activity against L. monocytogenes or Escherichia coli O157:H7 in vitro (13); however, lower populations of total bacteria, molds, and yeast were recovered from waxed apples than unwaxed apples throughout 5 months of storage at 1°C (13). Therefore, using unwaxed apples may not alter the growth rate of L. monocytogenes on the caramel-coated apples.
In addition, we hypothesize that some L. monocytogenes cells harbored in the stem area might be pushed into the core when the stick is inserted, where these bacterial cells would be protected from the heat of the caramel. Liquid could carry surviving L. monocytogenes cells to the surface, where they would be trapped under the caramel in a region where the local aw might be sufficient for listerial growth. Both moisture transfer (which is trapped under the caramel layer) and microbial growth are accelerated at room temperature compared to refrigeration.
We chose regions of the apple surface (calyx, stem, and peel areas) for inoculation because intact apples rarely harbor bacteria within the flesh (7), and the stem and calyx regions are common harborage sites for microbes on apples (14, 15). We also focused on these regions for microbial collection from the caramel apples by immersing them in buffer and massaging the caramel off the apple. L. monocytogenes present in this wash buffer was then enumerated. It is unlikely that L. monocytogenes was also present within the flesh of the fruit because of the surface inoculation method used in our study. In addition, the pH of the apple flesh used in our experiments was measured to be 3.2, and growth of L. monocytogenes below pH 4.0 has not been reported (16). A previous study reported L. monocytogenes inactivation in pH 3.4 apple juice but growth in Red Delicious apples slices (pH 4.7) stored at 10 or 20°C (17). Both Granny Smith and Gala apples were implicated in the 2014 listeriosis outbreak, but Granny Smith apples were chosen for these experiments because their exceptionally low pH represents a steeper hurdle for bacterial growth (3).
It is possible that other parts of the apple, such as the core or seeds, also hosted L. monocytogenes growth. These parts of the apple are not typically eaten completely, but may be bitten into by consumers. The pH of the core region of Granny Smith apples used in these experiments was not measured, but in other apple varieties, the core region pH may be 0.6 to 0.8 units higher than that in the apple flesh (18, 19). Future experiments are planned to investigate whether L. monocytogenes growth occurs in the core region.
It is unknown whether the strains of L. monocytogenes from this disease outbreak possess unusual resistance to low pH or exceptional virulence. Additional studies are in progress to determine the minimum pH for growth of the outbreak strains in laboratory media and apple juice and to determine if the addition of antimicrobials to the caramel dip can inhibit listerial growth. All outbreak strains tested were able to form biofilms, invade, and multiply within the human adenocarcinoma cell line Caco-2 and exhibit virulence in an established mouse model (N. G. Faith and C. Czuprynski, unpublished data), comparable to that of a different L. monocytogenes strain implicated in another significant foodborne disease outbreak (20).
The level of L. monocytogenes that was recovered from the surface of the apples following caramel dipping (3 to 3.4 log10 CFU per apple) represents a level that could potentially be found on produce. A review of 165 prevalence studies found a 0.17% probability for L. monocytogenes to be present on a fresh or minimally processed vegetable at 3 log10 CFU/g (21). Following 3 days of incubation at 25°C, some individual caramel apples with sticks had levels of L. monocytogenes as high as 7 log10 CFU/apple, which is sufficient to cause disease if the product is consumed by a susceptible individual.
Conclusions.
Our findings suggest that the 2014 listeriosis outbreak associated with caramel-coated apples can be explained by growth of L. monocytogenes occurring at the interface between two foods which, by themselves, are inhibitory to pathogen growth. If L. monocytogenes was present on or in the apple after coating with hot caramel, the typical extended storage at ambient temperature by the retailer, and perhaps the consumer, would be sufficient to allow the pathogen to grow to infectious levels. The insertion of the stick into the apples increased the growth rate of L. monocytogenes in caramel-coated apples, likely by enhancing the moisture migration to the caramel-apple interface and accelerating the development of optimal growth conditions. One might suggest eliminating the stick; however, this could hinder both production and consumption of the product and therefore may not be a useful strategy for the caramel apple industry. Practical intervention strategies might include validated disinfection of the apple, addition of growth inhibitors to the caramel coating or apple wax, or temperature-time controls to inhibit growth of L. monocytogenes on caramel apples.
Listeria monocytogenes inoculum preparation.
A four-strain mixture of L. monocytogenes clinical isolates was used in this study. The inoculum was composed of three strains from the 2014 caramel apple outbreak (573-035, 576-043, and 580-060; all serotype 4b) plus one additional strain (548-072, also a serotype 4b strain) that was not considered responsible for an outbreak case but matched the pulsed-field gel electrophoresis (PFGE) patterns of the outbreak strains (provided by the Wisconsin State Laboratory of Hygiene, Madison, WI). Stocks of these strains were maintained in ceramic beads (CRYO/M; Copan Diagnostics Inc., Murrieta, CA) stored at −80°C. For inoculum preparation, each individual strain bead was cultured in 10 ml of fresh Trypticase soy broth (TSB; Becton, Dickinson and Company, Sparks, MD, USA) at 37°C for 20 to 24 h. The freshly grown culture (0.1 ml) was further transferred into 10 ml of fresh TSB and incubated at 37°C for 18 to 22 h. Cells were harvested by centrifugation (4,000 × g, 20 min) and suspended in 4.5 ml 0.1% buffered peptone water (pH 7.1 ± 0.1). Equivalent populations of each isolate were combined to provide a four-strain mixture of L. monocytogenes. Purity and populations of each strain were verified by plating on Trypticase soy agar (TSA) and modified Oxford agar (MOX; Listeria selective agar base; Difco, BD Biosciences, Sparks, MD).
Inoculated apple preparation and testing.
Waxed Granny Smith apples (1.4-kg bags) and commercially prepared caramel apple dip (ingredients included high-fructose corn syrup, skim milk, corn syrup, palm oil, sugar, butter, modified corn starch, disodium phosphate, potassium sorbate, tert-butylhydroquinone, salt, mono- and diglycerides, and artificial flavors) were purchased from a local retailer. The pH of the apple flesh (skin removed) was 3.2, and the aw was 0.98; the caramel apple dip had a measured aw of 0.79 and a pH of 5.85. Apples with obvious damage/bruising were not used for these experiments. Granny Smith apples were chosen for tests because this variety was implicated in the listeriosis outbreak and because their high acidity represents a higher barrier for microbial growth.
In order to simulate/prepare L. monocytogenes-contaminated apples, 200 µl of L. monocytogenes cocktail was pipetted into the bottom calyx of the apple (~22°C). The inoculum was allowed to stand for 2 min; the residual volume was removed by pipette and applied to the stem region and allowed to sit for another 2 min; finally, the residual volume was applied over the surface of the apple using a sterile cotton swab. Apples were then divided into two groups; for one set of apples, wooden sticks (either flat sticks, 11.4 cm long by 0.95 cm wide by 0.2 cm high, or round sticks, 14 cm long by 0.6 cm in diameter; there was no difference in growth rates among apples with different stick dimensions) were inserted approximately 5 cm into the core region from the stem side, whereas no sticks were inserted into the second set of apples. The sticks were not sterilized or treated in any way before use, and the moisture content of the dry sticks was not measured in this study. All apples were air dried for a minimum 5 to 10 min at room temperature (visibly dry). L. monocytogenes populations were determined on triplicate inoculated apples after air drying as described below.
Caramel dip was placed in a 2.5-liter double-jacketed mixer (Universal Machine UMC-5; Stephan Machinery GmbH, Hameln, Germany) and heated with agitation to 95°C (commercial caramel apple makers typically use a temperature of 104 to 116°C, but temperatures can cool to <100°C during production). The caramel was removed from the heat once it reached 95°C, and apples were then dipped into the caramel using either the stick or kitchen tongs. During the process, the caramel temperature decreased to 85°C. The dipping process resulted in a caramel coating approximately 3 mm thick.
Coated apples were placed on individual sanitized polystyrene weighing boats, transferred to household polyethylene storage containers, lidded, and then stored at 25 or 7°C (without additional humidity control); triplicate samples for each treatment were assayed before and after coating and on days 3, 7, 11, and 14 for 25°C and at weeks 1, 2, 3, and 4 at 7°C. The study was performed three times.
L. monocytogenes populations were enumerated from inoculated apples by transferring to sterile polypropylene sample bags and adding 100 ml of sterile 1% buffered peptone water to each package. The contents of the bag were massaged externally by hand for about 3 min to release the caramel and cells from the surface. Rinsates were serially diluted, and L. monocytogenes populations were enumerated by surface plating serial dilutions of rinse material on MOX. Typical colonies recovered on MOX were considered confirmatory.
Statistical analysis.
Data were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. P values of 0.05 or less were considered statistically significant.
ACKNOWLEDGMENTS
This study was funded by the University of Wisconsin-Madison Food Research Institute (FRI).
We thank Amanda Skarlupka, Ming Mu, Subash Shrestha, Adam Bartling, Emily Merry, Katina Fisher, Anna Spensely, Abby Dabson, and Katie Osterbauer for technical assistance during inoculation and testing, Ken Brandenburg for statistical analysis, and Nan Faith for virulence and biofilm testing. We are grateful to Tim Monson, Wisconsin State Laboratory of Hygiene, for the L. monocytogenes isolates and Rachel Klos, Wisconsin Division of Public Health, for helpful discussion.
Footnotes
Citation Glass KA, Golden MC, Wanless B, Bedale W, Czuprynski C. 2015. Growth of Listeria monocytogenes within a caramel-coated apple microenvironment. mBio 6(5):e01232-15. doi:10.1128/mBio.01232-15.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2015. Multistate outbreak of listeriosis linked to commercially produced, prepackaged caramel apples made from Bidart Bros. apples. http://www.cdc.gov/listeria/outbreaks/caramel-apples-12-14/. Accessed 9 March 2015.
- 2.U.S. FDA. 2015. FDA investigated Listeria monocytogenes illnesses linked to caramel apples. http://www.fda.gov/Food/RecallsOutbreaksEmergencies/Outbreaks/ucm427573.htm. Accessed 9 June 2015.
- 3.Wu J, Gao H, Zhao L, Liao X, Chen F, Wang Z, Hu X. 2007. Chemical compositional characterization of some apple cultivars. Food Chem 103:88–93. doi: 10.1016/j.foodchem.2006.07.030. [DOI] [Google Scholar]
- 4.Ergun R, Lietha R, Hartel RW. 2010. Moisture and shelf life in sugar confections. Crit Rev Food Sci Nutr 50:162–192. doi: 10.1080/10408390802248833. [DOI] [PubMed] [Google Scholar]
- 5.Ryser ET, Buchanan RL. 2013. Listeria monocytogenes. In Doyle MP, Buchanan RL (ed), Food microbiology: fundamentals and frontiers. ASM Press, Washington, DC. [Google Scholar]
- 6.Strawn LK, Grohn YT, Warchocki S, Worobo RW, Bihn EA, Wiedmann M. 2013. Risk factors associated with Salmonella and Listeria monocytogenes contamination of produce fields. Appl Environ Microbiol 79:7618–7627. doi: 10.1128/AEM.02831-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doores S, Splittstoesser DF. 1983. The microbiology of apples and apple products. CRC Crit Rev Food Sci Nutr 19:133–149. doi: 10.1080/10408398309527372. [DOI] [PubMed] [Google Scholar]
- 8.Keller SE. 2014. Tree fruits and nuts: outbreaks, contamination sources, prevention, and remediation, p 291–312. In Matthews KR, Sapers GM, Gerba CP (ed), Produce contamination problem: causes and solutions, 2nd ed. Elsevier, Amsterdam, The Netherlands. doi: 10.1016/b978-0-12-404611-5.00013-0:291-312. [DOI] [Google Scholar]
- 9.Sivapalasingam S, Friedman CR, Cohen L, Tauxe RV. 2004. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J Food Prot 67:2342–2353. [DOI] [PubMed] [Google Scholar]
- 10.Farber JM, Ross WH, Harwig J. 1996. Health risk assessment of Listeria monocytogenes in Canada. Int J Food Microbiol 30:145–156. doi: 10.1016/0168-1605(96)01107-5. [DOI] [PubMed] [Google Scholar]
- 11.Williams D, Castleman J, Lee C, Mote B, Smith MA. 2009. Risk of fetal mortality after exposure to Listeria monocytogenes based on dose-response data from pregnant guinea pigs and primates. Risk Anal 29:1495–1505. doi: 10.1111/j.1539-6924.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- 12.Chae MS, Schraft H, Hansen L, Mackereth R. 2006. Effects of physicochemical surface characteristics of Listeria monocytogenes strains on attachment to glass. Food Microbiol 23:250–259. doi: 10.1016/j.fm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Jo W, Song H, Song N, Lee J, Min SC, Song KB. 2014. Quality and microbial safety of “Fuji” apples coated with carnauba-shellac wax containing lemongrass oil. LWT Food Sci Technol 55:490–497. doi: 10.1016/j.lwt.2013.10.034. [DOI] [Google Scholar]
- 14.Buchanan RL, Edelson SG, Miller RL, Sapers GM. 1999. Contamination of intact apples after immersion in an aqueous environment containing Escherichia coli O157:H7. J Food Protect 62:444–450. [DOI] [PubMed] [Google Scholar]
- 15.Baskaran SA, Upadhyay A, Kollanoor-Johny A, Upadhyaya I, Mooyottu S, Roshni Amalaradjou MA, Schreiber D, Venkitanarayanan K. 2013. Efficacy of plant-derived antimicrobials as antimicrobial wash treatments for reducing enterohemorrhagic Escherichia coli O157:H7 on apples. J Food Sci 78:M1399–M1404. doi: 10.1111/1750-3841.12174. [DOI] [PubMed] [Google Scholar]
- 16.Lado BH, Yousef AE. 2007. Characteristics of Listeria monocytogenes important to food processors, p 157–213. In Ryser ET, Marth EH (ed), Listeria, listeriosis, and food safety. CRC Press, Boca Raton, FL. [Google Scholar]
- 17.Conway WS, Leverentz B, Saftner RA, Janisiewicz WJ, Sams CE, Leblanc E. 2000. Survival and growth of Listeria monocytogenes on fresh-cut apple slices and its interaction with Glomerella cingulata and Penicillium expansum. Plant Dis 84:177–181. doi: 10.1094/PDIS.2000.84.2.177. [DOI] [PubMed] [Google Scholar]
- 18.Niem J, Miyara I, Ettedgui Y, Reuveni M, Flaishman M, Prusky D. 2007. Core rot development in red delicious apples is affected by susceptibility of the seed locule to Alternaria alternata colonization. Phytopathology 97:1415–1421. doi: 10.1094/PHYTO-97-11-1415. [DOI] [PubMed] [Google Scholar]
- 19.Dingman DW. 2000. Growth of Escherichia coli O157:H7 in bruised apple (Malus domestica) tissue as influenced by cultivar, date of harvest, and source. Appl Environ Microbiol 66:1077–1083. doi: 10.1128/AEM.66.3.1077-1083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald PDM, Whitwam RE, Boggs JD, MacCormack JN, Anderson KL, Reardon JW, Saah JR, Graves LM, Hunter SB, Sobel J. 2005. Outbreak of listeriosis among Mexican immigrants as a result of consumption of illicitly produced Mexican-style cheese. Clin Infect Dis 40:677–682. doi: 10.1086/427803. [DOI] [PubMed] [Google Scholar]
- 21.Crepet A, Albert I, Dervin C, Carlin F. 2007. Estimation of microbial contamination of food from prevalence and concentration data: application to Listeria monocytogenes in fresh vegetables. Appl Environ Microbiol 73:250–258. doi: 10.1128/AEM.00351-06. [DOI] [PMC free article] [PubMed] [Google Scholar]