Abstract
Significance: Cutaneous scarring is an almost inevitable end point of adult human wound healing. It is associated with significant morbidity, both physical and psychological. Pathological scarring, including hypertrophic and keloid scars, can be particularly debilitating. Manipulation of the chemokine system may lead to effective therapies for problematic lesions.
Recent Advances: Rapid advancement in the understanding of chemokines and their receptors has led to exciting developments in the world of therapeutics. Modulation of their function has led to clinically effective treatments for conditions as diverse as human immunodeficiency virus and inflammatory bowel disease. Potential methods of targeting chemokines include monoclonal antibodies, small-molecule antagonists, interference with glycosaminoglycan binding and the use of synthetic truncated chemokines. Early work has shown promising results on scar development and appearance when the chemokine system is manipulated.
Critical Issues: Chemokines are implicated in all stages of wound healing leading to the development of a cutaneous scar. An understanding of entirely regenerative wound healing in the developing fetus and how the expression of chemokines and their receptors change during the transition to the adult phenotype is central to addressing pathological scarring in adults.
Future Directions: As our understanding of chemokine/receptor interactions and scar formation evolves it has become apparent that effective therapies will need to mirror the complexities in these diverse biological processes. It is likely that sophisticated treatments that sequentially influence multiple ligand/receptor interactions throughout all stages of wound healing will be required to deliver viable treatment options.

Ardeshir Bayat, BSc (Hons), MBBS, MRCS, PhD
Scope and Significance
Progressive fibrosis resulting in scarring represents the end point of normal mammalian tissue repair after dermal injury. Although effective in restoring cutaneous barrier function, scar tissue is inferior to healthy skin.1 Fetal wound healing is entirely regenerative before 24 weeks gestation, without scar tissue formation.2,3 Behavioral discrepancies have been attributed to differing inflammatory responses and cytokine profiles of fetal and adult wounds. These are controlled by a range of bioactive molecules, including chemokines. This review summarizes current knowledge of chemokine behavior in acute and pathological cutaneous wounds before discussing their application as novel antifibrotic therapeutic agents.
Translational Relevance
Chemokines are bioactive molecules that play key roles throughout wound healing, but particularly within the inflammatory and proliferative phases. First identified by their ability to induce leukocyte migration they have now been shown to have vital roles in leukocyte recruitment, activation and effector function, as well as regulation of angiogenesis and myofibroblast localization.4–8 Chemokine behavior as agonists or antagonists is variable and dependent on the receptor they bind to.9 The formation of receptor/ligand dimers and oligomers also influences function.
Clinical Relevance
Chemokines are a large and potentially powerful family of targets for scar reducing therapeutics. Chemokines play a prominent role in normal wound healing, but altered expression is observed in keloid and hypertrophic scars as well as chronic wounds.10–12 Consequently iatrogenic manipulation of specific chemokine signaling pathways could offer an alternative means to reduce wound fibrosis, chronic wound development, and the incidence of pathological scarring.13 Complexities of chemokine physiology have delayed development of effective scar-reducing agents.
Discussion of Findings and Relevant Literature
Overview of chemokines
Chemokines are a large family of heparin-binding cytokines known for their small size (8–10 kDa) and four highly conserved cysteine residues.14 Since interleukin (IL)-8 was first described by Baggiolini15 knowledge of these complex interacting proteins has increased exponentially.
In 2000 a system of nomenclature was introduced in which each ligand and receptor is identified by its subfamily and an identifying number.16,17 Recent discoveries and advances, particularly in the area of atypical receptors, has necessitated an update.18 This method of nomenclature will be utilized throughout this review.
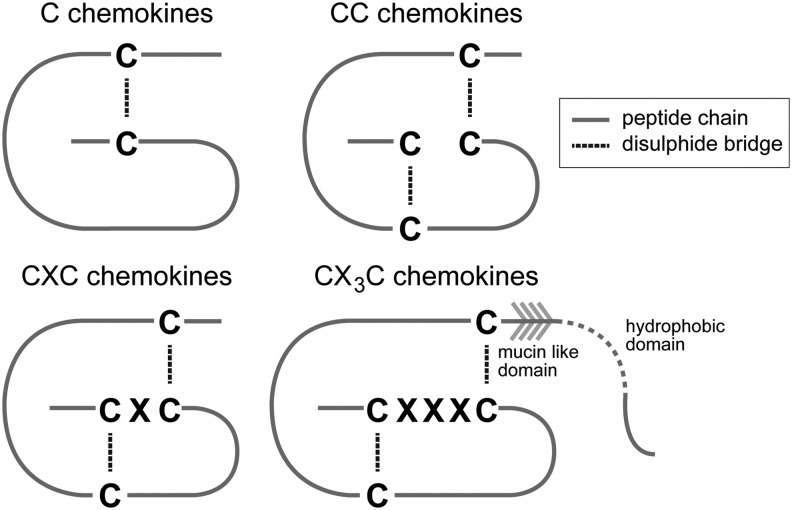
Over 50 chemokines have been identified to date, divided into 4 subgroups based on the arrangement of the first 2 of the 4 cysteine amino acids - CC, CXC, CX3C, and C19 (Fig. 1).20 The large CC chemokine family consists of chemokines with the first two cysteine residues adjacent to each other, in comparison to the CXC group, which has a single (variable) amino acid dividing them.21 The lone member of the CX3C group (CX3CL1) has three amino acids dividing the first two cysteines. The last group, C, is notable for its members, XCL1 and XCL2, possessing only two of the usual four cysteine residues.22,23 A detailed discussion of the structure of chemokines is beyond the scope of this review, but is covered comprehensively by Allen et al.24
Figure 1.
The four chemokine subfamilies, including basic structure (Adapted from Martins-Green et al. 2013).20
Chemokines may be further classified as either proinflammatory, homeostatic, or a mixture of both. Inflammatory chemokines are produced by activated cells during pathological conditions to recruit leukocytes to inflamed tissues. Homeostatic chemokines are constitutively produced to maintain homeostatic leukocyte trafficking and the architecture of secondary lymphoid organs.25
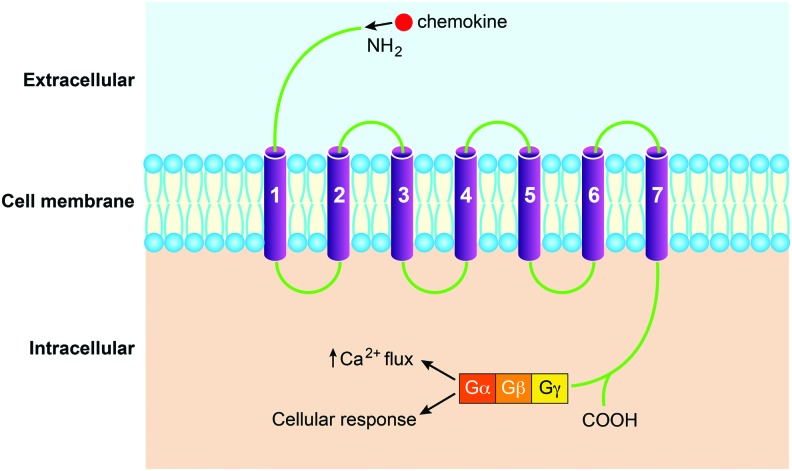
Chemokines exert their chemotactic affects by binding to chemokine receptors, of which over 20 have been discovered to date (Table 1).18 The classic chemokine receptors are seven transmembrane-spanning proteins coupled to heterotrimeric G proteins that is, G-protein coupled receptors (GPCRs).25 They are present in the lipid bilayer of target cells with both an extracellular and intracellular component. The extracellular domain consists of the N-terminus and three extracellular loops, while the intracellular region is composed of three loops and the C-terminus.26 These domains play a role in ligand binding and signal transduction, respectively. Atypical chemokine receptors have the ability to bind chemokines, but do not signal through G proteins.18 An example is atypical chemokine receptor 1 (ACKR1), previously duffy antigen receptor for chemokines (DARC). These atypical receptors fulfill a number of functions, including scavenging free chemokines in the blood stream.27
Table 1.
Chemokine receptors and their associated ligands
| Chemokine receptor | Associated ligands |
|---|---|
| CXC subfamily | |
| CXCR1 | CXCL5, CXCL6, CXCL8 |
| CXCR2 | CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, CXCL8 |
| CXCR3 | CXCL4, CXCL4L1, CXCL9, CXCL10, CXCL11 |
| CXCR4 | CXCL12 |
| CXCR5 | CXCL13 |
| CXCR6 | CXCL16 |
| Unknown receptor | CXCL14, CXCL17 |
| CC subfamily | |
| CCR1 | CCL3, CCL4, CCL5, CCL7, CCL8 CCL13, CCL14, CCL15, CCL16, CCL23 |
| CCR2 | CCL2, CCL5, CCL7, CCL8, CCL13, CCL16 |
| CCR3 | CCL4, CCL5, CCL7, CCL11, CCL13, CCL15, CCL24, CCL26, CCL28 |
| CCR4 | CCL17, CCL22 |
| CCR5 | CCL3, CCL4, CCL5, CCL7, CCL14, CCL16 |
| CCR6 | CCL20 |
| CCR7 | CCL19, CCL21 |
| CCR8 | CCL1, CCL18 |
| CCR9 | CCL25 |
| CCR10 | CCL27, CCL28 |
| C subfamily | |
| XCR1 | XCL1, XCL2 |
| CX3C subfamily | |
| CX3CR1 | CX3CL1 |
A two-step model of receptor activation has been proposed. Initially, the chemokine ligand specifically recognizes and binds to the receptor. Next a conformational change in the chemokine allows the N terminus to make the necessary interactions with the receptor resulting in activation.28 Activation of the receptor causes an exchange of bound guanosine diphosphate (GDP) for guanosine-5′-triphosphate (GTP) in the α-subunit of the G proteins. This results in dissociation of the G proteins and activation of several effector molecules downstream, which stimulates a cascade of signaling events within the cytoplasm of the cell (Fig. 2).26 What follows is a diverse range of physiological processes, including leukocyte migration and trafficking, leukocyte degranulation, cell differentiation, angiogenesis, and myofibroblast recruitment.29–31
Figure 2.
Typical chemokine receptor activation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Chemokines in acute wound healing
Cutaneous wound healing is a dynamic interactive process involving soluble mediators, infiltrating leukocytes, extracellular matrix (ECM), and resident cells—keratinocytes, fibroblasts, endothelial cells, and nerve cells.32 The traditional model of wound healing involves four overlapping phases, namely hemostasis, inflammation, cellular proliferation, and remodeling.33 This complex biological process, where multiple parallel and interrelated pathways are activated and synchronized to induce wound repair, requires strict control.34,35 Chemokines and their effects on leukocyte subsets and resident cells are an integral part of this system.
Inflammatory phase
During the inflammatory phase leukocytes migrate to the area of tissue insult in a characteristic temporal pattern.32 The predominant cell type transitions from neutrophils to macrophages and finally to lymphocytes.36,37 Directional cues for leukocyte migration are provided by chemokines, whereas immune cell responses to particular chemokines are determined by their complement of chemokine receptors.38 In murine models, knockouts of chemokines and their receptors, including CXCR2 and CXCR3, result in delayed or incomplete wound healing. In the case of CXCR4 and its ligand CXCL12, knockout results in perinatal death indicating concurrent roles in development.38–41
Leukocyte extravasation from the blood into the tissues is a regulated multistep process involving a series of coordinated interactions between leukocytes and endothelial cells under the guidance of chemokines through soluble gradients and/or immobilized molecules on endothelial luminal surfaces.42,43
Neutrophil and monocyte recruitment into inflamed tissues is mainly directed by CXC and CC chemokine subfamilies, respectively.38 CXC family members, particularly CXCL1, five and eight are released after wounding and are further induced in response to hypoxia, proinflammatory cytokines (IL-1 and tumor necrosis factor-alpha [TNF-α]) and lipopolysaccharides.44 These neutrophil-attractant chemokines are spatially and temporally expressed, suggesting a sophisticated multistep event in neutrophil recruitment. Proinflammatory cytokines such as TNF-α, suppress CXCR2 expression on neutrophils, whereas CXCL8 simultaneously stimulates the expression of CXCR1 on the cell membrane.14 This leads to a second chemotactic response enabling neutrophils to migrate to the superficial wound bed. Furthermore, CXCL8–CXCR1interactions induce granule exocytosis and the respiratory burst, thereby facilitating neutrophil immunological function.38,45
Production of monocyte chemoattractant proteins is provoked by similar stimuli to CXC chemokines and involves the secretion of CCL2, CCL7, CCL8, and CCL13.38 CCL2 is predominant and knockout studies in mice have demonstrated that its absence results in deficient monocyte recruitment.41 Mouse models have also demonstrated that variable CX3CR1 and CCR2 chemokine receptor expression confers functional heterogeneity upon monocytes.46 Monocytes with high CX3CR1 expression combined with low levels of CCR1 and CCR2 have been termed resident monocytes, which preferentially locate to normal skin and act as forerunners to resident tissue macrophages. When this pattern of receptor expression is reversed, monocytes migrate to inflamed tissue and trigger an immune response.47
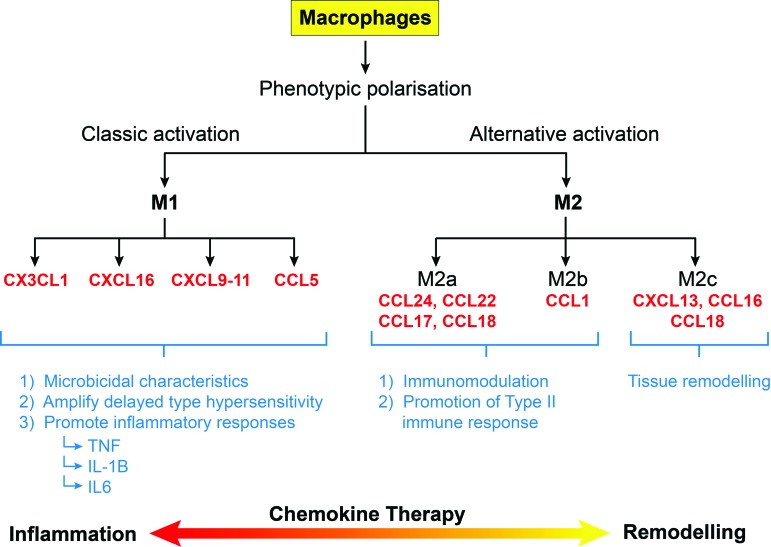
Macrophages are derived from monocyte differentiation and characterized by reduced CCR2 and increased CCR1 and CCR5 expression in humans.48,49 This change is likely to reflect stepwise migration into cutaneous tissues with CCL2–CCR2 interaction responsible for the initial recruitment and CCR1/CCR5 involvement to guide accurate tissue localization. Murine skin wound healing models have demonstrated that CCL2, CCL3, and CCL5 also play critical roles in macrophage recruitment.50–54 Macrophages are essential for normal wound repair providing a source of cytokines, growth factors, and chemokines.44,50,55 Once recruited, the wound microenvironment strongly influences phenotypic polarization of macrophages enabling functional heterogeneity through differential chemokine, cytokine, and receptor repertoires (Fig. 3).56 Macrophages of the M1 phenotype (classically activated macrophages) demonstrate powerful microbicidal characteristics, amplify delayed-type hypersensitivity reactions, and promote strong inflammatory responses through TNF-α, IL-1B, and IL-6 secretion. M1 polarization is accompanied by production of interferon (IFN)-γ responsive chemokines and members of the CC subfamily, including CX3CL1, CXCL9-11, CXCL16, and CCL5, which facilitate type I immune responses.47,57 In contrast, M2 macrophages (alternatively activated) promote tissue repair, resolution of inflammation, and immunoregulation through high endocytic clearance capacities and reduced proinflammatory cytokine secretion.47,57 Three M2 subtypes exist (M2a, M2b, and M2c) characterized by different chemokine profiles. M2a and M2b are predominantly associated with immunomodulation and promotion of type II immune responses through CCL17, CCL18, CCL22, CCL24, and CCL1 (specific to M2b) expression. M2c is more important in tissue remodeling involving CXCL13, CCL16, and CCL18.47 Consequently, manipulation of chemokine signaling to reduce inflammation and promote M2 macrophage polarization is an attractive therapeutic option to reduce fibrosis and scarring (Fig. 3).
Figure 3.
Phenotypic polarization of wound macrophages. Manipulation of chemokine signaling to promote M2 macrophage differentiation may be a potential target for reducing fibrosis and scarring. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Proliferative phase
Cellular proliferation represents the third phase of the classic wound healing model.58,59 Key physiological processes occurring predominantly during this phase include fibroplasia, angiogenesis, and granulation tissue formation. In the later stages, fibroblasts from wound edges or bone marrow (BM) assume the myofibroblast phenotype, which have contractile properties and contribute to collagen remodeling.35,60
Fibroplasia
Fibroplasia is the process resulting in the deposition of ECM proteins, including collagens, proteoglycans, and fibronectin by fibroblasts.61 It is dependent upon successful angiogenesis, upon which chemokines display key regulatory roles.
Knowledge of chemokine action upon, and secretion by, fibroblasts is poorly studied, but it is apparent that their expression is increased after injury contributing to conditioning of the cellular and cytokine environment within the healing tissue.62,63 Furthermore, resident fibroblasts are important in initiating inflammation secondary to chemokine-related recruitment of leukocytes through the secretion of CXCL8, CXCL12, CCL2, and CCL11.63,64 The extent of the inflammatory response is crucial in determining the outcome of fibroplasia, since fibrogenesis is influenced by cytokines, growth factors, and cellular mediators derived from the inflammatory phase. Prolonged ECM deposition results in fibrosis whereas insufficient production leaves wounds at the risk of dehiscence.65
Stoeckle and Barker demonstrated in a chorioamniotic membrane model that chicken chemotactic and angiogenic factor (cCAF) is highly expressed by fibroblasts in healing tissue and stimulated granulation tissue formation.66 It was subsequently found that exogenous cCAF administration stimulated fibroblast differentiation into myofibroblasts and accelerated wound closure in vivo.62 Given cCAF's structural similarity to CXCL8 a similar mechanism may exist in humans. Similarly, CXCR3 receptors expressed by fibroblasts and its ligands CXCL10 and CXCL11, may play a crucial role in wound healing. In vitro, CXCL10 has been observed to inhibit epidermal growth factor-related fibroblast motility secondary to the inhibition of calpain proteinases in fibroblasts, thus preventing fibroblast detachment.67 Furthermore, in mouse models CXCL10 administration limited fibrotic severity in bleomycin-induced pulmonary fibrosis whereas knockout of CXCR3 resulted in impaired wound healing with poor organization of the ECM, reduced collagen content, and altered biomechanical properties.68
Chemokines are also important in the control of absolute fibroblast number and consequently the extent of fibroplasia. As stated, CXCL8 may reduce the number of fibroblasts by stimulating the differentiation into myofibroblasts.62 In contrast, CCL21, a ligand for CCR7, is a potent stimulus for human fibrocyte chemotaxis in vitro and promotes migration to sites of injury in vivo when injected into cutaneous mouse wounds.69 Fibrocytes are precursors to fibroblasts and to a lesser extent myofibroblasts.33 Consequently, influx of these cells to the wound environment may increase the population of resident fibroblasts providing a larger pool of ECM secreting cells.
Angiogenesis
Reconstruction of wound microvasculature is vital during the proliferative phase of healing to restore nutrient supply to regenerating tissue, promote fibroplasias, and prevent sustained tissue hypoxia.33 The process by which this occurs is angiogenesis. Regulation depends on a dual, yet opposing balance of angiogenic and angiostatic factors that promote or inhibit neovascularization, respectively.70 During wound repair this balance is shifted in favor of proangiogenic factors.71 As wound healing reaches its conclusion there is a marked decline in angiogenesis. This suggests that during wound repair, angiogenesis is tightly controlled and temporally related to the imbalance of expression of angiogenic and angiostatic factors.70 A comprehensive review of molecular and cellular mechanisms driving angiogenesis has recently been published.33 It is well established that chemokines, particularly of the CXC subfamily, have a major role in the regulation of angiogenesis. The NH2 terminus of multiple CXC chemokines contains three amino acid residues, Glu-Leu-Arg, immediately before the first cysteine amino acid residue, termed the ELR motif.72 The CXC chemokines possessing the ELR motif (ELR+) promote angiogenesis, whereas those without the ELR motif (ELR-) inhibit it (Table 2).72,73 It has been demonstrated that CXCR2 is the primary functional chemokine receptor in mediating endothelial cell chemotaxis.74–76 When full-thickness excisional wounds were made on CXCR2−/− mice significant delays in wound healing resulted, including decreased neovascularization.39 The ELR chemokines exert their angiostatic effects by interaction with CXCR3.73 This receptor was originally identified on murine endothelial cells77 and exists in alternative splice forms, CXCR3A, CXCR3B, and CXCR3-alt.73 It is CXCR3B which mediates the angiostatic activity of most ELR chemokines on human microvascular endothelial cells.78 Another angiostatic mediator related to the CXC chemokines is ACKR1. This promiscuous receptor found on red blood cells sequesters the ELR+ CXC chemokines and indirectly inhibits angiogenesis.79,80
Table 2.
ELR+ and ELR chemokines
| ELR+ chemokines | ELR− chemokines |
|---|---|
| CXCL1 | CXCL4 |
| CXCL2 | CXCL4L1 |
| CXCL3 | CXCL9 |
| CXCL5 | CXCL10 |
| CXCL6 | CXCL11 |
| CXCL7 | CXCL14 |
| CXCL8 |
CC chemokines also have a role in angiogenesis. CCL2, acting on CCR2 present on endothelial cells, mediates neovascularization by effecting membrane type-1 matrix metalloproteinase (MT1-MMP).81 This chemokine also induces expression of vascular endothelial growth factor (VEGF)-A and the transcription factor, monocyte chemotactic protein 1 (MCP-1)-induced protein in vivo.82,83 Ishida et al.84 have demonstrated a significant role for the CCL5/CCR5 interaction during angiogenesis in wound healing. Endothelial progenitor cells (EPCs) have been presumed to be involved in a number of conditions requiring neovascularization, including wound healing, although to date, this has not been validated.85,86 EPCs are produced in the BM and home to specific injured tissue. In-vitro, CCL5 induces migration of EPCs by acting on CCR5 on their cell surface and CCR5-deficient mice exhibit impaired neovascularization during excisional wound healing.84 CCL11 and CCL16 also have a direct positive effect on angiogenesis.87
Remodeling phase
Remodeling begins 2–3 weeks after injury and can last for a year or more. Processes activated after injury slow down and stop as endothelial cells, macrophages, and myofibroblasts undergo apoptosis or exit the wound leaving a relatively acellular collagen-rich mass. This reduction in cellularity is influenced by interactions between CXC chemokines, particularly CXCL10 and CXCL11 with CXCR3, expressed by maturing endothelium and keratinocytes, respectively.40,88–91 These ligand–receptor interactions reduce fibroblast and endothelial motility while increasing keratinocyte migration.67,92,93 It is proposed that this signaling axis allows wounds to transition through remodeling resulting in the characteristic appearance of scar tissue with densely packed collagen bundles and reduced elastin.94,95 Wound contraction secondary to myofibroblast action begins in the proliferative phase, but continues during remodeling.
Re-epithelialization
Re-epithelialization involves keratinocyte migration and proliferation, followed by differentiation to regenerate the epidermis during wound closure. Migration begins within 3–6 h after injury and proceeds throughout wound healing until completed.96 It is well established that chemokines are crucial to the process of re-epithelialization. In an in vitro skin model CXCL1 and CXCL8 induced keratinocyte migration by their interactions with CXCR2.97 Several other ligand–receptor combinations appear to positively influence keratinocyte migration and proliferation in vitro.98,99 In vivo studies in CXCL11−/− and CXCR3−/− mice demonstrated a significant delay in re-epithelialization and deficient dermal maturation in excisional wounds.40,100
Chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3 are expressed on the surface of mouse keratinocytes and since the same keratinocytes are able to secrete their corresponding monospecific ligands a model of autocrine regulation of re-epithelialization has been postulated.101 Chemokines may also play a role during nonhematopoietic cell recruitment in wound healing. Bone-marrow derived stem cells (BMDSCs) possess the ability to self-renew and differentiate into multiple cell lines. They reside in adult BM and cutaneous injury leads to increased engraftment of these BMDSCs as epidermal cells.102 CCL27, and its interaction with CCR10, is the major regulator involved in this migration of keratinocyte precursor cells from the BM to the skin in excisional mice wounds.103 These BM cells are able to transdifferentiate into keratinocytes at the wound site and intradermal injection of CCL27 accelerated wound healing.103
CX3CL1/CX3CR1
CX3CL1 and its receptor CX3CR1, are the sole members of the CX3C group. It is discussed separately as it influences numerous stages of wound healing. CX3CL1 is expressed in a soluble chemokine domain form and as a membrane-bound form on the surface of inflamed endothelial cells, epithelial cells, macrophages, and vascular smooth muscle cells.104 Interaction of the membrane-bound ligand and its receptor mediates cell to cell adhesion of leukocytes.22,105,106 CX3CL1 is expressed in numerous organs, including the skin whereas the receptor is found on a variety of cells, including monocytes/macrophages.104 During the inflammatory stage it directly mediates macrophage accumulation and during the proliferative stage promotes granulation tissue formation, collagen deposition, myofibroblast accumulation, and angiogenesis.104 Finally, inoculation of wild-type mice with neutralizing anti-CX3CR1 antibodies had dramatic, detrimental, and overarching effects on wound healing.104
Contrasts with fetal wound healing
During fetal wound healing there are several key differences compared with the adult model. These are summarized in Table 3. Due to the underlying differences in cellular chemoattraction and inflammation between adult and fetal wound healing it is highly likely that chemokines are also differentially expressed. When human fetal and adult fibroblasts were cultured in vitro it was demonstrated that decreased levels of CXCL8 were expressed by fetal fibroblasts.107 This would certainly correlate with other literature suggesting a reduced role of inflammation and neutrophils during fetal wound healing and the absence of scar formation. It is clear that many growth factors and cytokines appear to vary in their expression in fetal and adult wounds. While each may be important, their overall significance may be obscured by the complexity of the cytokine milieu, including other unknown or unexamined factors acting in fetal and adult wound healing.108 It is highly likely that further, as yet undiscovered, chemokines play an important role in the scarless healing of fetal wound healing.
Table 3.
Comparison of adult and fetal wound healing
| Adult wound healing | Fetal wound healing | |||
|---|---|---|---|---|
| Collagen content | Type I +++ | Type III + | Type I + | Type III +++ |
| Hyaluronic acid | + | +++ | ||
| Extracellular matrix modulators | MMP + | TIMP +++ | MMP +++ | TIMP + |
| Adhesion proteins | ↓ upregulation | ↑ upregulation | ||
| Platelets | ↑ PDGF, TGF-β1 and TGF-β2 | ↓ degranulation and aggregation | ||
| Inflammatory cells | +++ | + | ||
| Interleukins | ↑ proinflammatory cytokines | ↑ anti-inflammatory cytokines | ||
| Transforming Growth Factor-β | TGF-β1 & β2+++ | TGF-β1 & β2 + TGF-β3 +++ |
||
| Gene expression | ↑ genes involved in cell growth & proliferation | ↑↑↑ genes involved in cell growth & proliferation | ||
| Progenitor cells | + | +++ | ||
| CXCL8 | +++ | + | ||
MMP, matrix metalloproteinases; TIMP, tissue inhibitor of metalloproteinase; PDGF, platelet-derived growth factor; TGF, transforming group factor; ↑, increase; ↓, decrease.
Chemokines in chronic wounds
A chronic wound is defined as a break in skin continuity of greater than 42 days duration or of frequent recurrence.109,110 These debilitating wounds have failed to progress through the normal stages of healing and, therefore, enter a state of pathological inflammation.111 This leads to delayed or incomplete healing with poor anatomical and functional outcomes as well as detrimental effects on patient quality of life.112,113 Multiple etiologies underlie chronic wound development, but over 90% are secondary to diabetes, venous insufficiency or pressure.114 Increasingly prevalent risk factors, including obesity and diabetes, in combination with an aging population, suggests chronic wounds will impose a progressively larger economic burden upon healthcare providers in the future.114
The normal function of the inflammatory phase is to prepare the wound bed for healing by removing debris, necrotic tissue, and bacterial contaminates, as well as recruiting fibroblasts.111 It is an essential process, but must be tightly controlled. Neutrophils play a vital role and during normal wound healing their population declines following successful transition into the proliferation stage. In chronic wounds, activated neutrophils persist throughout the healing process,115 leading to an excess of activated neutrophils, which cause an accumulation of MMPs.116 This, combined with downregulation of tissue inhibitor of metalloproteinase (TIMP) expression, creates an environment with a relative excess of MMP activity leading to the degradation of crucial wound components and further neutrophil recruitment generating a positive feedback loop and chronic inflammation.111,117
The use of MMP-deficient animals has demonstrated that MMPs can affect cytokines and chemokines as well as ECM proteins, establishing another method by which inflammatory processes can be influenced.118 More specifically MMPs have been shown to inactivate specific chemokines, generate antagonistic derivatives, and produce truncated chemokine variations that are more potent.118 Examples of chemokine inactivation by MMPs include CXCL12, which has been shown to be inactivated by MMP-1–3, MMP-9, MMP-13, and MMP-14.119 MMP-9 also inactivates CXCL chemokines, including CXCL1 and CXCL4.120 Finally, CXCL9 and CXCL10 are degraded by MMP-8 and MMP-9,121 which in the case of CXCL9, decreases chemotactic ability. A number of inactivated chemokines act as functional antagonists through binding to their original receptors, thereby blocking noncleaved chemokines or affecting chemotactic gradients. For example, MMP-2 has been shown to process CCL7 into an antagonistic form.122 Finally, MMPs have been shown to increase the biological activity of certain chemokines. MMP-9 has been demonstrated to influence CXCL8, resulting in significantly increased chemotactic activity.120 A truncated CXCL8 variant with increased activity has also been generated by MMP-8, MMP-13, and MMP-14.123,124 In summary, the variable actions of MMPs on chemokines affects the progression of inflammatory responses and influences the type of cells, which are recruited and activated.118
Much of our understanding of acute wound healing comes from in vivo animal models. Chronic wounds on the other hand are challenging to simulate as they do not occur naturally in the animal world.117 Despite this, a number of models have been developed although their findings are sometimes contradictory and cannot be directly translated to humans.
A murine model involving animals deficient in TNFSF14 (LIGHT) has been developed.125 LIGHT mediates VEGF, a growth factor that induces macrophage apoptosis in vitro.126 Since macrophage apoptosis is important in the resolution of inflammation during wound healing, the authors propose that LIGHT-deficient wounds mimic chronic healing/nonhealing wounds in humans.125 This work also implicates chemokines in chronic wound pathogenesis. Excess proinflammatory chemokine production (CXCL8, CCL2, and CXCL10) persisted from the early stages following injury, resulting in excess leukocyte infiltrates. Excessive neutrophil infiltration is associated with unregulated MMP production and high levels of reactive oxygen species leading to increased inflammation,127,128 whereas an increased duration of macrophage presence may lead to further protease-mediated damage of the healing tissue.129 Crucially, premature and excessive CXCL10, resulted in early chemoattraction of T-lymphocytes,125 which are usually associated with the remodeling phase of wound healing.13 This suggests a disorganized healing process. In contrast Pradhan et al., demonstrated significantly increased baseline expression of CXCL8 and its receptors CXCR1 and CXCR2 in diabetic rabbits compared with nondiabetic animals. However, after wounding there was almost unchanged expression of these chemokines.130 Consequently, the acute inflammatory response was significantly blunted with adverse effects on wound healing rates. Correction of this deficiency may represent a novel therapeutic approach. Indeed, the application of talactoferrin to wounded diabetic and nondiabetic mice modulated the early inflammatory response with evidence of increased CXCL8 expression associated with improved wound closure.131 Furthermore, improved healing has been demonstrated in diabetic ulcers with the application of a talactoferrin gel in humans.132
A consistent finding in pressure ulcers, the diabetic foot and venous stasis ulcers is an accumulation of senescent fibroblasts.133–135 These fibroblasts demonstrate decreased proliferative rates and a dysfunctional chemokine secretory profile involving excess CCL2 release and reduced CXCL8 production compared with wounds healing normally.136,137 Furthermore, high levels of functional CCL2, CCL5, CCL18, CCL20, CCL27, CXCL1, and CXCL12 have been reported in chronic wound debridement tissue. This extract was able to stimulate migration of healthy dermal fibroblasts and bioactive molecule secretion from cellular skin substitutes suggesting that senescent cells also show aberrant responses to chemokines.101 Interestingly, there was little variation in chemokine concentration between donors in this study, despite differing wound etiologies, size, and duration.101
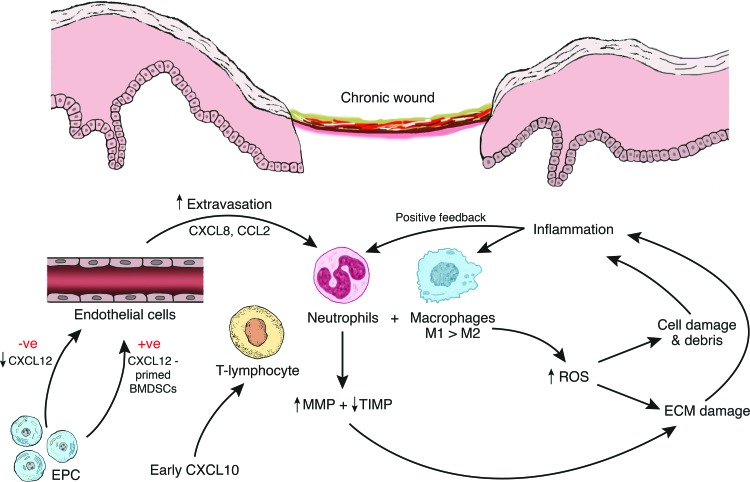
Finally, it is not just inflammatory processes that are deranged in chronic wounds. Altered angiogenesis is well recognized as a contributor to delayed healing in diabetic and venous ulcers.138,139 CXCL12, which exclusively binds to CXCR4, plays a crucial role in EPC migration140 and dysfunction in its signaling pathways has been implicated in aged and diabetic wound healing in preclinical models.141 A relative lack of CXCL12, as found in diabetic wounds, lead to decreased cellular migration and angiogenesis as well as increased inflammation.142 During diabetic wound healing, administration of CXCL12 directly to the wound reversed EPC recruitment impairment in mice.143 This beneficial effect has been replicated by lentiviral-mediated gene transfer of CXCL12 in diabetic mice wounds resulting in improved early healing.144 This hypothesis has been further developed using a novel cell-based therapy where ex-vivo BMDSCs primed with CXCL12 were injected subcutaneously into full-thickness diabetic mice wounds.145 These CXCL12-primed BMDSCs significantly promoted wound healing, neovascularization, and EPC recruitment.145 These and other cited literature, suggest a therapeutic role for CXCL12 and other chemokines in chronic wound management.146 This is summarized in Fig. 4.
Figure 4.
Chemokines in chronic wounds. Unbalanced MMP/TIMP expression leads to increased tissue degradation and inflammation. This results in further activated neutrophils/macrophages. A positive feedback loop is created leading to a chronic inflammatory milieu. Influencing chemokine expression may lead to therapeutic options. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Chemokines and pathological scarring
Hypertrophic scars are red, raised, uncomfortable scars that are confined to the boundaries of the original wound.11 Keloid scars are benign collagenous tumors that form in the reticular layer of the dermis during a prolonged wound healing process in persons with a genetic predisposition.147,148 They spread beyond the margins of the original wound and are associated with increased collagen deposition and fibroproliferation.149–151 Both are associated with significant morbidity.
Keloid scars are characterized by overproliferation of fibroblasts, leukocyte infiltration, and prolonged excessive collagen synthesis.152,153 Due to their fundamental function of chemoattraction, it has been hypothesized that chemokines are involved in this process.154 It has been shown that CXCL1 and its receptor CXCR2 are more abundant in keloid fibroblasts and, therefore, postulated that the inflammatory component of scarring is crucial to the development of keloid scars.154 Fibroblasts from keloid scars have been shown to have increased levels of CXCL8 compared with normal human fibroblasts.10 This highlights a possible role for CXCL8 in leukocyte recruitment and activation in keloid scars.
It is hypothesized that hypertrophic scarring results from the overproduction of ECM during fibroplasia, secondary to abnormalities in epidermal–dermal communication within which CXCR3 plays a critical role.95 To investigate the effect of a lack of CXCR3 receptors, wound healing and scarring was compared between CXCR3−/− and wild-type mice with full-thickness excisional wounds.95 In the absence of CXCR3, wounds remained immature with an inflammatory milieu and went on to develop a hypertrophic scar phenotype, similar to those observed in humans.95 Taking this idea to its logical conclusion, the authors postulated that transplantation of normal fibroblasts to a CXCR3-/- wound may lead to the correction of fibroblast-generated matrix dysfunction, resulting in improved healing. Not only did these normal fibroblasts survive within the wound milieu, they had a positive effect on healing, including matrix remodeling, improved collagen alignment, and increased tensile strength.155 This raises the possibility of using transplanted fibroblasts as cellular therapies to stimulate more functional and regenerative healing responses.155
Hypertrophic scars are hypercellular due to increased numbers of fibroblasts and recruitment of peripheral nonhematopoietic cells. Once again, the recruitment of cells hints at the possible role of chemokines during hypertrophic scar development. It has been previously demonstrated that CXCL12 can be beneficial in chronic wounds, where there is a lack of recruitment of effector cells. Therefore, it is logical to assume that it can be disadvantageous in situations where excessive cellular recruitment is a problem. Within biopsies of hypertrophic scars from human burn patients, increased CXCL12/CXCR4 signaling was demonstrated compared with normal skin.11 This increased signaling was downregulated following administration of IFN α 2B and coincided with remodeling of hypertrophic scars.11 Therefore, it is argued that CXCL12/CXCR4 interactions are involved in promoting recruitment of cells that contribute to the development of these abnormal scars. However, somewhat contradictory work into the role of CXCL12 in wound healing and scarring has been published.156 Following the evidence that CXCL12 was beneficial in chronic, hard-to-heal wounds, they studied the effect of continuously delivered CXCL12 mounted on an alginate dressing on wound healing and scar appearance in full-thickness incisional porcine wounds. They were able to show that treated pig wounds healed faster and with less fibrosis than control wounds.156 Interestingly, histological assessment did not reveal increased vascular density in CXCL12-treated wounds156 suggesting a mechanism other than EPC homing for the beneficial effects.
Chemokines as therapeutic targets
The excessive infiltration of leukocytes is a hallmark of many autoimmune and chronic inflammatory diseases. Several approaches have been postulated to prevent cellular recruitment to inflamed tissues, including the modulation of chemokines and their receptors.9 However, despite great interest, effective treatments that target the chemokine family have remained elusive.
Potential methods of influencing the chemokine system
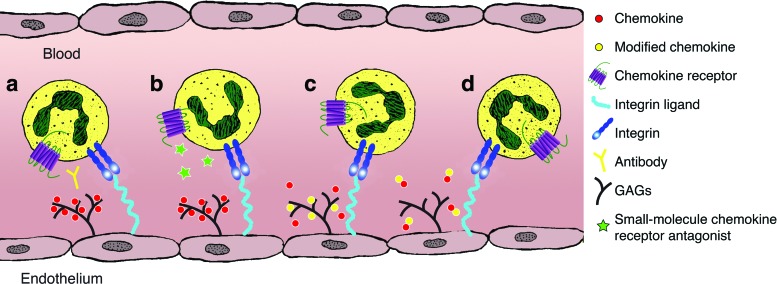
Potential strategies for influencing the chemokine system are highlighted in Fig. 5. In recent years the pharmaceutical industry has made many developments in the use of therapeutic monoclonal antibodies (mAbs) to inhibit specific aspects of immune cell function, although their use to intervene in the chemokine system is a relatively new strategy.25 These therapeutic antibodies can act either directly or indirectly on their targets. They can bind to and block their target protein, that is, direct targeted therapy, or they can influence critical biological processes that is, antibody-dependent cell-mediated cytotoxicity or complement-dependent toxicity.157 Certain characteristics of chemokine receptors, including their limited availability as purified proteins and low immunogenicity, have hampered the development of novel therapeutic agents.157 However, following increased interest in this area, neutralizing mAbs have been used to inhibit leukocyte recruitment in a number of disease processes in animal models and have been incorporated into human trials. A mAb to CXCL8 (ABX-IL8, Abgenix) has been shown to inhibit neutrophil and monocyte infiltration into the lungs of patients suffering from COPD, reducing the severity of dyspnea, but having no influence on lung function or health status.158 The same antibody has been tested in the context of cutaneous disease that is, psoriasis, see below.
Figure 5.
Diagrammatic representation of therapeutic strategies to influence the chemokine system (a) mAbs act either directly or indirectly to prevent ligand/receptor binding (b) small-molecule antagonists bind to an allosteric site, preventing chemokines binding to the main orthosteric site (c) modified, nonfunctioning chemokines prevent native chemokines from binding to GAGs (d) Truncated chemokines can bind to chemokine receptors acting as antagonists. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Unlike adhesion molecules and cytokine receptors, GPCRs can be blocked by small-molecule antagonists, and much work has been focused on this area of the chemokine system. Certain chemokine receptors, including CXCR3 and CCR5, have the ability to bind several ligands at nonoverlapping binding sites.159,160 Due to a discrepancy in size, small molecules are unable to compete with the larger chemokine ligands at an orthosteric site. However, they can successfully antagonize chemokine activity by interaction at an allosteric binding site causing modulation of the main chemokine orthosteric site.161 This induced change also overcomes the problem of ligand promiscuity as these inhibitors prevent all ligands from binding to a particular receptor.161 Following discovery of the chemical profile of chemokines, the identification of small molecules to antagonize their receptors has gathered momentum.162
Chemokines binding to glycosaminoglycans (GAGs) is vital for normal chemokine function.163 They help to establish a chemotactic gradient, protect chemokines from proteolytic cleavage, and have a role in oligomerization.164–167 It has been postulated that interfering with GAG-chemokine interactions could be a potential therapeutic target, however, inherent complexities of this system has prevented significant progress.25
While not affecting their ability to bind to their receptor(s), the truncation of the N terminus eliminates the signaling potential of many chemokines.25 It has been demonstrated that re-engineered chemokines can be biologically active as chemokine agonists,168 while this approach has also produced potent antagonists also.169
Obstacles to developing chemokine-based therapies
Most of the chemokine receptor targets identified for the treatment of human disease processes have been validated in animal models.170 The majority of compounds developed targeting these receptors have shown good efficacy in animal and in vitro models, but have ultimately failed to translate into clinically effective therapeutics. There are a number of reasons to explain this frustration. There are clear similarities between the chemokine systems of man and mice and work in murine models has contributed significantly to our understanding of chemokine biology. However, due to speciation and differing evolutionary pressures, the gene organization of human and mice chemokine clusters is very different.171 Clearly, not all observations made in murine models can be extrapolated to application in man and hence, many identified targets have failed to progress to fruition.
Many of the potential targets of therapeutic intervention in the chemokine system are based on the classical paradigm of a single ligand interacting with a single receptor leading to a consistent and predictable cellular response. Although they may function as monomers, it is now well established that many chemokine receptors also act as dimers or higher order oligomers.172 In fact, all tested chemokine receptors form oligomers in a ligand-independent manner,173–177 and heterodimers can form even between CC and CXC subclasses.172 Another relevant phenomenon is that of crosstalk between different receptors. This refers to the ability of a particular receptor to influence the signaling and function of another receptor.172 The principles of oligomerization and crosstalk challenges the logic of inhibiting one ligand/receptor to bring about a desired therapeutic effect.
Success stories in chemokine-targeted therapy
Undoubtedly, the most developed field of chemokine therapeutics is in the treatment of the human immunodeficiency virus (HIV). Two chemokine receptors have been implicated in viral entry into host cells, namely CXCR4 and CCR5,24 affecting T-cells and macrophages, respectively.178 A naturally occurring mutation of CCR5, CCR5-Δ32, is uncommon in HIV-1-infected patients and individuals homozygous for the mutation are rarely affected by HIV-1.179 This observation led to the development of numerous small-molecule antagonists of CCR5. The first to be approved by the European Medicines Agency has been Maraviroc, developed by Pfizer, which is used in treatment-experienced patients infected with CCR5-tropic viruses only.157 Further antagonists continue to be developed.180 When HIV translates into the more advanced AIDS, CXCR4 becomes the more relevant receptor.181 Although potent inhibitors of CXCR4 exist, their clinical utility is limited by severe side effects. Indeed, since CXCR4 or CXCL12 knockout mice are not viable, significant doubt remains over whether this pathway can be safely manipulated in the long term.157
The use of chemokine therapeutics in inflammatory disease processes is far less developed. The receptor CCR9 is expressed on a subset of circulating lymphocytes and is recognized as being responsible for mediating homing of these cells to the intestinal mucosa.182 Its sole ligand CCL25 is highly expressed within the intestine.183 CCX282-B (Vercirnon) is a small-molecule antagonist to CCR9, which inhibits CCR9- and CCL25-dependent chemotaxis. In a recent randomized control trial it was demonstrated to be safe, efficacious, and well tolerated by patients suffering from Crohn's disease through the oral route although it failed to reach its primary endpoints.184 This work has demonstrated that therapies targeting the chemokine system in inflammatory disease processes is a realistic goal.
Existing nonchemokine therapies for cutaneous scarring
Traditional approaches toward scar reduction are well established. They involve pressure/silicon dressings, onion extracts, vitamin E-based remedies, and corticosteroids. However, the clinical efficacy and evidence base for these strategies is limited.185 As detailed above, there are significant differences in the healing profiles of adult and fetal wounds. Recent therapies have attempted to reproduce fetal healing that is, regeneration, in adults. In particular, modulating the ratios of the subsets of transforming group factor beta (TGF-β) in the wound microenvironment has received much attention. Recombinant human TGF-β3, Avotermin, has been demonstrated to be safe and numerous double-blind, placebo-controlled prospective trials has demonstrated statistically significant improvement in scar appearance at a range of doses and dose frequencies.186
The key to any modulation of scar formation is the coordination of numerous cellular and molecular processes. Direct cytoplasmic coupling between cells mediated by gap junctions provides a direct line for the spread of biological information within tissues.187 This is now recognized as an important aspect of cutaneous injury response, and connexins appear to have an influence on scar formation.188 Several connexins appear to be expressed in the skin, including Cx43, found in the epidermis and dermis and the potential for targeting this connexin has been extensively researched.189–191 Numerous antisense-based therapies targeting Cx43 are in development.188
Summary
It is clearly apparent that chemokines are intrinsically involved in the process of cutaneous wound healing and scar formation. The way in which they mediate chemoattraction and cellular responses to injury are vital to a satisfactory outcome following wounding. It has been shown that they can have positive and negative impacts on this complex physiological process, and novel therapies that influence either the expression or suppression of these cytokines will likely play a significant role in the treatment of problem scars in the future. However, to date numerous works in the literature report contradicting observations of the functions of chemokines. This suggests that the exact biological pathways by which chemokines assert their influence are yet to be fully realized. This must be addressed before clinically effective, readily available therapies can be a realistic goal.
Research into other applications of chemokines in health may lead to benefit in the setting of wound healing. Work into bioengineering has demonstrated potential gains producing synthetic chemokines that are more effective than their native counterparts.168
Take-Home Messages.
• Chemokines and their receptors have been implicated in all aspects of wound healing and scarring, both normal and pathological. Despite our ever-expanding knowledge in this large family of chemotactic cytokines, their exact nature and influences remain elusive.
• Current novel therapeutics for the treatment of cutaneous scarring attempt to revert adult wound healing to the regenerative phenotype of the fetus. There is currently a lack of knowledge with regard to the role of chemokines in fetal healing. Advances in this area may lead to clinically effective treatments in the future.
• Future therapies will need to address the issues of chemokine/receptor oligomerization and cross talk. It is likely that they will need to influence multiple ligand/receptor interactions simultaneously or sequentially.
Something which has become clear from work in other areas of wound healing therapy for example, growth factors, is that targeting a single element of a complex process is unlikely to yield clinically significant results outside of experimental conditions. It is likely that to effectively interfere with the aberrant physiology leading to problematic scars, multiple chemokines and/or receptors will need to be targeted either simultaneously or sequentially.18 Despite these obstacles, chemokines will almost certainly play a significant role in wound healing therapies of the future.
Abbreviations and Acronyms
- ACKR1
atypical chemokine receptor 1
- AIDS
acquired immunodeficiency syndrome
- BM
bone marrow
- BMDSCs
bone marrow-derived stem cells
- cCAF
chicken chemotactic and angiogenic factor
- Cx43
connexin 43
- DARC
duffy antigen receptor for chemokines
- ECM
extracellular matrix
- EPCs
endothelial progenitor cells
- GAGs
glycosaminoglycans
- GDP
guanosine diphosphate
- GPCRs
G-protein-coupled receptors
- GTP
guanosine-5′-triphosphate
- HIV
human immunodeficiency virus
- IFN-γ
interferon gamma
- IL
interleukin
- mAbs
monoclonal antibodies
- MCP-1
monocyte chemotactic protein 1
- MMPs
matrix metalloproteinases
- MT1-MMP
membrane type 1-matrix metalloproteinase
- PDGF
platelet-derived growth factor
- TGF-β
transforming group factor-beta
- TIMP
tissue inhibitor of metalloproteinase
- TNFSF14
tumor necrosis factor ligand superfamily member 14
- TNF-α
tumor necrosis factor-alpha
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
No funding was required for this review article.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Ardeshir Bayat is a clinician as well as a scientist. He is currently a principal investigator at the University of Manchester, England, UK. The focus of his research has been in wound repair, fibrosis, and tissue regeneration. His laboratory has an independent capacity in carrying out molecular, cellular, histological, genetic, and protein research in tissue biology utilizing state-of-the-art equipment plus access to an in vitro organotypic and organ culture models of skin developed in his group. His lab is based at the Manchester Institute of Biotechnology (MIB) at the University of Manchester. His mission is to develop research programs that combine high-quality basic research with strong clinical interactions to make a major impact on human health through translation into better outcomes for patients suffering from chronic wound healing, abnormal cutaneous scarring, and fibrosis. His research thrives upon the multidisciplinary and interactive research environment that currently exists, which allows translation of basic science research to major therapeutic developments that will have direct impact and specific utilization in clinical practice. Dr. Peter Adam Rees is a surgical research fellow at the University Hospital South Manchester in Manchester, UK. Dr. Nicholas Stuart Greaves is a surgical research fellow at the University Hospital South Manchester and the University of Manchester in Manchester, UK. Dr. Mohamed Baguneid is a consultant vascular surgeon at the University Hospital South Manchester in Manchester, UK.
References
- 1.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995;108 (Pt 3):985–1002 [DOI] [PubMed] [Google Scholar]
- 2.Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol 1979;381:353–361 [DOI] [PubMed] [Google Scholar]
- 3.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg 2010;126:1172–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson PJ, Krensky AM. Chemokines, chemokine receptors, and allograft rejection. Immunity 2001;14:377–386 [DOI] [PubMed] [Google Scholar]
- 5.Keane MP, Strieter RM. The role of CXC chemokines in the regulation of angiogenesis. Chem Immunol 1999;72:86–101 [DOI] [PubMed] [Google Scholar]
- 6.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol 2000;12:336–341 [DOI] [PubMed] [Google Scholar]
- 7.Segerer S, Nelson PJ, Schlondorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol 2000;11:152–176 [DOI] [PubMed] [Google Scholar]
- 8.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol 2000;18:217–242 [DOI] [PubMed] [Google Scholar]
- 9.Godessart N. Chemokine receptors: attractive targets for drug discovery. Ann N Y Acad Sci 2005;1051:647–657 [DOI] [PubMed] [Google Scholar]
- 10.Lim CP, Phan TT, Lim IJ, Cao X. Cytokine profiling and Stat3 phosphorylation in epithelial-mesenchymal interactions between keloid keratinocytes and fibroblasts. J Invest Dermatol 2009;129:851–861 [DOI] [PubMed] [Google Scholar]
- 11.Ding J, Hori K, Zhang R, et al. Stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 in the formation of postburn hypertrophic scar (HTS). Wound Repair Regen 2011;19:568–578 [DOI] [PubMed] [Google Scholar]
- 12.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med 2008;14:222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood 2000;95:3032–3043 [PubMed] [Google Scholar]
- 15.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest 1989;84:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy PM, Baggiolini M, Charo IF, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 2000;52:145–176 [PubMed] [Google Scholar]
- 17.Bacon K, Baggiolini M, Broxmeyer H, et al. Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res 2002;22:1067–1068 [DOI] [PubMed] [Google Scholar]
- 18.Bachelerie F, Ben-Baruch A, Burkhardt AM, et al. International Union of Pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev 2014;66:1–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devalaraja MN, Richmond A. Multiple chemotactic factors: fine control or redundancy? Trends Pharmacol Sci 1999;20:151–156 [DOI] [PubMed] [Google Scholar]
- 20.Martins-Green M, Petreaca M, Wang L. Chemokines and their receptors are key players in the orchestra that regulates wound healing. Adv Wound Care (New Rochelle) 2013;2:327–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baggiolini M. Chemokines and leukocyte traffic. Nature 1998;392:565–568 [DOI] [PubMed] [Google Scholar]
- 22.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997;385:640–644 [DOI] [PubMed] [Google Scholar]
- 23.Kelner GS, Kennedy J, Bacon KB, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science 1994;266:1395–1399 [DOI] [PubMed] [Google Scholar]
- 24.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol 2007;25:787–820 [DOI] [PubMed] [Google Scholar]
- 25.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol 2008;48:171–197 [DOI] [PubMed] [Google Scholar]
- 26.Mellado M, Rodriguez-Frade JM, Manes S, Martinez AC. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol 2001;19:397–421 [DOI] [PubMed] [Google Scholar]
- 27.Furie MB, Randolph GJ. Chemokines and tissue injury. Am J Pathol 1995;146:1287–1301 [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol 2002;42:469–499 [DOI] [PubMed] [Google Scholar]
- 29.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol 2001;2:102–107 [DOI] [PubMed] [Google Scholar]
- 30.Mackay CR. Chemokines: immunology's high impact factors. Nat Immunol 2001;2:95–101 [DOI] [PubMed] [Google Scholar]
- 31.Szekanecz Z, Koch AE. Chemokines and angiogenesis. Curr Opin Rheumatol 2001;13:202–208 [DOI] [PubMed] [Google Scholar]
- 32.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 33.Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci 2013;72:206–217 [DOI] [PubMed] [Google Scholar]
- 34.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514–525 [DOI] [PubMed] [Google Scholar]
- 35.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 36.Engelhardt E, Toksoy A, Goebeler M, et al. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 1998;153:1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol 2001;69:513–521 [PubMed] [Google Scholar]
- 38.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 2006;354:610–621 [DOI] [PubMed] [Google Scholar]
- 39.Devalaraja RM, Nanney LB, Du J, et al. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol 2000;115:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yates CC, Whaley D, Hooda S, et al. Delayed reepithelialization and basement membrane regeneration after wounding in mice lacking CXCR3. Wound Repair Regen 2009;17:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters W, Dupuis M, Charo IF. A mechanism for the impaired IFN-gamma production in C-C chemokine receptor 2 (CCR2) knockout mice: role of CCR2 in linking the innate and adaptive immune responses. J Immunol 2000;165:7072–7077 [DOI] [PubMed] [Google Scholar]
- 42.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med 1998;338:436–445 [DOI] [PubMed] [Google Scholar]
- 43.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood 2002;100:3853–3860 [DOI] [PubMed] [Google Scholar]
- 44.Zaja-Milatovic S, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol 2008;23:1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones SA, Wolf M, Qin S, Mackay CR, Baggiolini M. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci U S A 1996;93:6682–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science 2010;327:656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004;25:677–686 [DOI] [PubMed] [Google Scholar]
- 48.Fantuzzi L, Borghi P, Ciolli V, et al. Loss of CCR2 expression and functional response to monocyte chemotactic protein (MCP-1) during the differentiation of human monocytes: role of secreted MCP-1 in the regulation of the chemotactic response. Blood 1999;94:875–883 [PubMed] [Google Scholar]
- 49.Kaufmann A, Salentin R, Gemsa D, Sprenger H. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1 alpha during differentiation of human monocytes to macrophages. J Leukoc Biol 2001;69:248–252 [PubMed] [Google Scholar]
- 50.DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock 1995;4:233–240 [PubMed] [Google Scholar]
- 51.DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest 1998;101:1693–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank S, Stallmeyer B, Kampfer H, Kolb N, Pfeilschifter J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J Clin Invest 2000;106:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackman SH, Yoak MB, Keerthy S, Beaver BL. Differential expression of chemokines in a mouse model of wound healing. Ann Clin Lab Sci 2000;30:201–207 [PubMed] [Google Scholar]
- 54.Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol 2000;115:245–253 [DOI] [PubMed] [Google Scholar]
- 55.Riches DWH. The Molecular and Cellular Biology of Wound Repair. 2nd ed. London: Springer, 1996. [Google Scholar]
- 56.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 2008;13:453–461 [DOI] [PubMed] [Google Scholar]
- 57.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity 2005;23:344–346 [DOI] [PubMed] [Google Scholar]
- 58.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 2007;25:9–18 [DOI] [PubMed] [Google Scholar]
- 59.Telgenhoff D, Shroot B. Cellular senescence mechanisms in chronic wound healing. Cell Death Differ 2005;12:695–698 [DOI] [PubMed] [Google Scholar]
- 60.Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J 2005;19:1561–1563 [DOI] [PubMed] [Google Scholar]
- 61.Clark RA. Fibrin and wound healing. Ann N Y Acad Sci 2001;936:355–367 [DOI] [PubMed] [Google Scholar]
- 62.Feugate JE, Wong L, Li QJ, Martins-Green M. The CXC chemokine cCAF stimulates precocious deposition of ECM molecules by wound fibroblasts, accelerating development of granulation tissue. BMC Cell Biol 2002;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol 1997;151:317–322 [PMC free article] [PubMed] [Google Scholar]
- 64.Brouty-Boye D, Pottin-Clemenceau C, Doucet C, Jasmin C, Azzarone B. Chemokines and CD40 expression in human fibroblasts. Eur J Immunol 2000;30:914–919 [DOI] [PubMed] [Google Scholar]
- 65.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–289 [DOI] [PubMed] [Google Scholar]
- 66.Stoeckle MY, Barker KA. Two burgeoning families of platelet factor 4-related proteins: mediators of the inflammatory response. New Biol 1990;2:313–323 [PubMed] [Google Scholar]
- 67.Shiraha H, Glading A, Chou J, Jia Z, Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol 2002;22:2716–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davidson JM. Can scarring be turned off? Am J Pathol 2010;176:1588–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–7562 [DOI] [PubMed] [Google Scholar]
- 70.Belperio JA, Keane MP, Arenberg DA, et al. CXC chemokines in angiogenesis. J Leukoc Biol 2000;68:1–8 [PubMed] [Google Scholar]
- 71.Leibovich SJ, Wiseman DM. Macrophages, wound repair and angiogenesis. Prog Clin Biol Res 1988;266:131–145 [PubMed] [Google Scholar]
- 72.Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 1995;270:27348–27357 [DOI] [PubMed] [Google Scholar]
- 73.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev 2005;16:593–609 [DOI] [PubMed] [Google Scholar]
- 74.Addison CL, Daniel TO, Burdick MD, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol 2000;165:5269–5277 [DOI] [PubMed] [Google Scholar]
- 75.Murdoch C, Monk PN, Finn A. Cxc chemokine receptor expression on human endothelial cells. Cytokine 1999;11:704–712 [DOI] [PubMed] [Google Scholar]
- 76.Heidemann J, Ogawa H, Dwinell MB, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem 2003;278:8508–8515 [DOI] [PubMed] [Google Scholar]
- 77.Soto H, Wang W, Strieter RM, et al. The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc Natl Acad Sci U S A 1998;95:8205–8210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lasagni L, Francalanci M, Annunziato F, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 2003;197:1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Du J, Luan J, Liu H, et al. Potential role for Duffy antigen chemokine-binding protein in angiogenesis and maintenance of homeostasis in response to stress. J Leukoc Biol 2002;71:141–153 [PMC free article] [PubMed] [Google Scholar]
- 80.Horton LW, Yu Y, Zaja-Milatovic S, Strieter RM, Richmond A. Opposing roles of murine duffy antigen receptor for chemokine and murine CXC chemokine receptor-2 receptors in murine melanoma tumor growth. Cancer Res 2007;67:9791–9799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res 2011;317:575–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 2005;105:1405–1407 [DOI] [PubMed] [Google Scholar]
- 83.Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J Biol Chem 2008;283:14542–14551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ishida Y, Kimura A, Kuninaka Y, et al. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest 2012;122:711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999;85:221–228 [DOI] [PubMed] [Google Scholar]
- 86.Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal 2008;10:1869–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol 2008;28:1928–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Satish L, Yager D, Wells A. Glu-Leu-Arg-negative CXC chemokine interferon gamma inducible protein-9 as a mediator of epidermal-dermal communication during wound repair. J Invest Dermatol 2003;120:1110–1117 [DOI] [PubMed] [Google Scholar]
- 89.Tensen CP, Flier J, Van Der Raaij-Helmer EM, et al. Human IP-9: A keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3). J Invest Dermatol 1999;112:716–722 [DOI] [PubMed] [Google Scholar]
- 90.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med 1995;182:219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yates CC, Whaley D, Kulasekeran P, et al. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol 2007;171:484–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Satish L, Blair HC, Glading A, Wells A. Interferon-inducible protein 9 (CXCL11)-induced cell motility in keratinocytes requires calcium flux-dependent activation of mu-calpain. Mol Cell Biol 2005;25:1922–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res 2006;98:617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yates CC, Bodnar R, Wells A. Matrix control of scarring. Cell Mol Life Sci 2011;68:1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yates CC, Krishna P, Whaley D, et al. Lack of CXC chemokine receptor 3 signaling leads to hypertrophic and hypercellular scarring. Am J Pathol 2010;176:1743–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin P. Wound healing—aiming for perfect skin regeneration. Science 1997;276:75–81 [DOI] [PubMed] [Google Scholar]
- 97.Steude J, Kulke R, Christophers E. Interleukin-1-stimulated secretion of interleukin-8 and growth-related oncogene-alpha demonstrates greatly enhanced keratinocyte growth in human raft cultured epidermis. J Invest Dermatol 2002;119:1254–1260 [DOI] [PubMed] [Google Scholar]
- 98.Florin L, Maas-Szabowski N, Werner S, Szabowski A, Angel P. Increased keratinocyte proliferation by JUN-dependent expression of PTN and SDF-1 in fibroblasts. J Cell Sci 2005;118(Pt 9):1981–1989 [DOI] [PubMed] [Google Scholar]
- 99.Fujimoto S, Uratsuji H, Saeki H, et al. CCR4 and CCR10 are expressed on epidermal keratinocytes and are involved in cutaneous immune reaction. Cytokine 2008;44:172–178 [DOI] [PubMed] [Google Scholar]
- 100.Yates CC, Whaley D A YC, et al. ELR-negative CXC chemokine CXCL11 (IP-9/I-TAC) facilitates dermal and epidermal maturation during wound repair. Am J Pathol 2008;173:643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kroeze KL, Boink MA, Sampat-Sardjoepersad SC, et al. Autocrine regulation of re-epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J Invest Dermatol 2012;132:216–225 [DOI] [PubMed] [Google Scholar]
- 102.Borue X, Lee S, Grove J, et al. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol 2004;165:1767–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inokuma D, Abe R, Fujita Y, et al. CTACK/CCL27 accelerates skin regeneration via accumulation of bone marrow-derived keratinocytes. Stem Cells 2006;24:2810–2816 [DOI] [PubMed] [Google Scholar]
- 104.Ishida Y, Gao JL, Murphy PM. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J Immunol 2008;180:569–579 [DOI] [PubMed] [Google Scholar]
- 105.Fong AM, Robinson LA, Steeber DA, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med 1998;188:1413–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997;91:521–530 [DOI] [PubMed] [Google Scholar]
- 107.Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res 1998;77:80–84 [DOI] [PubMed] [Google Scholar]
- 108.Satish L, Kathju S. Cellular and Molecular Characteristics of Scarless versus Fibrotic Wound Healing. Dermatol Res Pract 2010;2010:790234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fonder MA, Lazarus GS, Cowan DA, et al. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008;58:185–206 [DOI] [PubMed] [Google Scholar]
- 110.Singh A, Halder S, Menon GR, et al. Meta-analysis of randomized controlled trials on hydrocolloid occlusive dressing versus conventional gauze dressing in the healing of chronic wounds. Asian J Surg 2004;27:326–332 [DOI] [PubMed] [Google Scholar]
- 111.Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol 2007;25:19–25 [DOI] [PubMed] [Google Scholar]
- 112.Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994;130:489–493 [PubMed] [Google Scholar]
- 113.Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev 2001;17:246–249 [DOI] [PubMed] [Google Scholar]
- 114.Nwomeh BC, Yager DR, Cohen IK. Physiology of the chronic wound. Clin Plast Surg 1998;25:341–356 [PubMed] [Google Scholar]
- 115.Diegelmann RF. Excessive neutrophils characterize chronic pressure ulcers. Wound Repair Regen 2003;11:490–495 [DOI] [PubMed] [Google Scholar]
- 116.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 1996;107:743–748 [DOI] [PubMed] [Google Scholar]
- 117.Greaves NS, Iqbal SA, Baguneid M, Bayat A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen 2013;21:194–210 [DOI] [PubMed] [Google Scholar]
- 118.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol 2007;82:1375–1381 [DOI] [PubMed] [Google Scholar]
- 119.McQuibban GA, Butler GS, Gong J-H, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem 2001;276:43503–43508 [DOI] [PubMed] [Google Scholar]
- 120.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood 2000;96:2673–2681 [PubMed] [Google Scholar]
- 121.Van den Steen PE, Husson SJ, Proost P, Van Damme J, Opdenakker G. Carboxyterminal cleavage of the chemokines MIG and IP-10 by gelatinase B and neutrophil collagenase. Biochem Biophys Res Commun 2003;310:889–896 [DOI] [PubMed] [Google Scholar]
- 122.McQuibban GA, Gong JH, Tam EM, et al. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 2000;289:1202–1206 [DOI] [PubMed] [Google Scholar]
- 123.Tam EM, Morrison CJ, Wu YI, Stack MS, Overall CM. Membrane protease proteomics: Isotope-coded affinity tag MS identification of undescribed MT1-matrix metalloproteinase substrates. Proc Natl Acad Sci U S A 2004;101:6917–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tester AM, Cox JH, Connor AR, et al. LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PLoS One 2007;2:e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Petreaca ML, Do D, Dhall S, et al. Deletion of a tumor necrosis superfamily gene in mice leads to impaired healing that mimics chronic wounds in humans. Wound Repair Regen 2012;20:353–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Petreaca ML, Yao M, Ware C, Martins-Green MM. Vascular endothelial growth factor promotes macrophage apoptosis through stimulation of tumor necrosis factor superfamily member 14 (TNFSF14/LIGHT). Wound Repair Regen 2008;16:602–614 [DOI] [PubMed] [Google Scholar]
- 127.Abbott RE, Corral CJ, MacIvor DM, et al. Augmented inflammatory responses and altered wound healing in cathepsin G-deficient mice. Arch Surg 1998;133:1002–1006 [DOI] [PubMed] [Google Scholar]
- 128.Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury? Thromb Haemost 2004;92:275–280 [DOI] [PubMed] [Google Scholar]
- 129.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol 2007;19:289–295 [DOI] [PubMed] [Google Scholar]
- 130.Pradhan L, Cai X, Wu S, et al. Gene expression of pro-inflammatory cytokines and neuropeptides in diabetic wound healing. J Surg Res 2011;167:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Engelmayer J, Blezinger P, Varadhachary A. Talactoferrin stimulates wound healing with modulation of inflammation. J Surg Res 2008;149:278–286 [DOI] [PubMed] [Google Scholar]
- 132.Lyons TE, Miller MS, Serena T, et al. Talactoferrin alfa, a recombinant human lactoferrin promotes healing of diabetic neuropathic ulcers: a phase 1/2 clinical study. Am J Surg 2007;193:49–54 [DOI] [PubMed] [Google Scholar]
- 133.Vande Berg JS, Rudolph R, Hollan C, Haywood-Reid PL. Fibroblast senescence in pressure ulcers. Wound Repair Regen 1998;6:38–49 [DOI] [PubMed] [Google Scholar]
- 134.Loots MA, Lamme EN, Mekkes JR, Bos JD, Middelkoop E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch Dermatol Res 1999;291:93–99 [DOI] [PubMed] [Google Scholar]
- 135.Stanley A, Osler T. Senescence and the healing rates of venous ulcers. J Vasc Surg 2001;33:1206–1211 [DOI] [PubMed] [Google Scholar]
- 136.Schwarz F, Jennewein M, Bubel M, et al. Soft tissue fibroblasts from well healing and chronic human wounds show different rates of myofibroblasts in vitro. Mol Biol Rep 2013;40:1721–1733 [DOI] [PubMed] [Google Scholar]
- 137.Almqvist S, Werthen M, Johansson A, et al. Evaluation of a near-senescent human dermal fibroblast cell line and effect of amelogenin. Br J Dermatol 2009;160:1163–1171 [DOI] [PubMed] [Google Scholar]
- 138.Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 2004;164:1935–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Drinkwater SL, Burnand KG, Ding R, Smith A. Increased but ineffectual angiogenic drive in nonhealing venous leg ulcers. J Vasc Surg 2003;38:1106–1112 [DOI] [PubMed] [Google Scholar]
- 140.Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med 2005;15:57–63 [DOI] [PubMed] [Google Scholar]
- 141.Loh SA, Chang EI, Galvez MG, et al. SDF-1 alpha expression during wound healing in the aged is HIF dependent. Plast Reconstr Surg 2009;123(2 Suppl):65s–75s [DOI] [PubMed] [Google Scholar]
- 142.Bermudez DM, Xu J, Herdrich BJ, et al. Inhibition of stromal cell-derived factor-1alpha further impairs diabetic wound healing. J Vasc Surg 2011;53:774–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest 2007;117:1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Badillo AT, Chung S, Zhang L, Zoltick P, Liechty KW. Lentiviral gene transfer of SDF-1alpha to wounds improves diabetic wound healing. J Surg Res 2007;143:35–42 [DOI] [PubMed] [Google Scholar]
- 145.Castilla DM, Liu ZJ, Tian R, et al. A novel autologous cell-based therapy to promote diabetic wound healing. Ann Surg 2012;256:560–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wong VW, Crawford JD. Vasculogenic cytokines in wound healing. Biomed Res Int 2013;2013:190486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murray JC, Pollack SV, Pinnell SR. Keloids: a review. J Am Acad Dermatol 1981;4:461–470 [DOI] [PubMed] [Google Scholar]
- 148.Niessen FB, Spauwen PH, Schalkwijk J, Kon M. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 1999;104:1435–1458 [DOI] [PubMed] [Google Scholar]
- 149.Cosman B, Crikelair GF, Ju DMC, Gaulin JC, Lattes R. The surgical treatment of keloids. Plast Reconstr Surg 1961;27:335–358 [Google Scholar]
- 150.Diegelmann RF, Cohen IK, McCoy BJ. Growth kinetics and collagen synthesis of normal skin, normal scar and keloid fibroblasts in vitro. J Cell Physiol 1979;98:341–346 [DOI] [PubMed] [Google Scholar]
- 151.Calderon M, Lawrence WT, Banes AJ. Increased proliferation in keloid fibroblasts wounded in vitro. J Surg Res 1996;61:343–347 [DOI] [PubMed] [Google Scholar]
- 152.Tredget EE, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids, and contractures. The cellular and molecular basis for therapy. Surg Clin North Am 1997;77:701–730 [DOI] [PubMed] [Google Scholar]
- 153.Machesney M, Tidman N, Waseem A, Kirby L, Leigh I. Activated keratinocytes in the epidermis of hypertrophic scars. Am J Pathol 1998;152:1133–1141 [PMC free article] [PubMed] [Google Scholar]
- 154.Nirodi CS, Devalaraja R, Nanney LB, et al. Chemokine and chemokine receptor expression in keloid and normal fibroblasts. Wound Repair Regen 2000;8:371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yates CC, Whaley D, Wells A. Transplanted fibroblasts prevents dysfunctional repair in a murine CXCR3-deficient scarring model. Cell Transplant 2012;21:919–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rabbany SY, Pastore J, Yamamoto M, et al. Continuous delivery of stromal cell-derived factor-1 from alginate scaffolds accelerates wound healing. Cell Transplant 2010;19:399–408 [DOI] [PubMed] [Google Scholar]
- 157.Scholten DJ, Canals M, Maussang D, et al. Pharmacological modulation of chemokine receptor function. Br J Pharmacol 2012;165:1617–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mahler DA, Huang S, Tabrizi M, Bell GM. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: a pilot study. Chest 2004;126:926–934 [DOI] [PubMed] [Google Scholar]
- 159.Cox MA, Jenh CH, Gonsiorek W, et al. Human interferon-inducible 10-kDa protein and human interferon-inducible T cell alpha chemoattractant are allotopic ligands for human CXCR3: differential binding to receptor states. Mol Pharmacol 2001;59:707–715 [DOI] [PubMed] [Google Scholar]
- 160.Blanpain C, Doranz BJ, Bondue A, et al. The core domain of chemokines binds CCR5 extracellular domains while their amino terminus interacts with the transmembrane helix bundle. J Biol Chem 2003;278:5179–5187 [DOI] [PubMed] [Google Scholar]
- 161.Allegretti M, Cesta MC, Garin A, Proudfoot AE. Current status of chemokine receptor inhibitors in development. Immunol Lett 2012;145:68–78 [DOI] [PubMed] [Google Scholar]
- 162.Gao Z, Metz WA. Unraveling the chemistry of chemokine receptor ligands. Chem Rev 2003;103:3733–3752 [DOI] [PubMed] [Google Scholar]
- 163.Johnson Z, Proudfoot AE, Handel TM. Interaction of chemokines and glycosaminoglycans: a new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev 2005;16:625–636 [DOI] [PubMed] [Google Scholar]
- 164.Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today 1992;13:291–294 [DOI] [PubMed] [Google Scholar]
- 165.Cadene M, Boudier C, de Marcillac GD, Bieth JG. Influence of low molecular mass heparin on the kinetics of neutrophil elastase inhibition by mucus proteinase inhibitor. J Biol Chem 1995;270:13204–13209 [DOI] [PubMed] [Google Scholar]
- 166.Proudfoot AE, Handel TM, Johnson Z, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A 2003;100:1885–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Campanella GS, Grimm J, Manice LA, et al. Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol 2006;177:6991–6998 [DOI] [PubMed] [Google Scholar]
- 168.Hiesinger W, Goldstone AB, Woo YJ. Re-engineered stromal cell-derived factor-1alpha and the future of translatable angiogenic polypeptide design. Trends Cardiovasc Med 2012;22:139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Proudfoot AE, Power CA, Hoogewerf AJ, et al. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J Biol Chem 1996;271:2599–2603 [DOI] [PubMed] [Google Scholar]
- 170.Koelink PJ, Overbeek SA, Braber S, et al. Targeting chemokine receptors in chronic inflammatory diseases: an extensive review. Pharmacol Ther 2012;133:1–18 [DOI] [PubMed] [Google Scholar]
- 171.Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol 2006;7:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Salanga CL, O'Hayre M, Handel T. Modulation of chemokine receptor activity through dimerization and crosstalk. Cell Mol Life Sci 2009;66:1370–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Issafras H, Angers S, Bulenger S, et al. Constitutive agonist-independent CCR5 oligomerization and antibody-mediated clustering occurring at physiological levels of receptors. J Biol Chem 2002;277:34666–34673 [DOI] [PubMed] [Google Scholar]
- 174.Babcock GJ, Farzan M, Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem 2003;278:3378–3385 [DOI] [PubMed] [Google Scholar]
- 175.El-Asmar L, Springael JY, Ballet S, et al. Evidence for negative binding cooperativity within CCR5-CCR2b heterodimers. Mol Pharmacol 2005;67:460–469 [DOI] [PubMed] [Google Scholar]
- 176.Wilson S, Wilkinson G, Milligan G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J Biol Chem 2005;280:28663–28674 [DOI] [PubMed] [Google Scholar]
- 177.Sohy D, Yano H, de Nadai P, et al. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem 2009;284:31270–31279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol 1999;17:657–700 [DOI] [PubMed] [Google Scholar]
- 179.Gorry PR, Zhang C, Wu S, et al. Persistence of dual-tropic HIV-1 in an individual homozygous for the CCR5 Delta 32 allele. Lancet 2002;359:1832–1834 [DOI] [PubMed] [Google Scholar]
- 180.Klibanov OM. Vicriviroc, a CCR5 receptor antagonist for the potential treatment of HIV infection. Curr Opin Investig Drugs 2009;10:845–859 [PubMed] [Google Scholar]
- 181.Jekle A, Keppler OT, De Clercq E, et al. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J Virol 2003;77:5846–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zabel BA, Agace WW, Campbell JJ, et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med 1999;190:1241–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zaballos A, Gutierrez J, Varona R, Ardavin C, Marquez G. Cutting edge: identification of the orphan chemokine receptor GPR-9-6 as CCR9, the receptor for the chemokine TECK. J Immunol 1999;162:5671–5675 [PubMed] [Google Scholar]
- 184.Keshav S, Vanasek T, Niv Y, et al. A randomized controlled trial of the efficacy and safety of CCX282-B, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn's disease. PLoS One 2013;8:e60094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tziotzios C, Profyris C, Sterling J. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part II. Strategies to reduce scar formation after dermatologic procedures. J Am Acad Dermatol 2012;66:13–24; quiz 25–16 [DOI] [PubMed] [Google Scholar]
- 186.Occleston NL, O'Kane S, Laverty HG, et al. Discovery and development of avotermin (recombinant human transforming growth factor beta 3): a new class of prophylactic therapeutic for the improvement of scarring. Wound Repair Regen 2011;19 (Suppl 1):s38–s48 [DOI] [PubMed] [Google Scholar]
- 187.Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol 2004;20:811–838 [DOI] [PubMed] [Google Scholar]
- 188.Rhett JM, Ghatnekar GS, Palatinus JA, et al. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol 2008;26:173–180 [DOI] [PubMed] [Google Scholar]
- 189.Kretz M, Maass K, Willecke K. Expression and function of connexins in the epidermis, analyzed with transgenic mouse mutants. Eur J Cell Biol 2004;83:647–654 [DOI] [PubMed] [Google Scholar]
- 190.Mori R, Power KT, Wang CM, Martin P, Becker DL. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci 2006;119(Pt 24):5193–5203 [DOI] [PubMed] [Google Scholar]
- 191.Qiu C, Coutinho P, Frank S, et al. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol 2003;13:1697–1703 [DOI] [PubMed] [Google Scholar]