Abstract
Significance: Fetal wounds heal with a regenerative phenotype that is indistinguishable from surrounding skin with restored skin integrity. Compared to this benchmark, all postnatal wound healing is impaired and characterized by scar formation. The biologic basis of the fetal regenerative phenotype can serve as a roadmap to recapitulating regenerative repair in adult wounds. Reduced leukocyte infiltration, likely mediated, in part, through changes in the chemokine milieu, is a fundamental feature of fetal wound healing.
Recent Advances: The contributions of chemokines to wound healing are a topic of active investigation. Recent discoveries have opened the possibility of targeting chemokines therapeutically to treat disease processes and improve healing capability, including the possibility of achieving a scarless phenotype in postnatal wounds.
Critical Issues: Successful wound healing is a complex process, in which there is a significant interplay between multiple cell types, signaling molecules, growth factors, and extracellular matrix. Chemokines play a crucial role in this interplay and have been shown to have different effects in various stages of the healing process. Understanding how these chemokines are locally produced and regulated during wound healing and how the chemokine milieu differs in fetal versus postnatal wounds may help us identify ways in which we can target chemokine pathways.
Future Directions: Further studies on the role of chemokines and their role in the healing process will greatly advance the potential for using these molecules as therapeutic targets.

Sundeep G. Keswani, MD
Scope and Significance
Fetal tissue is unique since it has the ability to heal without scarring, with total regeneration of epidermal and dermal layers, including dermal appendages such as hair follicles and sweat glands.1 There are key differences between the fetal scarless wound healing phenotype and adult wound healing, and although the exact mechanisms are unknown, a better understanding of these mechanisms will lead to more successful therapeutic interventions in the future. Chemokines, a class of signaling molecules known for leukocyte recruitment, are pivotal in regulating the wound healing process. However, new and more complex roles for chemokines have been discovered in wound healing, including the regulation of resident cell migration, neovascularization, inflammation, and tissue repair.2 This review will begin with a brief introduction to chemokines and then discuss their roles in the wound healing process. Furthermore, we will discuss some key differences between chemokine expression and function in the fetal versus the adult wound environment that may contribute to the characteristic differences in the wound healing phenotype.
Translational Relevance
Chemokines are intimately involved in the orchestration of wound healing. They are the important regulators of inflammation, neovascularization, reepithelialization, and repair processes during wound healing. If we can identify how chemokine expression patterns are different in fetal versus adult physiological wound healing, these regulatory molecules and the G-protein–coupled receptors they signal through could be targeted therapeutically to recreate a more fetal-like wound milieu and stimulate regenerative wound healing. Moreover, coadministration of chemokines (or chemokine pathway inhibitors) might complement the local application of growth factors to improve healing through promoting the migration of resident and inflammatory cells. Despite the great promise of chemokine-targeted therapy, much work still needs to be done to determine whether control of this signaling system will prove to be fruitful from bench to bedside.
Clinical Relevance
In the developed world, more than 100 million patients acquire scars each year, many of which cause significant morbidity. There are an estimated 11 million keloid scars and 4 million burn scars.3,4 Although global figures are unknown, they are likely much higher. Furthermore, although there is a paucity of data that adequately describes the negative physical impact of scars on a patient's quality of life, scars can also cause psychological impact with anxiety and negative impact on self-consciousness.5 By understanding the underlying molecular mechanisms in the fetal regenerative response to injury, we may be able to develop therapies based on this knowledge to recapitulate the fetal phenotype in postnatal tissues. Patients would benefit from improved healing, and care providers would be able to have more influence over the healing process after invasive procedures. Chemokines undoubtedly play a pivotal role in the healing process and further investigations into their mechanisms of action may allow us to improve outcomes in our patients.
Discussion of Findings and Relevant Literature
Chemokine classification and function
Chemokines are a diverse family of chemotactic cytokines. They are small (8–10 kDa) positively charged secreted proteins that facilitate cell–cell communication through both autocrine and paracrine mechanisms with the specific endpoint of cell trafficking. Chemokines have been the subject of much research since the 1970s and 1980s, when they were first discovered,6–8 and a great deal has been learned about their role in diverse processes, including angiogenesis, inflammation, leukocyte trafficking and differentiation, and the response to infection and autoimmune disease.
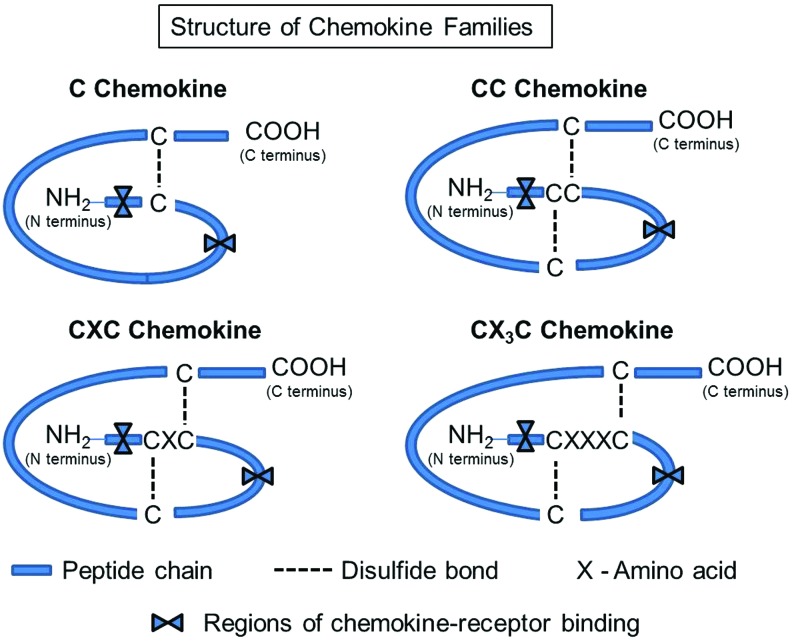
Chemokines share certain structural characteristics. They share 20–50% homology in amino acid sequence,2,9 including the presence of cysteine residues in conserved locations that determine their shape.10 They are classified into four families based on the number and positioning of the cysteine residues: C, CC, CXC, and the CX3C (Fig. 1). Members of the C chemokine group, such as XCL1 (lymphotacin) and XCL2 (SCM-1β), have only two cysteines, one N-terminal and one downstream. CC chemokines have two adjacent cysteines near their N-terminal end. An example of a CC chemokine is CCL-2, also known as the monocyte chemoattractant protein-1 (MCP-1), which induces transendothelial migration of monocytes into sites of tissue remodeling to become macrophages.11 Members of the CXC group share two conserved cysteines separated by an amino acid.9 The CXC family is further divided into two groups based on the presence of glutamic acid (E), leucine (L), and arginine (R) immediately before the first cysteine. This ELR+ sequence is important for receptor selectivity and binding. An example of an ELR+ CXC chemokine is interleukin (IL)-18, which is a chemoattractant for neutrophils.12 An example of an ELR− chemokine is CXCL13, which induces lymphocyte egress into surrounding tissues.13 Of note, ELR+ CXC chemokines are said to be angiogenic, whereas chemokines lacking this sequence (ELR− CXC chemokines) are said to be angiostatic.14 The CX3C family contains two cysteines separated by three amino acids. The only CX3C chemokine identified to date is CX3CL1 (fractalkine). Over 45 chemokines have been reported to date and many of them impact the behavior of cell types that influence cutaneous wound healing (Table 1).
Figure 1.
Chemokine family structure. Chemokines contain cysteines (usually four) in conserved positions. The spacing between the first two cysteines determines the type of chemokine. The C subfamily contains only one of the proximal n-terminal cysteines. In the CC subfamily, the first two cysteines are adjacent to each other, in the CXC family, there is one amino acid between the first two cysteines, and in the CX3C family, the two cysteines are separated by three amino acids. The first cysteine (C) in the sequence forms a covalent bond with the third, the second and the fourth cysteines also form a disulfide bond to create the tertiary structure characteristic of chemokines. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Table 1.
Chemokines and chemokine receptors in wound healing
| Chemokines | ||||
|---|---|---|---|---|
| Systemic name | Human ligand | Mouse ligand | Chemokine receptors | Target cells in wound healing |
| CCL1 | I-309/TCA-3 | TCA-3 | CCR8 | Monocytes, macrophages |
| CCL2 | MCP-1 | MCP-1 | CCR2 | Monocytes, T lymphocytes, mast cells, keratinocytes, endothelial cells, fibroblasts |
| CCL3 | MIP-1α | MIP-1α | CCR4, CCR5 | Monocytes, macrophages |
| CCL4 | MIP-1β | MIP-1β | CCR1, CCR5 | Monocytes, macrophages |
| CCL5 | RANTES | RANTES | CCR1, CCR3, CCR4, CCR5 | Monocytes, macrophages |
| CCL7 | MCP-3 | MARC | CCR1, CCR2, CCR3, CCR5 | Neutrophils |
| CCL8 | MCP-2 | MCP-2 | CCR1, CCR2, CCR3, CCR5 | Mast cells, monocytes, T lymphocytes |
| CCL22 | MDC | ABCD-1 | CCR4 | T lymphocytes |
| CCL23 | MPIF-1 | CCL6, C10 | CCR1 | Neutrophils, monocytes, T lymphocytes |
| CCL27 | CTACK/ILC | CTACK/ILC | CCR10 | T lymphocytes |
| CXCL1 | GROa/MGSAa | GRO/KC | CXCR2 | Neutrophils, keratinocytes, endothelial cells, fibroblasts |
| CXCL2 | GROb/MGSAb | GRO/KC | CXCR2 | Endothelial cells |
| CXCL3 | GROg/MGSAg | GRO/KC | CXCR2 | Endothelial cells |
| CXCL4 | PF4 | PF4 | CXCR3b | Fibroblasts |
| CXCL5 | ENA-78 | GCP-2/LIX | CXCR2 | Neutrophils |
| CXCL6 | GCP-2 | GCP-2/LIX | CXCR1, CXCR2 | Neutrophilic granulocytes |
| CXCL7 | NAP-2 | NAP-2 | CXCR2 | Neutrophils, leucocytes, macrophages, keratinocytes, endothelial cells, fibroblasts |
| CXCL8 | IL-8 | MIP-2 | CXCR1, CXCR2 | Neutrophils, leucocytes, macrophages, keratinocytes, endothelial cells, fibroblasts |
| CXCL9 | MIG | MIG | CXCR3 | T lymphocytes, endothelial cells, fibroblasts |
| CXCL10 | IP-10 | IP-10 | CXCR3 | T lymphocytes, endothelial cells, fibroblasts |
| CXCL11 | I-TAC | I-TAC | CXCR3 | T lymphocytes |
| CXCL12 | SDF-1a/b | SDF-1a/b | CXCR4 | T lymphocytes, keratinocytes, endothelial cells, endothelial progenitor cells |
| CXCL13 | BLC/BCA-1 | BLC/BCA-1 | CXCR5 | B cells, T lymphocytes |
| CX3CL1 | Fractalkine | Fractalkine | CX3CR1 | Fibroblasts, NK cells, T cells, endothelial cells, epithelial cells, macrophages, and vascular smooth muscle cells |
IL, interleukin; MCP, monocyte chemoattractant protein; MIP-1α or β, macrophage inflammatory protein-1 (MIP-1α=CCL3; MIP-1β=CCL4); RANTES, regulated on activation, normal T expressed and secreted (CCL5); SDF-1, stromal cell-derived factor-1.
Along with the composition of the local chemokine milieu, chemokine diffusion and clearance are also tightly regulated. Within tissues and at the cell surface, chemokines are bound to extracellular matrix (ECM) proteoglycans, particularly those containing the heparin sulfate glycosaminoglycan. The resulting sequestration and diffusion of chemokines create gradients within tissues that govern cell chemotaxis.15 All chemokines signal through the G-protein-linked transmembrane receptors. As with the chemokines themselves, the chemokine receptors are likewise divided into four families with distinct chemokine-binding properties: XCR1 binds XC chemokines, CCR1-10 receptors bind CC chemokines, CXCR1-7 receptors bind CXC chemokines, and CX3CR1 binds CX3CL1. Although some chemokine–chemokine receptor interactions are selective, many chemokine receptors bind multiple chemokines, engendering both redundancy and plasticity in chemotactic responses.16
On a functional basis, chemokines can be categorized as either being homeostatic, in which case they are constitutively expressed, or inflammatory, in which case they are present only in the setting of an inflammatory response. These distinctions are likely to be highly relevant to chemotaxis during wound healing responses, where an inflammatory influx occurs into previously homeostatic tissues.9 However, in debilitating ischemic or chronic wounds that are associated with poor wound healing outcome, such as in diabetes, the distinctions between basal and inflammatory chemokine levels blur, with important consequences for immune regulation at these sites.17 Along with these contextual associations, chemokines have particular tropisms in terms of the cell types they impact, reflecting the distribution of chemokine receptors (Table 1).
Chemokine expression/function during the different phases of wound healing
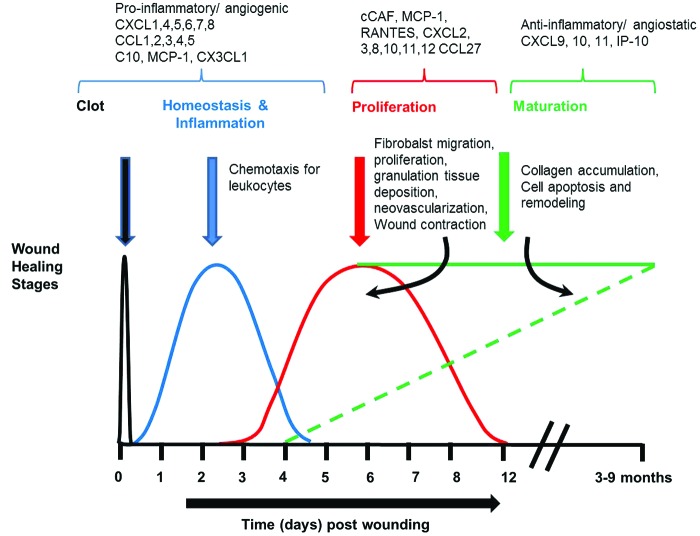
Normal wound healing occurs through a series of sequential overlapping stages (Fig. 2). During these stages, chemokines control the trafficking of specialized cell types to local sites of injury in a time- and context-dependent manner. In this study, we will address the fundamental biology of chemokines in wound healing, organized according to the stages of a normal wound response.
Figure 2.
Chemokines and their roles in the various phases of wound healing. Normal wound healing progresses through a series of sequential overlapping stages. The initial stage of a wound response is hemostasis, occurring within minutes to hours of injury. Clot formation occurs that sets the stage for the inflammatory phase of healing, which begins with neutrophils coming in first followed by macrophages. This phase is followed by proliferative and maturation phases. Reepithelialization and granulation tissue formation in which the keratinocytes migrate to cover the wound, and the wound tissue begins its repair by cell proliferation, ECM production, and blood vessel development. Finally, during the remodeling, many of the extracellular elements are removed by apoptosis, and the ECM is remodeled. During these stages, chemokines control the trafficking of specialized cell types to local sites of injury in a time- and context-dependent manner. ECM, extracellular matrix. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The hemostasis stage of wound healing
The initial stage of a wound response is hemostasis, occurring within minutes to hours of injury. Bleeding as a consequence of tissue injury results in the release of platelets, plasma fibronectin, and prothrombin and in the formation of a clot that serves as a provisional matrix for the initiation of the inflammatory phase. Chemokine CXCL4 participates in the initial coagulation process and prevents the premature development of blood vessels. Platelet degranulation releases a plethora of cytokines that activate the tissue resident cells, including the macrophages, keratinocytes, and fibroblasts. These activated cells in turn produce inflammatory mediators such as the proinflammatory chemokines CXCL8 and CCL2, which promote the directional migration of inflammatory cells and endothelial cells to the wounded area, further regulating the inflammation and angiogenesis at the tissue repair site.
There are some suggestions that chemokine expression in fetal wounds during hemostasis is different from that seen in postnatal wounds. Porcine fetal platelets may release less cytokines (and presumably chemokines).18 It may also be the case that fetal tissues inhibit platelet-mediated chemotaxis. Olutoye et al.19 had demonstrated that hyaluronan (HA), the predominant glycosaminoglycan GAG in the fetal dermal matrix, inhibits platelet aggregation and cytokine release. The authors concluded that the HA-induced inhibition of platelet aggregation and resultant attenuation of inflammatory response may explain, in part, the minimal inflammation and modest cellular infiltrates, characteristic of fetal wounds. However, fetal platelets may also produce factors that actively promote tissue regeneration, in part, through the recruitment of mesenchymal stem cells (MSCs).20 Further elucidation of the chemokine profile from fetal tissues during homeostatic conditions and from the midgestation scarless wound healing, late-gestation transitional wound healing, and postnatal stages is required to understand the factors leading to reduced inflammatory infiltrates in fetal wounds.
The inflammatory stage of wound healing
Hemostasis sets the stage for the inflammatory stage of wound healing, occurring in the first 72 h of a wound response. The inflammatory stage is characterized by an influx of inflammatory cell types and the increase in proinflammatory mediators is highly regulated by chemokines. However, in fetal wound healing, numbers of inflammatory cells are reduced, they are less activated, and have more short-lived responses.21
The rapid influx of inflammatory cell types begins acutely after injury with the release of platelets and neutrophils directly from circulation into the wound. Activated platelets release a multitude of inflammatory mediators, which promote the directional migration of leukocytes to the wounded area. Neutrophils dominate the initial influx of leukocytes, and at day 1 postinjury, neutrophils have been shown to constitute nearly 50% of wound cells.22 Neutrophils express CXC chemokine receptor 2 and are recruited in response to CXCL8 (IL-8), a potent proinflammatory chemokine that is involved in this process initially.2 In humans, CXCL8 is a potent activator and chemoattractant of neutrophils and as neutrophils arrive at the new wound, they also secrete CXCL8, further augmenting the effect.23 It is suggested that CXCL8 also plays a role in the inhibition of neutrophil apoptosis, thus potentiating the inflammatory process.24 CXCL8 also stimulates endothelial permeability, thereby facilitating leukocyte migration into injured tissues,25,26 and has been shown to have increased expression levels in chronic wounds.9 Other inflammation-related chemokines that may play a role after injury are CXCL1, 4, 5, 6, and 7. Each of these chemokines, along with CXCL8, forms gradients through interactions with proteoglycans, rendering directional movement of leukocytes into wounded areas.23,27,28
Neutrophils are also present in fetal wound healing, but there are important differences in the expression pattern and function of neutrophils in fetal versus adult wound healing29 that may distinguish whether a wound heals regeneratively or with a scar.30 Adult neutrophils are characterized by a much higher expression of cell adhesion molecules than the fetal neutrophils,31 which may induce increased neutrophil–endothelial interaction and thereby increased migration of neutrophils into the wound bed that then can contribute to scarring phenotype of the adult wounds.32 In comparison, fetal wounds have in fact been demonstrated to have a smaller fraction of neutrophils. In addition, the expression levels of proinflammatory chemokine IL-8 (CXCL8) are higher during adult wound repair compared to a much lower expression in the midgestational fetal wounds that heal scarlessly.33,29 Since CXCL8 is the main neutrophil chemoattractant, this could explain the difference between the number and activation of neutrophils in fetal versus adult wounds.
Following the initial influx of neutrophils to kill potential pathogens and decontaminate the wound, a switch in the leukocyte recruitment occurs that favors the recruitment of cells of monocyte/macrophage lineage to the wound.22 The differential release of their respective chemoattractants in the wound microenvironment, which is, in part, orchestrated by IL-6 and its soluble receptor,34 facilities the temporal switch in the leukocyte recruitment from neutrophils to monocytes. Neutrophils themselves are thought to mediate this switch by releasing soluble factors into the early inflammatory wound microenvironment that then initiates monocyte recruitment.35 MCP-1, also referred to as CCL-2, is a predominant chemokine implicated in monocyte recruitment. Monocytes express chemokine receptor 2 that plays an important role in their response to MCP-1. In addition to infiltrating neutrophils, MCP-1 is also known to be predominantly produced in situ by tissue-resident macrophages at the site of wound healing.36 Monocyte chemotaxis is also partly regulated by other CC chemokine family members such as CCL5 (regulated on activation, normal T expressed and secreted [RANTES]), CCL3 (macrophage inflammatory protein [MIP]-1α), CCL4 (MIP-1β), CCL1 (I-309), and CCL-7 (MCP-3).23 Monocytes differentiate into macrophages once within the wound and play an essential role in the removal of debris and dead cells, as antigen-presenting cells, and as secretors of additional growth factors. A study by Kaesler et al.37 demonstrated that injury induced the expression of chemokine CCL-6 (C10), which is a potent chemoattractant for macrophages and may contribute to strong macrophage influx observed in cutaneous wound healing. There are differences between monocytes and macrophages within the fetal and adult wounds, with a decrease in the number and persistence of these cell types, as well as macrophages in their active and mature forms in fetal wounds.38 It is unclear whether these differences are attributable to differences in chemokine secretion.
Another cell type, mast cells, is not only resident in the skin but also recruited to wounds and is one of the initial cell types to respond to trauma with the release of inflammatory mediators. There is growing evidence to demonstrate that chemokines and their receptors regulate mast cell tissue localization and function. Human mast cells of different origin express several chemokine receptors (CXCR1, CXCR2, CXCR3, CXCR4, CX3CR1, CCR1, CCR3, CCR4, and CCR5). Seven chemokines (CXCL1, CXCL5, CXCL8, CXCL14, CX3CL1, CCL5, and CCL11) have been shown to act on some of these receptors and to induce mast cell migration.39 The chemokine MCP-1 has been shown to be a potent attractant for mast cells, which are increasingly considered to be important mediators in wound healing.40 Mast cells degranulate after injury releasing multiple proinflammatory mediators and vasoactive amines41 and have also shown to be abundant in both keloids and hypertrophic scars.42 Mast cells also release chemokines such as IL-8, MIP-1α, and MIP-1β independent of degranulation, when stimulated through the activation of the CD30 pathway.43 Mast cells synthesize IL-4 and stimulate fibroblast proliferation.40 Mast cells can have both stimulatory and inhibitory functions in skin inflammation that are regulated by chemokines (reviewed by Harvima et al.44). In fetal skin that heals with the scarless phenotype (embryonic day 15 [E15] in murine model], mast cells differ in size (smaller), number (decreased), granularity (less), and maturity when compared with mast cells in more mature skin (E18) and they do not degranulate as effectively. Consistent with this, Wulff et al. demonstrated that injection of mast cell lysates into E15 wounds disrupted the scarless healing phenotype and that mice deficient in mast cells when injured at E18, healed with significantly smaller scars, suggesting that mast cells may influence the scarless healing phenotype.42 Mast cells are also increased in the skin in chronic skin inflammatory conditions, for example, psoriasis, basal cell carcinoma, and chronic ulcers. Disodium cromoglycate, a pharmacologic inhibitor of mast cell activation and degranulation, has been used to decrease the amount of inflammatory cytokines such as IL-1β and chemokine CXCL1 (Murine IL-8)41 in the wounds, which resulted in the augmented healing response, characterized by improved collagen fiber organization and reduction in wound scar width, without negative effects on wound breaking strength. These data demonstrate that chemokine regulation can offer a potential therapeutic benefit for wound healing.
Lymphocytes are the final inflammatory cell type to be recruited to the wound, where they serve as the immunological effector cells and producers of additional growth factors. CCL3, CCL4, and CCL5 are also chemoattractants of lymphocytes and are produced in granulation tissue.2 Specifically, B lymphocytes produce antibody responses to antigens present in the wound, and T lymphocytes produce cytokines and promote the cytolytic activity. There are also differences in lymphocyte responses between fetal and adult wounds. While B lymphocytes were seen in adult mouse wounds, they were not present in fetal wounds.38
In addition to the specific roles for specific cell types in wound healing, there are data implicating specific chemokines. CX3CL1 and its receptor CX3CR1 is particularly highly expressed in murine models of skin wound healing.45 CX3CL1 is expressed by endothelial cells, epithelial cells, macrophages, and vascular smooth muscle cells. CX3CR1 is expressed by monocytes/macrophages, fibroblasts, NK cells, a subpopulation of T cells, and smooth muscle cells. Loss of CX3CR1 function delayed wound closure in CX3CR1 knockout mice, characterized by reduced macrophages and macrophage products, such as transforming growth factor-β (TGF-β)1 and vascular endothelial growth factor, reduced myofibroblasts and collagen deposition, and reduced neovascularization. The transfer of normal bone marrow for wild-type mice rescued the phenotype in these mice, suggesting an important profibrotic and angiogenic role for the CX3CL1/CX3CR1 axis in cutaneous wound healing. Since the CX3CL1/CX3CR1 axis is associated with several cell types and signaling factors (including TGF-β1, myofibroblasts, and collagen deposition) that distinguish the fetal wound healing phenotype from adult phenotype, it can be hypothesized that targeted manipulations of this axis may facilitate recapitulating fetal-like wound healing in postnatal wounds.
Along with differences in cell types, fetal and adult wounds also differ in terms of their ECM milieu in ways that likely influence wound healing and inflammatory responses. The fetal wound ECM is significantly different from the adult wound environment. The ECM of fetal scarless wounds is characterized by the increased levels of type-III to type-I collagen ratio and high and persistent levels of high-molecular-weight HA. This distinct ECM serves as structural scaffolding that facilitates increased cellular migration. On the other hand, in adult wounds, HA levels are only transiently elevated and low-molecular-weight (LMW) HA predominates. The LMW form of HA (<200 kDa) is known to accumulate at sites of inflammation, influencing various activities, including monocyte activation, leukocyte adhesion to endothelium, and smooth muscle cell migration, after wound healing. In addition, it has also been shown to induce chemokines such as MCP-1, RANTES, and IL-8, which can alter the wound healing phenotype. HA is also shown to suppress platelet aggregation and the release of growth factors in a dose-dependent manner. Matrix turnover also regulates the wound repair outcome. Dang et al.46 demonstrated that the E16 midgestation murine wounds that completely reepithelialize within 72 h and heal without a scar have greater expression of matrix metalloproteinases (MMPs) relative to tissue inhibitors of metalloproteinases (TIMP), which favors ECM turnover, faster migration of cells, and promotes scarless repair compared to the late-gestation scarring wounds at E19. A study by Bullard et al.47 demonstrated that the fetal skin has greater amounts of MMPs (interstitial collagenase, stomelysin-1, and gelatinase-A) than adult skin, with the expression pattern localized to dermal cells, keratinocytes, and around vascular structures, implicating a significant role in wound healing and neovascularization. Chemokine signaling can be significantly influenced by MMPs in several ways, including the release of chemokines bound to the cell surface or ECM, which can impact chemokine gradients, inactivation of the chemokine, and/or generation of more powerful chemotactic or antagonistic derivatives (reviewed by Van Lint and Libert48). These findings reiterate the association between inflammation, ECM deposition, ECM turnover, and remodeling, and suggest that chemokines may be the major regulators of the interdependence between these wound healing processes.
The proliferation and maturation stage of wound healing
Inflammation sets the stage for the proliferative stage, occurring in the first 2–3 weeks after wounding. The proliferation phase is characterized by granulation tissue formation, reepithelialization, and neovascularization.
Granulation tissue formation
The chemokine milieu regulates the movement of resident and infiltrating wound cells as well as influences tissue remodeling. Fibroblasts are important both for remodeling and granulation tissue formation. They are also known to regulate the inflammatory process by the release of inflammatory mediators.49 They are one of the more potent effector cells that manipulate the chemokine expression using autocrine and paracrine mechanisms. Fibroblasts also manipulate chemokine signaling by being target cells for chemokines. For example, MCP-1 can induce the expression of TGF-β and collagen synthesis by rat fibroblasts.50 MCP-1 has also been shown to enhance MMP-1 and TIMP-1 gene expression in primary human dermal fibroblasts.51 This MCP-1-mediated fibroblast signaling can further influence the chemokine milieu. CXCL11 is important in dermal–epidermal interactions and in maturation of the healing tissue. Feugate et al.52,53 demonstrated that the chicken chemotactic and angiogenic factor, a CXC chemokine ortholog of human IL-8, stimulates fibroblasts to produce early granulation tissue and ECM components, including tenacin, fibronectin, and collagen, as well as stimulates the differentiation of fibroblasts to myofibroblasts.
Previous reports have described functional differences between adult and fetal fibroblasts, including more efficient migration. Fetal fibroblasts stimulated by lipopolysaccharide or platelet-derived growth factor have an attenuated IL-6 and IL-8 response compared to adult fibroblasts, suggesting that even when stimulated to release proinflammatory cytokines, the response of the fetal fibroblast is blunted.33,54 It is suggested that some functional differences observed between adult and fetal fibroblasts may be a direct result of the unique HA-rich ECM observed in the fetus. Fibroblasts have also been shown to have differences in gene expression between murine (E15) and (E18) stages, revealing upregulation of several inflammatory genes, for example, cyclooxygenase-1 (COX-1) in the E18 fibroblasts versus E15.49,55 Although not much is known about the specific role of chemokines in the fetal scarless healing phenotype, differences between all the inflammatory cell subtypes and fibroblasts suggest that chemokine differences are likely and may serve as potential targets for therapeutic intervention in the healing process.
Reepithelialization
Reepithelialization of the wound during the proliferative stage is an important process, which occurs through the proliferation and migration of keratinocytes from wound edges to cover the wound. Factors released by platelets such as epidermal growth factor and TGF-β stimulate the keratinocytes near the wound edge to proliferate, and other factors released by other cell types in the wound, including CXCL1 (growth-related oncogene alpha [Gro-α]), CXCL8 (IL-8), fibroblast growth factor, and keratinocyte growth factor, further stimulate the proliferation and migration of keratinocytes.2 CXCL8, specifically, is expressed highly along the denuded wound surface and both CXCL8 and CXCL1 bind to CXCR2, expressed on keratinocytes.23,56 In addition, CXCL1 and CXCR2 are expressed in normal human epidermis and they have been shown to be induced during the wound healing phases of inflammation, reepithelialization, and neovascularization of human burn wounds.57 Mice lacking CXCR2 receptors were observed to have severely impaired reepithelialization in vivo.58 Conversely, topical application of CXCL8 or CXCL1 appears to have favorable effects on wound healing in mouse models.59,60
Keratinocytes migrate through the new ECM through integrin interaction with receptors on the cells, and reepithelialization is therefore dependent upon the presence of proteases such as plasmin and MMPs for breakdown of ECM during migration.2,61 Another chemokine, CXCL11, is produced by basal keratinocytes and promotes the migration of undifferentiated keratinocytes into the wound and therefore playing an active role in reepithelialization.62 Interestingly, another chemokine, CCL27, has been reported to regulate the recruitment of bone marrow-derived keratinocyte stem cells to wounds through its receptor CCR10, suggesting chemokine-mediated recruitment of keratinocytes from outside of the wound.63
Undoubtedly, chemokines play a vital role in the reepithelialization process. Little is known about how chemokines impact keratinocytes in fetal scarless wound healing. Studies have shown that activated epidermal keratinocytes near the wound edge in fetal wounds have an upregulation of epidermal integrin receptor, specific for fibronectin, tenascin, collagen, laminin, and other proteins in the provisional ECM, allowing rapid proliferation and migration of keratinocytes in fetal wounds.30 Another study, using allogenic peripheral blood mononuclear cell proliferation tests, demonstrated that fetal keratinocytes and fibroblasts possessed immunosuppressive properties, in part, mediated by IL-8, vascular endothelial growth factor (VEGF), and granulocyte macrophage colony stimulating factor (GM-CSF),64 suggesting that these cells polarize the cytokine/chemokine milieu.
Neovascularization
During granulation tissue formation, new blood vessels are formed to revascularize the wound tissue by means of endothelial cell proliferation and sprouting from preexisting microvasculature in a process called angiogenesis. Multiple chemokines and cytokines produced by a variety of wound cells influence the angiogenesis process.65 The tissue-resident cells, including macrophages, keratinocytes, and fibroblasts, produce angiogenic growth factors such as VEGF, while in parallel, release of the proteolytic enzymes allows ECM degradation and endothelial cell migration.
When the wound defect is filled, angiogenesis has to cease. A temporal change in the balance between proangiogenic and angiostatic factors regulated by chemokines plays an important role in the orchestration of this cessation. Endothelial cells differentially express several chemokines in response to the wound microenvironmental stimuli.66 These include MCP-1 and RANTES, as well as IL-8, Gro-α, Gro-β (CXCL2), and Gro-γ (CXCL3). CTAP-III, β thromboglobulin, and NAP-2 have also been described to induce endothelial cell proliferation in vitro and angiogenesis in vivo. ELR+ chemokines IL-8 and Gro-α are significantly induced at the neovascularization sites in the wound granulation tissue immediately after wounding and then markedly decline after day 4.22 The CXCR2 chemokine receptor, which binds to all ELR+ chemokines, is also believed to play an important role in the mediation of neovascularization (reviewed by Gillitzer and Goebeler23). Furthermore, chemokines indirectly influence neovascularization by facilitating the recruitment of support cells that are essential for tissue revascularization. DiPietro et al.67 reported that depletion of MIP-1α significantly reduced the angiogenic activity of murine wounds. It was suggested that MIP-1α promotes recruitment of macrophages to wound sites, which in turn act as a source of angiogenic cytokines.
Several ELR− CXC chemokines, including interferon-γ-inducible cytokines IP-10, CXCL-9 (Mig), CXCL4, 9, 10, and 11 or I-TAC exhibit growth inhibitory functions, including inhibition of angiogenesis. IP-10 and Mig have also been shown to be highly expressed after day 4 during the later stages of normal wound healing,22 with an inhibitory action on fibroblast motility and recruitment. While there is clearly a role for chemokines in the regulation of wound neovascularization and maturation, the exact differences between the fetal and adult phenotypes are not completely understood.
Remodeling
During wound maturation and remodeling, the ECM turnover occurs with disorganized collagen fibers being rearranged and crosslinked, along with a decrease in cellularity and regression of the neovasculature. Fibroblasts and myofibroblasts synthesize mature connective tissue as well as produce metalloproteinases that remove the nascent matrix and facilitate matrix maturation. In postnatal tissues, this results in scar formation, while fetal wound healing is associated with regeneration of specialized dermal structures. Chemokines CXCL9, CXCL10, and CXCL11 are important in the process of epidermal and dermal maturation and regression of neovasculature. The wound repair and regeneration is thought to be further influenced by stem cell recruitment and mechanical forces that impact the wound bed, both of which are governed, in part, by chemokines.
Stem cell recruitment
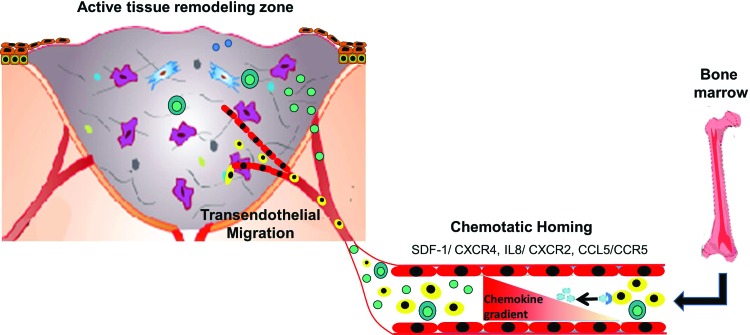
Tissue repair and regeneration involve selective recruitment of circulating or resident stem cell populations (Fig. 3). Transition from regenerative to scar forming cutaneous repair coincides most evidently with the development and maturation of the hair follicle. The role of chemokines in the folliculogenesis is not elucidated. In response to wound healing, stem cells from the basal layer of the epidermis and from the bulge area of the hair follicles get activated and migrate into the wound to contribute to the reepithelialization and wound healing processes.68,69 Chemokines and chemoattractant signals also regulate the mobilization and homing of bone marrow-derived as well as circulating stem cell populations to the tissue repair sites, including MSCs and endothelial progenitor cells (EPCs). Under the guidance of chemokine signaling, MSCs can selectively migrate to injured sites, including skin wound healing,63,70,71 bone fractures,72,73 myocardial infarctions,74,75 and ischemic cerebral injuries,76 where they engraft and contribute to tissue recovery.76 Interestingly, recent reports demonstrated that mechanical stretching can upregulate stromal cell-derived factor-1 alpha (SDF-1α) and hypoxia-inducible factor-1 alpha in skin and recruit circulating MSCs and other stem cells through the SDF-1α/CXCR4 pathway.77 The signaling between SDF-1 and its receptor CXCR4 is critical for the homing and recruitment of circulating progenitor cells after tissue injury and ischemia. SDF-1 is a potent chemoattractant for EPCs. The other major chemokines and respective receptors that regulate EPC activation and homing are IL-8 and CXCR2, growth-regulated oncogene-a and CXCR1, CCL5, CCR5, and C–C chemokine and chemokine (C–C motif) receptors 2 and 5.78 Upon interaction with tissue-specific chemokines, EPCs become activated and initiate integrin-mediated adhesion to endothelial vascular cells and, consequently, transendothelial migration into sites of vascular and tissue remodeling. CXCL8 stimulates vascular permeability, which is important for both angiogenesis and vasculogenesis. EPC invasion into the vascular injury site depends on the breakdown and remodeling of the vessel basement membrane and the interstitial wound space mediated by extracellular proteases. As discussed previously, chemokines play a major role in the regulation of the ECM remodeling and may thereby influence wound neovascularization mediated through both angiogenesis and stem cell-dependent vasculogenesis mechanisms.
Figure 3.
Chemokine gradient. Establishment of a chemokine concentration gradient after injury and hypoxia, which facilitates directional migration and recruitment of bone marrow-derived as well as circulating stem and progenitor cells, inflammatory cells, as well as tissue-resident cells to the sites of tissue repair. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Amniotic stem cells are known to enhance fetal wound healing responses in animal models.79 Furthermore, Roubelakis et al.20 recently reported that platelet-rich plasma promotes regenerative wound healing through recruitment of MSCs. Together these data suggest that there may be a role for circulating stem and progenitor cells in the fetal response that may similarly be, in part, regulated by chemokines. This is an exciting area for future research.
Mechanotransduction
Not all midgestation fetal wounds heal without scars. In fact, larger excisional wounds in the fetus heal with a characteristic postnatal scar formation at gestational age that should heal without scar. The larger scarring fetal wound may have increased shear forces and mechanical stress, suggesting that biomechanical forces may be, in part, an underlying mechanism of the fetal regenerative phenotype. In fact it has been well established that during the remodeling phase of wound healing, myofibroblasts undergo apoptosis, which is thought to result from a release of mechanical tension after their contraction of the nascent matrix.80–82 Increased mechanical force induces local production of profibrotic growth factors, such as an increase in TGF-β1 and TGF-β2. Several studies have demonstrated that mechanical stretch can change the expression pattern of chemokines. IL-1β, tumor necrosis factor-α, IL-6, IL-8, COX-2, prostaglandin E2, pre-B-cell colony-enhancing factor, monocyte chemotactic and activating factor/MCP-1, granulocyte colony-stimulating factor, GM-CSF, and macrophage colony-stimulating factor are now well recognized as stretch-responsive genes.83,84 Interestingly, several studies have demonstrated in both in vitro and in vivo models that mechanical stretch increases the production of LMW HA as well as an increase in IL-8 production.85
The implications of decreasing transduction of profibrogenic mechanical stress are readily translated to postnatal wounds in the effort to ameliorate scar formation. Mechanomodulatory devices designed to offload wound stress have demonstrated a significant decrease in scarring with reduced area of fibrosis in large animal studies.86 However, the exact regulation of the chemokine expression by these devices has to be elucidated.
Future Directions
The studies reviewed here show that normal wound healing requires the sequential stimulation and resolution of multiple phases, which are influenced by regulatory molecules such as the chemokines. Chemokines play multiple roles in injury repair, not confined to their well-characterized roles in leukocyte chemotaxis and angiogenesis. It is becoming clear that chemokines also play an integral part in reepithelialization, granulation tissue formation, and remodeling of the healing wounds. Alteration in chemokine expression, longevity, and localization can result in pathological healing states. In a recent murine model, overproduction of CXCL10, very early after wounding, resulted in abnormal initiation and resolution of inflammation and impaired healing, presumably because the normal function of CXCL10 is to attract lymphocytes to the wound tissue in the later stages of healing to finalize the process. It has been suggested that the change in the expression of this chemokine resulted in the presence of lymphocytes at the same time as neutrophils and macrophages, leading to confusion in the orchestrated progression of wound healing, ultimately resulting in impaired healing. Chemokines may also play a critical role in the pathogenesis of chronic wounds, not only by altering the inflammatory and angiogenesis pathways, but also through their role in combating with biofilm forming multicellular organisms. In addition, chemokine overexpression has been demonstrated to be associated with fibrosis in multiple organs. CXCL1, 2 and their receptors CXCR1, 2, respectively, have been shown to be increased in burn wounds and keloids, which may contribute to the excessive scarring seen in these conditions. Overexpression of CXCL8 and CCL3 in the lavage fluid of lungs is characteristic of progressive idiopathic pulmonary fibrosis in patients.87 CXCL10 and CXCL9 have been shown to be upregulated in bleomycin-induced lung fibrosis. CXCL1, 5, and 8 are overexpressed in fibrotic pancreatitis.88 Targeted manipulation of the chemokine activity can be a novel therapeutic strategy to reduce the fibrosis in these diseases. In this context, understanding the differences in the chemokine profile in fetal versus adult wounds can take us one step closer to using the fetal regenerative wound healing as a roadmap to recapitulate scarless healing in postnatal wounds and, in fact, in any process characterized by excessive fibrosis.
Take Home Messages.
Basic science advances
Chemokines are a family of small cytokines that could be considered as one of the major regulators of wound healing. They play a significant role as soluble mediators and adhesion factors involved in the recruitment and trafficking of inflammatory as well as tissue-resident cells. Chemokine expression and function vary in a temporal and spatial manner during the different phases of wound healing. They are both mechanosensitive and mechanomodulatory. They regulate the sequential orchestration of wound healing by governing angiogenesis, granulation tissue production, and matrix catabolism that together mediate the remodeling of the wound tissue.
The midgestation fetus is capable of regenerative wound healing and is characterized by an anti-inflammatory milieu with decreased levels of IL-8 and IL-6, decreased neovascularization, and significantly reduced mechanical stresses, suggesting a preeminent role for chemokines in regulating the fetal scarless phenotype. Further understanding of this can lead to the development of targeted therapeutics to recapitulate fetal-like scarless wound healing in postnatal wounds with multiple etiologies.
Clinical science advance
Chemokine activation of G-protein receptors makes them very amenable to the development of small molecules that serve as agonists or antagonists of function for therapeutic wound healing purposes. In addition, neutralizing antibodies directed to either the chemokine or its receptor can be added to the wounds to promote wound healing. For example, CCR5 and CXCR4 antagonists are being tested in several preclinical animal models and in clinical trials to improve healing outcomes.
More studies on the role of chemokines and their receptors need to be performed to use them effectively in the clinic.
Relevance to clinical care
The therapeutic applications of chemokines may be beneficial to any pathology that is characterized by unresolved wound healing and excess fibrosis, including cancer, chronic wounds, pulmonary fibrosis, renal fibrosis, hypertrophic scarring, and keloids. Chronic inflammation and alteration in angiogenesis can potentially be reduced or eliminated by interfering with proinflammatory or angiogenic chemokines and/or their receptors. Similarly, neutralization of profibrotic chemokine activity can greatly reduce fibrosis. However, one chemokine may have different downstream effects on different cell types in the wound, and targeted inhibition/or activation of chemokines may alter multiple wound outcomes. Thus, further detailed study of the mechanisms and effects of chemokine manipulation is necessary before targeting these pathways and can become a clinical reality.
Abbreviations and Acronyms
- COX
cyclooxygenase
- E15
embryonic day 15
- ECM
extracellular matrix
- EPC
endothelial progenitor cell
- Gro-α
growth-related oncogene alpha
- HA
hyaluronan
- IL
interleukin
- LMW
low molecular weight
- MCP
monocyte chemoattractant protein
- MIP-1α or β
macrophage inflammatory protein-1 (MIP-1α=CCL3; MIP-1β=CCL4)
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cell
- RANTES
regulated on activation, normal T expressed and secreted (CCL5)
- SDF-1α
stromal cell-derived factor-1 alpha
- TGFβ
transforming growth factor beta
- TIMP
tissue inhibitors of metalloproteinases
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
This work is supported by the NIH/NIGMS awards K08 GM098831-03 and 1R01GM111808-01 (S.G.K.), the Wound Healing Society Foundation 3M Award (S.G.K.), the NIH/NIDDK award 1R01DK096087 (P.L.B.), and the NIH/NHLBI award 1R01HL113294-01A1 (P.L.B.).
Author Disclosure and Ghostwriting
There are no competing financial interests. The content of this article is expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Swathi Balaji, PhD, is a postdoctoral research fellow in the laboratory for regenerative wound healing at CCHMC specializing in mechanisms underlying the fetal regenerative wound healing. Carey L. Watson, MD, is a University of Cincinnati General Surgery Resident who is completing a two-year postdoctoral research fellowship at Cincinnati Children's Hospital Medical Center (CCHMC). Rajeev Ranjan, PhD, is a postdoctoral research fellow in the laboratory for regenerative wound healing at CCHMC. Alice King, MD, is a University of Cincinnati General Surgery Resident who has completed a two-year postdoctoral research fellowship in the laboratory for regenerative wound healing at CCHMC. Paul L. Bollyky, MD, DPhil, is an immunologist and infectious disease specialist at Stanford University whose laboratory has a special interest in the relationship of inflammation and the extracellular matrix in wound healing. Sundeep G. Keswani, MD, is a pediatric and fetal surgeon at CCHMC. He is the Principal Investigator of the laboratory for regenerative wound healing at CCHMC focused on elucidating the mechanisms underlying the fetal regenerative phenomenon and is also the director of Pediatric Wound Care Center at CCHMC.
References
- 1.Leung A, Crombleholme TM, Keswani SG. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediat 2012;24:371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martins-Green M, Petreaca M, Wang L. Chemokines and their receptors are key players in the orchestra that regulates wound healing. Adv Wound Care 2013;2:327–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ 2003;326:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sund B. New Developments in Wound Care. London, United Kingdom: PJB Publications, 2000:1–255 [Google Scholar]
- 5.Brown BC, McKenna SP, Siddhi K, McGrouther DA, Bayat A. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg 2008;61:1049–1058 [DOI] [PubMed] [Google Scholar]
- 6.Anisowicz A, Bardwell L, Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A 1987;84:7188–7192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deuel TF, Keim PS, Farmer M, Heinrikson RL. Amino acid sequence of human platelet factor 4. Proc Natl Acad Sci U S A 1977;74:2256–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugano S, Stoeckle MY, Hanafusa H. Transformation by Rous sarcoma virus induces a novel gene with homology to a mitogenic platelet protein. Cell 1987;49:321–328 [DOI] [PubMed] [Google Scholar]
- 9.Fivenson DP, Faria DT, Nickoloff BJ, et al. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Repair Regen 1997;5:310–322 [DOI] [PubMed] [Google Scholar]
- 10.Vinader V, Afarinkia K. A beginner's guide to chemokines. Future Med Chem 2012;4:845–852 [DOI] [PubMed] [Google Scholar]
- 11.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009;29:313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin F, Nguyen CM, Wang SJ, Saadi W, Gross SP, Jeon NL. Effective neutrophil chemotaxis is strongly influenced by mean IL-8 concentration. Biochem Biophys Res Commun 2004;319:576–581 [DOI] [PubMed] [Google Scholar]
- 13.Ebisuno Y, Tanaka T, Kanemitsu N, et al. Cutting edge: the B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer's patches and affects B cell trafficking across high endothelial venules. J Immunol 2003;171:1642–1646 [DOI] [PubMed] [Google Scholar]
- 14.Mehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thromb Haemost 2007;97:755–762 [PMC free article] [PubMed] [Google Scholar]
- 15.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity 2002;16:1–4 [DOI] [PubMed] [Google Scholar]
- 16.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol 2007;25:787–820 [DOI] [PubMed] [Google Scholar]
- 17.Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol 2000;156:1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olutoye OO, Yager DR, Cohen IK, Diegelmann RF. Lower cytokine release by fetal porcine platelets: a possible explanation for reduced inflammation after fetal wounding. J Pediatr Surg 1996;31:91–95 [DOI] [PubMed] [Google Scholar]
- 19.Olutoye OO, Barone EJ, Yager DR, Uchida T, Cohen IK, Diegelmann RF. Hyaluronic acid inhibits fetal platelet function: implications in scarless healing. J Pediatr Surg 1997;32:1037–1040 [DOI] [PubMed] [Google Scholar]
- 20.Roubelakis MG, Trohatou O, Roubelakis A, et al. Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev 2014;10:417–428 [DOI] [PubMed] [Google Scholar]
- 21.Rolfe KJ, Grobbelaar AO. A review of fetal scarless healing. ISRN Dermatol 2012;2012:698034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 1998;153:1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol 2001;69:513–521 [PubMed] [Google Scholar]
- 24.Conus S, Perozzo R, Reinheckel T, et al. Caspase-8 is activated by cathepsin D initiating neutrophil apoptosis during the resolution of inflammation. J Exp Med 2008;205:685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petreaca ML, Yao M, Liu Y, Defea K, Martins-Green M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell 2007;18:5014–5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol 2001;280:L1094–L1103 [DOI] [PubMed] [Google Scholar]
- 27.Patel DD, Koopmann W, Imai T, Whichard LP, Yoshie O, Krangel MS. Chemokines have diverse abilities to form solid phase gradients. Clin Immunol 2001;99:43–52 [DOI] [PubMed] [Google Scholar]
- 28.Proudfoot AE, Handel TM, Johnson Z, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A 2003;100:1885–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satish L, Kathju S. Cellular and molecular characteristics of scarless versus fibrotic wound healing. Dermatol Res Pract 2010;2010:790234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo DD, Zimmermann AS, Nauta A, Longaker MT, Lorenz HP. Scarless fetal skin wound healing update. Birth Defects Res 2012;96:237–247 [DOI] [PubMed] [Google Scholar]
- 31.Naik-Mathuria B, Gay AN, Zhu X, Yu L, Cass DL, Olutoye OO. Age-dependent recruitment of neutrophils by fetal endothelial cells: implications in scarless wound healing. J Pediatr Surg 2007;42:166–171 [DOI] [PubMed] [Google Scholar]
- 32.Olutoye OO, Zhu X, Cass DL, Smith CW. Neutrophil recruitment by fetal porcine endothelial cells: implications in scarless fetal wound healing. Pediatr Res 2005;58:1290–1294 [DOI] [PubMed] [Google Scholar]
- 33.Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine 2000;12:671–676 [DOI] [PubMed] [Google Scholar]
- 34.Hurst SM, Wilkinson TS, McLoughlin RM, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001;14:705–714 [DOI] [PubMed] [Google Scholar]
- 35.Ryan GB, Majno G. Acute inflammation. A review. Am J Pathol 1977;86:183–276 [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood 2003;102:328–335 [DOI] [PubMed] [Google Scholar]
- 37.Kaesler S, Regenbogen J, Durka S, Goppelt A, Werner S. The healing skin wound: a novel site of action of the chemokine C10. Cytokine 2002;17:157–163 [DOI] [PubMed] [Google Scholar]
- 38.Cowin AJ, Brosnan MP, Holmes TM, Ferguson MW. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev Dyn 1998;212:385–393 [DOI] [PubMed] [Google Scholar]
- 39.Juremalm M, Nilsson G. Chemokine receptor expression by mast cells. Chem Immunol Allergy 2005;87:130–144 [DOI] [PubMed] [Google Scholar]
- 40.Trautmann A, Toksoy A, Engelhardt E, Brocker EB, Gillitzer R. Mast cell involvement in normal human skin wound healing: expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J Pathol 2000;190:100–106 [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Schrementi ME, Ranzer MJ, Wilgus TA, DiPietro LA. Blockade of mast cell activation reduces cutaneous scar formation. PLoS One 2014;9:e85226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wulff BC, Parent AE, Meleski MA, DiPietro LA, Schrementi ME, Wilgus TA. Mast cells contribute to scar formation during fetal wound healing. J Invest Dermatol 2012;132:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer M, Harvima IT, Carvalho RF, et al. Mast cell CD30 ligand is upregulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion. J Clin Invest 2006;116:2748–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvima IT, Nilsson G. Mast cells as regulators of skin inflammation and immunity. Acta Derm Venereol 2011;91:644–650 [DOI] [PubMed] [Google Scholar]
- 45.Ishida Y, Gao JL, Murphy PM. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J Immunol 2008;180:569–579 [DOI] [PubMed] [Google Scholar]
- 46.Dang CM, Beanes SR, Lee H, Zhang X, Soo C, Ting K. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast Reconstr Surg 2003;111:2273–2285 [DOI] [PubMed] [Google Scholar]
- 47.Bullard KM, Cass DL, Banda MJ, Adzick NS. Transforming growth factor beta-1 decreases interstitial collagenase in healing human fetal skin. J Pediatr Surg 1997;32:1023–1027 [DOI] [PubMed] [Google Scholar]
- 48.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol 2007;82:1375–1381 [DOI] [PubMed] [Google Scholar]
- 49.Wulff BC, Yu L, Parent AE, Wilgus TA. Novel differences in the expression of inflammation-associated genes between mid- and late-gestational dermal fibroblasts. Wound Repair Regen 2013;21:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem 1996;271:17779–17784 [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T, Eckes B, Mauch C, Hartmann K, Krieg T. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loop. J Immunol 2000;164:6174–6179 [DOI] [PubMed] [Google Scholar]
- 52.Feugate JE, Wong L, Li QJ, Martins-Green M. The CXC chemokine cCAF stimulates precocious deposition of ECM molecules by wound fibroblasts, accelerating development of granulation tissue. BMC Cell Biol 2002;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feugate JE, Li Q, Wong L, Martins-Green M. The cxc chemokine cCAF stimulates differentiation of fibroblasts into myofibroblasts and accelerates wound closure. J Cell Biol 2002;156:161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res 1998;77:80–84 [DOI] [PubMed] [Google Scholar]
- 55.Hu MS, Januszyk M, Hong WX, et al. Gene expression in fetal murine keratinocytes and fibroblasts. J Surg Res 2014;190:344–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaja-Milatovic S, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol 2008;23:1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nanney LB, Mueller SG, Bueno R, Peiper SC, Richmond A. Distributions of melanoma growth stimulatory activity of growth-regulated gene and the interleukin-8 receptor B in human wound repair. Am J Pathol 1995;147:1248–1260 [PMC free article] [PubMed] [Google Scholar]
- 58.Devalaraja RM, Nanney LB, Du J, et al. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol 2000;115:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rennekampff HO, Hansbrough JF, Kiessig V, Dore C, Sticherling M, Schroder JM. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J Surg Res 2000;93:41–54 [DOI] [PubMed] [Google Scholar]
- 60.Rennekampff HO, Hansbrough JF, Woods V, Jr., Dore C, Kiessig V, Schroder JM. Role of melanoma growth stimulatory activity (MGSA/gro) on keratinocyte function in wound healing. Arch Dermatol Res 1997;289:204–212 [DOI] [PubMed] [Google Scholar]
- 61.Romer J, Bugge TH, Pyke C, et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med 1996;2:287–292 [DOI] [PubMed] [Google Scholar]
- 62.Satish L, Blair HC, Glading A, Wells A. Interferon-inducible protein 9 (CXCL11)-induced cell motility in keratinocytes requires calcium flux-dependent activation of mu-calpain. Mol Cell Biol 2005;25:1922–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inokuma D, Abe R, Fujita Y, et al. CTACK/CCL27 accelerates skin regeneration via accumulation of bone marrow-derived keratinocytes. Stem Cells 2006;24:2810–2816 [DOI] [PubMed] [Google Scholar]
- 64.Zuliani T, Saiagh S, Knol AC, Esbelin J, Dreno B. Fetal fibroblasts and keratinocytes with immunosuppressive properties for allogeneic cell-based wound therapy. PLoS One 2013;8:e70408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carmeliet P. Angiogenesis in health and disease. Nat Med 2003;9:653–660 [DOI] [PubMed] [Google Scholar]
- 66.Goebeler M, Yoshimura T, Toksoy A, Ritter U, Brocker EB, Gillitzer R. The chemokine repertoire of human dermal microvascular endothelial cells and its regulation by inflammatory cytokines. J Invest Dermatol 1997;108:445–451 [DOI] [PubMed] [Google Scholar]
- 67.DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest 1998;101:1693–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 2005;11:1351–1354 [DOI] [PubMed] [Google Scholar]
- 69.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 2009;10:207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, and Shimizu H: Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008;180:2581. [DOI] [PubMed] [Google Scholar]
- 71.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004;10:858–864 [DOI] [PubMed] [Google Scholar]
- 72.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 2009;60:813–823 [DOI] [PubMed] [Google Scholar]
- 73.Ito H. Chemokines in mesenchymal stem cell therapy for bone repair: a novel concept of recruiting mesenchymal stem cells and the possible cell sources. Mod Rheumatol 2011;21:113–121 [DOI] [PubMed] [Google Scholar]
- 74.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 2004;110:3300–3305 [DOI] [PubMed] [Google Scholar]
- 75.Kollar K, Cook MM, Atkinson K, Brooke G. Molecular mechanisms involved in mesenchymal stem cell migration to the site of acute myocardial infarction. Int J Cell Biol 2009;2009:904682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Backly RM, Cancedda R. Bone marrow stem cells in clinical application: harnessing paracrine roles and niche mechanisms. Adv Biochem Eng/Biotechnol 2010;123:265–292 [DOI] [PubMed] [Google Scholar]
- 77.Zhou SB, Wang J, Chiang CA, Sheng LL, Li QF. Mechanical stretch upregulates SDF-1alpha in skin tissue and induces migration of circulating bone marrow-derived stem cells into the expanded skin. Stem Cells 2013;31:2703–2713 [DOI] [PubMed] [Google Scholar]
- 78.Ishida Y, Kimura A, Kuninaka Y, et al. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest 2012;122:711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein JD, Turner CG, Steigman SA, et al. Amniotic mesenchymal stem cells enhance normal fetal wound healing. Stem Cells Dev 2011;20:969–976 [DOI] [PubMed] [Google Scholar]
- 80.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 2005;13:7–12 [DOI] [PubMed] [Google Scholar]
- 81.Bride J, Viennet C, Lucarz-Bietry A, Humbert P. Indication of fibroblast apoptosis during the maturation of disc-shaped mechanically stressed collagen lattices. Arch Dermatol Res 2004;295:312–317 [DOI] [PubMed] [Google Scholar]
- 82.Carlson MA, Longaker MT, Thompson JS. Wound splinting regulates granulation tissue survival. J Surg Res 2003;110:304–309 [DOI] [PubMed] [Google Scholar]
- 83.Kendal-Wright CE. Stretching, mechanotransduction, and proinflammatory cytokines in the fetal membranes. Reprod Sci 2007;14:35–41 [DOI] [PubMed] [Google Scholar]
- 84.Okada M, Matsumori A, Ono K, et al. Cyclic stretch upregulates production of interleukin-8 and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler Thromb Vasc Biol 1998;18:894–901 [DOI] [PubMed] [Google Scholar]
- 85.Mascarenhas MM, Day RM, Ochoa CD, et al. Low molecular weight hyaluronan from stretched lung enhances interleukin-8 expression. Am J Respir Cell Mol Biol 2004;30:51–60 [DOI] [PubMed] [Google Scholar]
- 86.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg 2011;254:217–225 [DOI] [PubMed] [Google Scholar]
- 87.Antoniou KM, Tzanakis N, Tzortzaki EG, et al. Different angiogenic CXC chemokine levels in bronchoalveolar lavage fluid after interferon gamma-1b therapy in idiopathic pulmonary fibrosis patients. Pulm Pharmacol Ther 2008;21:840–844 [DOI] [PubMed] [Google Scholar]
- 88.Saurer L, Reber P, Schaffner T, et al. Differential expression of chemokines in normal pancreas and in chronic pancreatitis. Gastroenterology 2000;118:356–367 [DOI] [PubMed] [Google Scholar]