Abstract
Our previous study suggests that ginger root extract can reverse behavioral dysfunction and prevent Alzheimer's disease (AD)-like symptoms induced by the amyloid-β protein (Aβ) in a rat model. 6-Gingerol is the major gingerol in ginger rhizomes, but its effect on the treatment of AD remains unclear. In this study, we aimed to determine if 6-gingerol had a protective effect on Aβ1–42-induced damage and apoptotic death in rat pheochromocytoma cells (PC12 cells) and to investigate the underlying mechanisms by which 6-gingerol may exert its neuroprotective effects. Our results indicated that pre-treatment with 6-gingerol significantly increased cell viability and reduced cell apoptosis in Aβ1–42-treated cells. Moreover, 6-gingerol pretreatment markedly reduced the level of intracellular reactive oxygen species (ROS) and malondialdehyde (MDA), the production of nitric oxide (NO), and the leakage of lactate dehydrogenase (LDH) and increased superoxide dismutase (SOD) activity compared with the Aβ1–42 treatment group. In addition, 6-gingerol pretreatment also significantly enhanced the protein levels of phosphorylated Akt (p-Akt) and glycogen synthase kinase-3β (p-GSK-3β). Overall, these results indicate that 6-gingerol exhibited protective effects on apoptosis induced by Aβ1–42 in cultured PC12 cells by reducing oxidative stress and inflammatory responses, suppressing the activation of GSK-3β and enhancing the activation of Akt, thereby exerting neuroprotective effects. Therefore, 6-gingerol may be useful in the prevention and/or treatment of AD.
Introduction
Alzheimer's disease (AD), the most common form of dementia, is a progressive neurodegenerative disorder of the brain characterized by progressive memory impairment, disordered cognitive function, and altered behavior.1 Data have suggested that there are 26.6 million AD patients as of 2009, and this number will quadruple by 2050 if no cure or preventive measure is found.2 Currently, no effective anti-AD drugs are available to either stop or reverse the progression of AD, although the development of anti-AD drugs has been slightly successful in aspects of symptomatic improvement, such as the development of acetylcholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists.3,4
AD is characterized pathologically by deposition of extracellular senile plaques and intracellular neurofibrillary tangles.5 Amyloid-β (Aβ), the major component of senile plaques, is derived from sequential proteolysis of the amyloid precursor protein by sequential cleavages of β-secretase and γ-secretase and plays a critical role in the pathophysiology of AD.6,7 Despite the abundance of studies that have been designed to investigate the underlying mechanisms of the neurotoxicity induced by Aβ, the precise mechanisms still remain unclear. Accumulating evidence suggests that oxidative stress and inflammatory responses are the major mechanisms of Aβ-induced neurotoxicity.8,9 For instance, the accumulation of Aβ in primary neurons could induce oxidative stress.10 Aβ accelerates additional inflammatory pathways by activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1β, tumor necrosis factor-α, and a nuclear factor-κB (NF-κB) mechanism in the rat brain.11 Moreover, evidence also indicates that Aβ-induced neurotoxicity is associated with the PI3K/Akt/GSK-3β signaling pathway.12,13 Therefore, investigating novel anti-AD drugs to reduce the Aβ-induced neurotoxicity may aid in AD prevention and/or treatment.
Ginger (Zingiber officinale Roscoe), the rhizome of the plant Z. officinale, is one of the most popular species and has been utilized in traditional medicines, especially in Asia and Africa.14 Our preliminary study reveals that ginger root extract could reverse behavioral dysfunction and prevent AD-like symptoms in a rat model of AD.15 6-Gingerol, a phenolic compound, is the major gingerol in ginger rhizomes with diverse pharmacological activities, including anti-tumor, anti-inflammatory, and anti-oxidant effects.16–19 In a recent study, 6-gingerol exhibited neuroprotective effects against cell apoptosis induced by Aβ through its anti-oxidative role.20
In this study, we aimed to determine if 6-gingerol exhibited protective effects of 6-gingerol on Aβ1–42-induced damages and apoptotic death in pheochromocytoma cells (PC12) cells, and not only investigated the role of 6-gingerol in the process of anti-oxidative stress but also proved its role of anti-inflammatory responses and Akt/GSK-3β signaling pathway to investigate the underlying mechanisms by which 6-gingerol may exert its neuroprotective effects.
Materials and methods
Materials
The PC12 cell line was obtained from the Shanghai Institute of Cell Biology at the Chinese Academy of Sciences (Shanghai, China). 6-Gingerol (C17H26O4, purity ≥98%) was purchased from Shanghai Fu life Industry Co. Ltd; Aβ1–42 was purchased from Wuhan Moon Biosciences Co. Ltd; Ham's F-12K (Kaighn's) Medium was purchased from BOSTER (Wuhan, China); fetal bovine serum (FBS) and horse serum were obtained from Gibco (Grand Island, NY); 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was obtained from Amresco (Solon, OH); nerve growth factor 2.5S protein (NGF) was obtained from Merck Millipore (Billerica, MA); the Annexin V-FITC/PI Apoptosis Detection Kit was purchased from KeyGEN BioTECH (Nanjing, China); Hoechst 33258 was obtained from the Beyotime Institute of Biotechnology (Shanghai, China); the reactive oxygen species (ROS) assay kit, nitric oxide (NO) assay kit, lactate dehydrogenase (LDH) assay kit, superoxide dismutase (SOD) assay kit, and cell malondialdehyde (MDA) assay kit were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China); the phospho-Akt (Ser473) antibody, Akt antibody, GSK-3β (Ser9) antibody, and GSK-3β rabbit monoclonal antibody (mAb) were obtained from Cell Signaling Technology (Danvers, MA); and the anti-β-actin rabbit polyclonal antibody was purchased from EarthOx (Millbrae, CA).
Preparation of aggregated Aβ
Aβ1–42 was dissolved in deionized distilled water at a concentration of 1 mM and incubated at 37°C for 3 days to form aggregated amyloid. After aggregation, the solution was stored at −20°C until use.
Cell culture
PC12 cells were cultured in F12K medium containing 10% horse serum, 5% fetal bovine serum (FBS), and 100 U/mL of penicillin-streptomycin at 37°C with a 5% CO2 atmosphere in a humidified incubator. PC12 cells were sub-cultured approximately once a week and split 1:3 when the culture was 80–90% confluent.
MTT assay
PC12 cells were harvested from flasks and plated onto poly-D-lysine–coated 96-well plates with approximately 20,000 cells in 100 μL of serum free F12K medium supplemented with 50 ng/mL of nerve growth factor (NGF) per well. After 5 days of induced differentiation by NGF, the cells were treated with prepared Aβ1–42 (5, 10, and 20 μM) for 24, 48, and 72 hr, and cell viability was then evaluated with an MTT assay. To determine the protective effects of 6-gingerol on Aβ1–42-induced cell injury, PC12 cells were treated with 6-gingerol at different concentrations (40, 80, 120, 200, and 300 μM) for 4 hr, and the cells were then incubated with 10 μM Aβ1–42 for another 48 hr. Then, the cells were incubated in fresh F12K medium containing 0.5 mg/mL MTT at 37°C for 4 hr. Next, the reaction mixture was carefully removed, and 150 μL of dimethylsulfoxide (DMSO) was added to each well. The absorption values were read at 570 nm using a Multiskan Multiwell microplate reader (Thermo Scientific, USA), and the experiments were repeated in triplicate. The data were expressed as the mean percent of viable cells versus control.
Nuclear staining for cell apoptosis by Hoechst 33258
To detect the effect of 6-gingerol on Aβ1–42-induced cell apoptosis, the chromosomal condensation and morphological changes of the cell nucleus were assessed by staining with Hoechst 33258. The differentiated PC12 cells were treated with 6-gingerol at different concentrations (80, 120, and 200 μM) for 4 hr, after which 10 μM Aβ1–42 was added, and the cells were incubated for an additional 48 hr. After being washed with phosphate-buffered saline (PBS), the cells were fixed with immunostaining fix solution (Beyotime, Shanghai, China) at room temperature for 15 min. The cells were washed twice with PBS, and then stained with Hoechst 33258 fluorochrome (10 μg/mL) at 37°C for 10 min in the dark. After three rinses with PBS, the Hoechst-stained nuclei were imaged with a confocal laser scanning microscope (Nikon, Japan).
Flow cytometric analysis of cell apoptosis
Apoptosis was determined using an Annexin V-FITC/PI assay kit. Briefly, cells were harvested after treatment, rinsed twice with cold PBS, and then re-suspended in binding buffer at a density of 1 × 106 cells/mL. After incubation with 5 μL of Annexin V-FITC and 5 μL of propidium iodide (PI) working solution for 15 min in the dark at room temperature, the stained cells were analyzed with a flow cytometer (BD Biosciences, USA).
Measurement of intracellular ROS
Intracellular ROS were measured using the dichloro-dihydro-fluorescein diacetate (DCFH-DA) method. PC12 cells (1 × 105) were seeded in six-well plates, and differentiation was induced by NGF for 5 days in a CO2 incubator at 37°C. Then, the cells were treated with 6-gingerol at different concentrations (80, 120, and 200 μM) for 4 hr, after which 10 μM of Aβ1–42 was added, and the cells were incubated for another 48 hr. After the medium was removed, the cells were washed twice with serum-free F12K medium and then incubated with serum-free F12K medium containing 10 μM DCFH-DA in the dark at 37°C for 45 min. The cells were washed twice with PBS, and finally, fluorescence was measured by photofluorography and quantified with ImageJ software (NIH Image, Bethesda, MD).
NO, LDH, SOD, and MDA assay
PC12 cells were seeded in six-well plates, and differentiation was induced by NGF for 5 days in a CO2 incubator at 37°C. Then, the cells were treated with 6-gingerol at different concentrations (80, 120, and 200 μM) for 4 hr, after which 10 μM Aβ1–42 was added, and the cells were incubated for another 48 hr. At the end of the drug treatment, the culture supernatant and cells were collected separately by centrifugation. Then, the levels of NO, LDH, and SOD activity in the medium were measured using a NO assay kit, LDH assay kit, and SOD assay kit in accordance with the manufacturer's instructions, respectively. The level of intracellular MDA was also detected with an MDA assay kit according to the manufacturer's instructions.
Western blotting
PC12 cells were harvested and washed twice with PBS solution after drug treatment. Then, the cells were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM phenylmethylsulfonyl fluoride [PMSF], sodium orthovanadate, sodium fluoride, EDTA, and leupeptin). Protein concentrations in the supernatants were determined using the BCA Protein Assay kit according to the protocol provided by the manufacturer. Before immunoblotting, the cell lysate was boiled in SDS-polyacrylamide gel electrophoresis (PAGE) Sample Loading Buffer for 5 min. The cell lysates were electrophoresed by 8% SDS-PAGE for 2.5 hr at 60 V and then electrotransferred onto a nitrocellulose filter membrane. After blocking for 1 hr in a solution of 5% (wt/vol) bovine serum albumin (BSA) in Tris-buffered saline containing 0.05% Tween-20 (TBST) at room temperature, the membrane was subsequently incubated at 4°C overnight with the appropriate amount of primary antibodies against p-Akt (Ser 473), Akt, p-GSK-3β (Ser9), GSK-3β, and β-actin. After washing four times with TBST, the membrane was incubated with horseradish peroxidase–conjugated goat anti-rabbit immunoglobulin G (IgG) at room temperature for 1.5 hr and again washed four times. The blotted proteins were visualized using the electrochemiluminescence (ECL) western blotting detection reagents, and the band intensities were quantified with ImageJ software (NIH Image, Bethesda, MD).
Statistical analysis
All of the data are expressed as the means ± standard deviation (SD). The significance was determined by one-way analysis of variance (ANOVA), and the least significant difference (LSD) multiple-range tests were used to analyze the differences between groups. The data were analyzed using SPSS 16.0 software, and a value of p < 0.05 or p < 0.01 was considered to indicate a statistically significant difference.
Results
MTT assay for cell viability
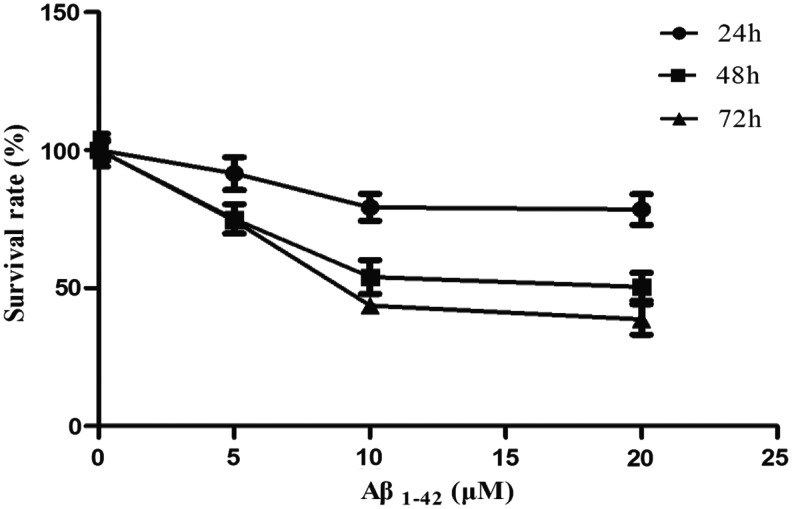
PC12 cells were treated with various concentrations of Aβ1–42 (5, 10, and 20 μM) for 24 hr, 48 hr, and 72 hr, and cell viability was evaluated with an MTT assay. Within different time groups, the cell survival rate decreased as dose increased, and the Aβ1–42 treatment group of 10 and 20 μM significantly reduced the viability of PC12 cells compared with the control group. The cell survival rate also decreased as time increased, and the viability of the PC12 cells in Aβ1–42 treatment groups at 48 hr and 72 hr were significantly reduced compared with the 24-hr groups, as shown in Fig. 1.
FIG. 1.
Effect of amyloid-β (Aβ1–42) on PC12 cell viability.
Effect of 6-gingerol on Aβ1–42-induced cell injury
To verify the protective effect of 6-gingerol on Aβ1–42-induced cell injury, the PC12 cells were pretreated with serial concentrations of 6-gingerol (40, 80, 120, 200, and 300 μM) for 4 hr before treatment with Aβ1–42 (10 μM) for 48 hr, and cellular viability was detected by MTT assay. As shown in Fig. 2, the Aβ1–42 treatment group significantly reduced the cell viability compared with the control group; the groups pretreated with 80, 120, and 200 μM of 6-gingerol exhibited significantly higher cell survival rates compared with the Aβ1–42 treatment group. However, the cell survival rate in the 300 μM 6-gingerol–pretreated group was lower than the Aβ1–42 treatment group. The results revealed that 6-gingerol could prevent PC12 cells from Aβ1–42-induced cell death and apoptosis. However, higher concentrations of 6-gingerol (300 μM) also had cytotoxic effects on PC12 cells. Therefore, we selected the optimal concentrations of 6-gingerol at 80, 120, and 200 μM for the following studies.
FIG. 2.
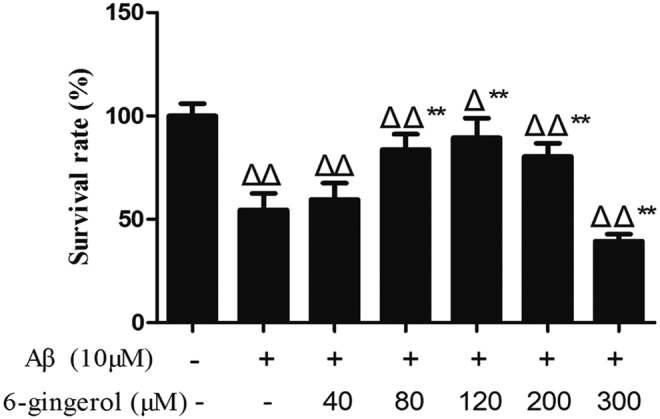
The MTT assay detects the effect of 6-gingerol on amyloid-β (Aβ1–42)-induced cell injury. PC12 cells were pretreated with or without 6-gingerol (40, 80, 120, 200, and 300 μM) for 4 hr before treatment with Aβ1–42 for 48 hr. The data are presented as the means ± standard deviation (SD) of four individual experiments. (Δ) p < 0.05 compared with the control group; (ΔΔ) p < 0.01 compared with the control group; (**) p < 0.01 compared with the Aβ1–42 treatment group.
Hoechst 33258 staining for cell apoptosis
Hoechst 33258 dye was used to assess the changes in nuclear morphology because it can diffuse through intact membranes of cells and stain their chromatin. We measured the effects of 6-gingerol on Aβ1–42-induced cell apoptosis through Hoechst 33258 nuclear staining. The nuclei of PC12 cells in the control group were uniformly stained and appeared to have a regular conformation except for a few abnormal nuclei (Fig. 3A), whereas most of the nuclei in the Aβ1–42 treatment group exhibited pyknosis and karyorrhexis (Fig. 3B), and this phenomenon was decreased in the 6-gingerol–pretreated group (Fig. 3C–E). The results suggested that 6-gingerol could attenuate Aβ1–42-induced cell apoptosis.
FIG. 3.
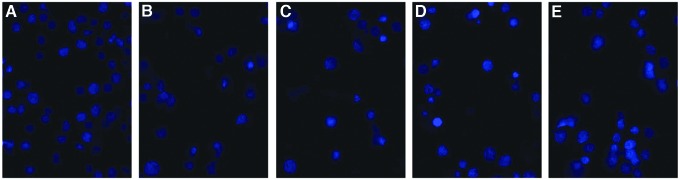
Hoechst 33258 staining measures the effect of 6-gingerol on amyloid-β (Aβ1–42)-induced apoptosis in PC12 cells. Confocal microscope images (magnification, 400 × ): (A) Blank control group; (B) Aβ1–42 (10 μM) group; (C) Aβ1–42 (10 μM) + 6-gingerol (80 μM) group; (D) Aβ1–42 (10 μM) + 6-gingerol (120 μM) group; (E) Aβ1–42 (10 μM) + 6-gingerol (200 μM) group. Color images available online at www.liebertpub.com/rej
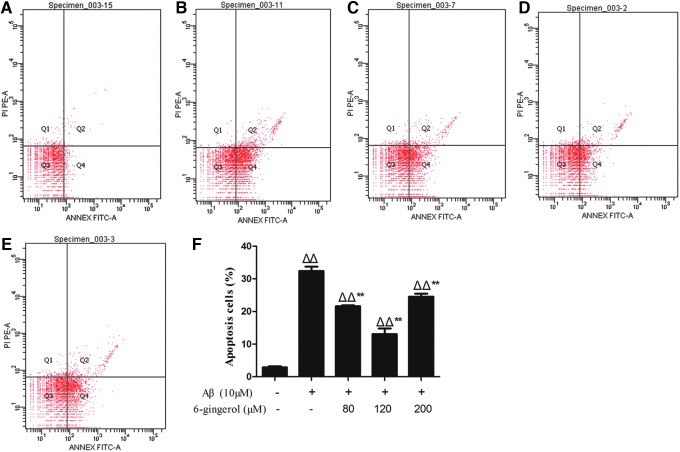
Effect of 6-gingerol on Aβ1–42-induced cell apoptosis by flow cytometry
The anti-apoptotic effects of 6-gingerol in PC12 cells were further studied using an Annexin V-FITC/PI assay. As shown in Fig. 4, flow cytometry detected that 2.87% of the cells were apoptotic in the control group, and cell apoptosis was significantly increased to 32.4% after treatment with 10 μM Aβ1–42. After pretreatment with 6-gingerol (80, 120, and 200 μM); however, apoptosis was significantly reduced to 21.67%, 13.07%, and 24.57%, respectively (p < 0.01).
FIG. 4.
Flow cytometric analysis. The effect of 6-gingerol on amyloid-β (Aβ1–42)-induced cell apoptosis. (A) Blank control group; (B) Aβ1–42 (10 μM) group; (C) Aβ1–42 (10 μM) + 6-gingerol (80 μM) group; (D) Aβ1–42 (10 μM) + 6-gingerol (120 μM) group; (E) Aβ1–42 (10 μM) + 6-gingerol (200 μM) group; (F) percentages of apoptotic cells. The data are presented as the means ± standard deviation (SD) of three individual experiments. (ΔΔ) p < 0.01 compared with the control group; (**) p < 0.01 compared with the Aβ1–42 treatment group. Color images available online at www.liebertpub.com/rej
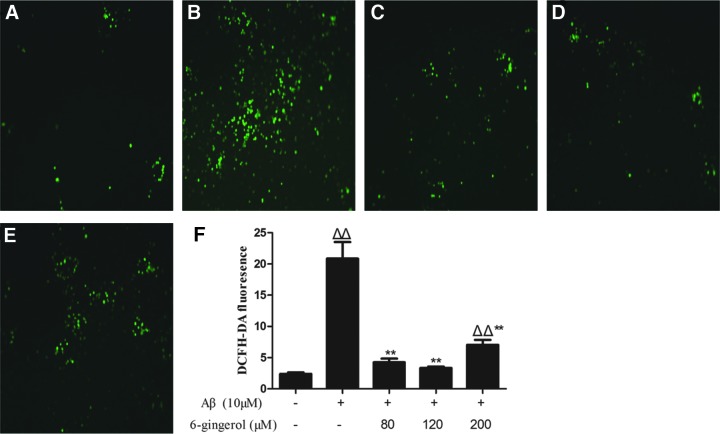
Effect of 6-gingerol on Aβ1–42-induced ROS production
To evaluate the effect of 6-gingerol on Aβ1–42-induced ROS, PC12 cells were pretreated with serial concentrations of 6-gingerol (80, 120, and 200 μM) for 4 hr before treatment with Aμ1–42 (10 μM) for 48 hr, and cellular viability was detected by fluorescence assay. As shown in Fig. 5, Aβ1–42 (10 μM) treatment for 48 hr induced a significant increase of the DCFH-DA fluorescence intensity in cells compared with the control group. After pretreatment with 6-gingerol (80, 120, and 200 μM), however, all of the DCFH-DA fluorescence intensity was decreased significantly. The results indicated that 6-gingerol could attenuate Aβ1–42-induced ROS production.
FIG. 5.
Effect of 6-gingerol on amyloid-β (Aβ1–42)-induced reactive oxygen species (ROS) production (magnification, 100 × ). (A) Blank control group; (B) Aβ1–42 (10 μM) group; (C) Aβ1–42 (10 μM) + 6-gingerol (80 μM) group; (D) Aβ1–42 (10 μM) + 6-gingerol (120 μM) group; (E) Aβ1–42 (10 μM) + 6-gingerol (200 μM) group; (F) quantitative analysis of the mean fluorescence intensity of dichloro-dihydro-fluorescein diacetate (DCFH-DA) from three random fields was performed using ImageJ software. The values are presented as the means ± standard deviation (SD). (Δ) p < 0.05 compared with the control group; (ΔΔ) p < 0.01 compared with the control group; (**) p < 0.01 compared with the Aβ1–42 treatment group. Color images available online at www.liebertpub.com/rej
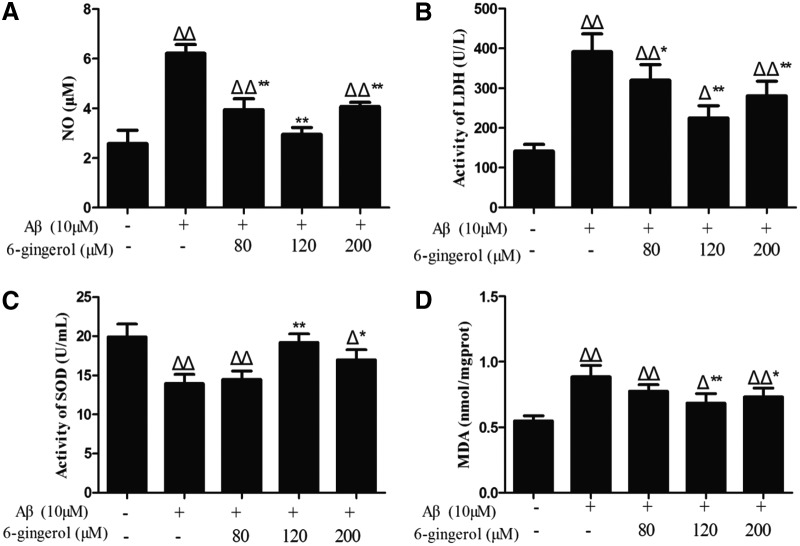
Effect of 6-gingerol on Aβ1–42-induced NO generation and LDH and SOD activity in the medium and intracellular MDA production
PC12 cells treated with Aβ1–42 for 48 hr showed a significant increase in NO level in the supernatant compared with the control group (6.23 ± 0.35 μM versus 2.58 ± 0.53 μM, p < 0.01). After pretreatment with 6-gingerol (80, 120, and 200 μM), however, the levels of NO were decreased to 3.95 ± 0.43 μM, 2.95 ± 0.28 μM, and 4.08 ± 0.16 μM, respectively (Fig. 6A).
FIG. 6.
PC12 cells were pretreated with 6-gingerol (80, 120, and 200 μM) for 4 hr followed by exposure to 10 μM amyloid-β (Aβ1–42) for 48 hr. The data are presented as the means ± standard deviation (SD) (n = 3). (Δ) p < 0.05 compared with the control group; (ΔΔ) p < 0.01 compared with the control group; (*) p < 0.01 compared with the Aβ1–42 treatment group; (**) p < 0.01 compared with the Aβ1–42 treatment group.
As shown in Fig. 6B, a significant increase (p < 0.01) of LDH activity was found in the medium in the Aβ1–42 treatment group compared with the control group. After pretreatment with 6-gingerol (80, 120, and 200 μM) for 4 hr, the LDH activity was significantly decreased compared with the Aβ1–42 treatment group.
As shown in Fig. 6C, SOD activity in the medium was significantly decreased in the Aβ1–42 treatment group compared with the control group. Pretreatment with 6-gingerol (120 and 200 μM) inhibited the decrease of SOD activity caused by Aβ1–42.
As shown in Fig. 6D, treatment with Aβ1–42 (10 μM) for 48 hr led to a significant increased production of MDA in PC12 cells, which was decreased by pretreatment with 6-gingerol (120 and 200 μM).
Effect of 6-gingerol on Aβ1–42-induced phosphorylation of Akt and GSK-3β
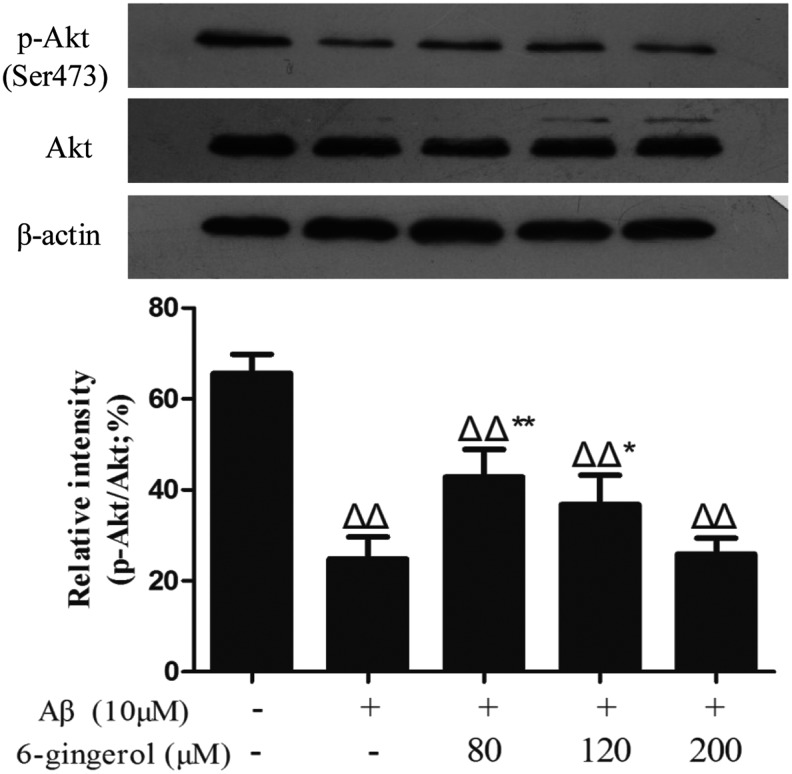
To determine the effects of 6-gingerol on Aβ1–42-induced activation of Akt and GSK-3β in PC12 cells, phosphorylated Akt (p-Akt) and phosphorylated GSK-3β (p-GSK-3β) were examined by western blot analysis using phospho-specific antibodies. The results showed that treatment with 10 μM Aβ1–42 for 48 hr significantly decreased the phosphorylation of Akt and GSK-3β compared with the control group, whereas pretreatment with 6-gingerol (80 and 120 μM) markedly inhibited the decrease in the expression of p-Akt and p-GSK-3β caused by Aβ1–42 (Figs. 7 and 8).
FIG. 7.
Effect of 6-gingerol on amyloid-β (Aβ1–42)-induced activation of Akt in PC12 cells. The data are presented as the means ± standard deviation (SD) of three individual experiments. (ΔΔ) p < 0.01 compared with the control group; (*) p < 0.05 compared with the Aβ1–42 treatment group; (**) p < 0.01 compared with the Aβ1–42 treatment group.
FIG. 8.
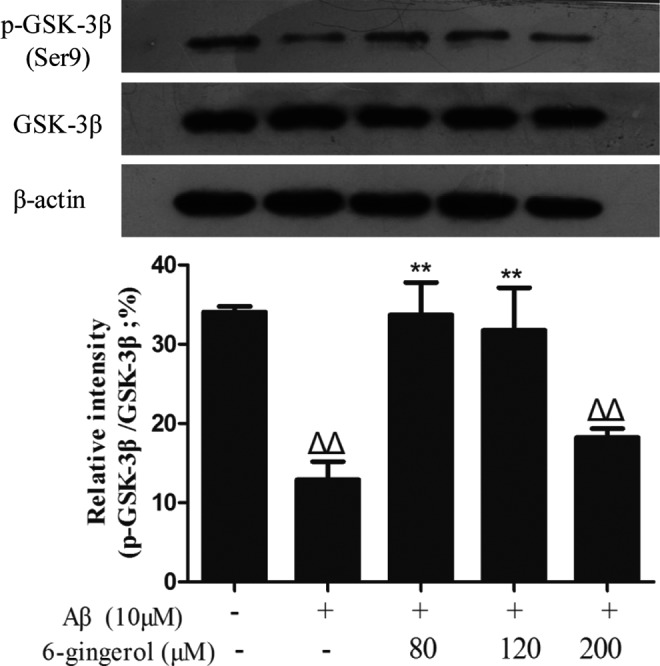
Effect of 6-gingerol on amyloid-β (Aβ1–42)-induced activation of GSK-3β in PC12 cells. The data are presented as the mean ± standard deviation (SD) of three individual experiments. (ΔΔ) p < 0.01 compared with the control group; (**) p < 0.01 compared with the Aβ1–42 treatment group.
Discussion
PC12 cells are clonal cells originating from a transplantable rat pheochromocytoma and exhibit the characteristics of neuronal cells in morphological, physiological, and biochemical aspects.21 Although undifferentiated PC12 cells do not have neurites or synapses, the cells respond to NGF with a dramatic change in phenotype and could acquire a number of properties characteristic of sympathetic neurons.22 In our experiment, PC12 cells were differentiated using NGF (50 ng/mL) for 5 days and were then used as the in vitro cell models to investigate the protective effects of 6-gingerol on Aβ-induced neurotoxicity.
Extensive studies into the apoptotic and necrotic processes induced by Aβ in neuronal cell lines have been performed.23–25 However, it is still unknown about the exact molecular mechanisms of Aβ-mediated neuronal apoptosis. Thus, our study was first started from the two aspects of cellular viability and survival rate. Figures 1 and 2 showed that the cell viability was decreased in the Aβ1–42 treatment group, whereas the cellular survival rate was markedly increased when pretreated with 6-gingerol (80, 120, and 200 μM). With Hoechst 33258 staining and flow cytometric analysis (Figs. 3 and 4), the apoptosis rate was significantly decreased in 6-gingerol–pretreated group (80, 120, and 200 μM) compared with the Aβ1–42 analysis group. These results revealed that 6-gingerol significantly attenuates Aβ1–42 -induced neurotoxicity by preventing cell damage.
Because the neuropathology of AD is widely associated with many factors such as inflammatory response and oxidative stress, we focused our study on whether 6-gingerol had the role of anti-inflammatory, anti-oxidative damage in PC12 cells induced by Aβ1–42. Studies have indicated that NO can generate a high level of pro-inflammatory cytokines to strengthen neurotoxicity, and this increase is consequently related to the development of AD.26 Aβ blocks the normally reparative effects of up-regulated vascular endothelial growth factor and nitric oxide synthases (NOS) and may accelerate in vivo vascular pathophysiology in AD.27 Excessive NO generated by NOS could strengthen the neurotoxicity because of the inhibition of glutamate reuptake, hence contributing to neuronal death and injury.28 In the study, it showed that 6-gingerol significantly reduced the levels of NO (Fig. 6A), indicating that 6-gingerol may have anti-inflammatory effects of attenuating the cytotoxicity of Aβ1–42 in PC12 cells.
In addition, oxidative stress is often defined as an imbalance between the cellular production of ROS and the ability of cells to efficiently defend against them.29 Studies suggest that Aβ exerts neuronal toxicity through the generation of excessive ROS following mitochondria superoxide accumulation.30 Oxidative stress can cause cellular damage because the ROS oxidizes vital cellular components, including lipids and nucleic acids, and consequently contributes to the pathophysiology of neurodegenerative diseases such as AD.31 The ROS can destroy the integrity of the neuronal cell membrane because of lipid oxidation, resulting in the release of bioactive substances into the extracellular space, such as LDH. Thus, LDH activity in the medium reflects the damage of cell membrane lipids. What's more, SOD, an endogenous anti-oxidant, has an important role in the reduction of oxidative stress and prevention of lipid damage.32 MDA is the degradation product of the oxygen-derived free radicals and lipid oxidation, and its levels are reflective of the overall levels of oxidative stress.15 In this study, it was first shown that 6-gingerol not only reduced the production of ROS, LDH, and MDA, but also increased the levels of SOD (Fig. 5 and Fig. 6B–D). This demonstrated that 6-gingerol may have a protective effect on PC12 cells induced by Aβ1–42 from two aspects via reducing the release of superoxide and the level of superoxide accumulation in mitochondria.
Recently, the notion that GSK-3β is associated with the neuropathology of AD has been generally accepted. Research has demonstrated that the hyper-activation of GSK-3β plays important roles in tau hyper-phosphorylation.33 Studies have also indicated that GSK-3β expression by Aβ relates to abnormal amyloid precursor protein (APP) processing and synaptic failure.34 The inhibition of GSK-3β reduces the β-site APP cleaving enzyme 1–mediated cleavage of APP through a NF-κB signaling-mediated mechanism, which subsequently reduces Aβ neuropathology and alleviates memory deficits in AD model mice.35 In addition, the pathological activation of GSK-3β has been reported to induce apoptosis and is inhibited by phosphorylation.36 Therefore, the up-regulation of p-GSK-3β inhibits its kinase's activity, and this may confer a protective effect on AD. Griffin RJ et al. (2005) indicated that the activation of Akt, shown by an increase in the expression of p-Akt, exhibited neuroprotective effects in AD.37 In addition, evidence suggests that the inhibition of the PI3K/Akt signaling pathway increases GSK-3β activity, resulting in tau protein hyperphosphorylation.38 In this experiment, our data showed that treatment with Aβ1–42 significantly suppressed the expression of p-GSK-3β (Ser9) and p-Akt (Ser473), whereas pre-treatment with 6-gingerol (80 and 120 μM) significantly inhibited the decreased expression of p-GSK-3β and p-Akt caused by Aβ1–42 (Figs. 7 and 8). These results suggested that 6-gingerol may enhance neuroprotection through up-regulating the phosphorylation levels of Akt and GSK-3β.
In physiological and pathological physiology, various processes are related to the AKT/GSK-3β pathway, including metabolic control, embryogenesis, cell death, and oncogenesis.39 Hyper-phosphorylated Tau is the major component of the paired helical filaments that accumulate in degenerating neurons in AD and other neurodegenerative diseases.40 Thus, the AKT/GSK-3β pathway seems to be vital for AD because it regulates Tau hyper-phosphorylation in cells. Our study indicated that 6-gingerol could activate the AKT/GSK-3β pathway through activation of AKT and inhibition of GSK-3β, indicating that 6-gingerol exerted neuroprotective effects through the AKT/GSK-3β pathway.
In summary, this study focused on three aspects to investigate the protective effects of 6-gingerol on inhibiting Aβ1–42-induced apoptosis in PC12 cells. First, the decreased levels of NO showed its anti-inflammatory role. Second, the results of ROS, MDA, SOD, and LDH assays reflected the anti-oxidative effects of 6-gingerol. Last, we clarified the molecular mechanisms of 6-gingerol on Aβ1–42-induced apoptosis in PC12 cells via Akt/GSK-3β signaling pathway in our experiments.
Ginger is generally considered a safe herbal medicine, and is on the FDA's Generally Recognized As Safe (GRAS) list.41,42 Moreover, ginger is found in a large variety of foods and can be ingested in considerable amounts (250 mg to 1 gram) daily in the human diet.43 Although some adverse effects of ginger have been reported in pregnant rats, no maternal toxicity was observed.44 As the major gingerol in ginger rhizomes, 6-gingerol might be reasonable in the treatment of AD patients without having any serious side effects.
Conclusions
In summary, our study revealed that 6-gingerol may exhibit a protective effect on the apoptosis induced by Aβ1–42 in PC12 cells, possibly by reducing oxidative stress and inflammatory responses, suppressing the activation of GSK-3β, and enhancing the activation of Akt through the AKT/GSK-3β pathway. Thus, 6-gingerol may be a valuable candidate drug for the treatment of AD. In addition, further studies are needed to investigate the underlying molecular mechanism concerning how 6-gingerol affects the progress of AD in vivo before clinical trials can be done.
Acknowledgments
We gratefully acknowledge the Guangxi postdoctoral special funds of Guangxi, China.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Selkoe DJ. Alzheimer's disease: Genes, proteins, and therapy. Physiol Rev 2001;81:741–766 [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 2007;3:186–191 [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Wang L, Su W, Xie XQ. Advances in recent patent and clinical trial drug development for Alzheimer's disease. Pharm Pat Anal 2014;3:429–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi X. Alzheimer disease: Update on basic mechanisms. J Am Osteopath Assoc 2010;110:S3–S9 [PubMed] [Google Scholar]
- 5.Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci 1997;20:154–159 [DOI] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791 [DOI] [PubMed] [Google Scholar]
- 7.Seubert P, Oltersdorf T, Lee MG, Barbour R, Blomquist C, Davis DL, Bryant K, Fritz LC, Galasko D, Thal LJ, Lieberburg I, Schenk DB. Secretion of beta-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature 1993;361:260–263 [DOI] [PubMed] [Google Scholar]
- 8.Couturier J, Paccalin M, Morel M, Terro F, Milin S, Pontcharraud R, Fauconneau B, Page G. Prevention of the beta-amyloid peptide-induced inflammatory process by inhibition of double-stranded RNA-dependent protein kinase in primary murine mixed co-cultures. J Neuroinflammation 2011;8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia M, Wang M, Yang Y, Chen Y, Liu D, Wang X, Song L, Wu J, Yang Y. rAAV/ABAD-DP-6His attenuates oxidative stress-induced injury of PC12 cells. Neural Regen Res 2014;9:481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldsbury C, Whiteman IT, Jeong EV, Lim YA. Oxidative stress increases levels of endogenous amyloid-beta peptides secreted from primary chick brain neurons. Aging Cell 2008;7:771–775 [DOI] [PubMed] [Google Scholar]
- 11.Carrero I, Gonzalo MR, Martin B, Sanz-Anquela JM, Arevalo-Serrano J, Gonzalo-Ruiz A. Oligomers of beta-amyloid protein (Abeta1-42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1beta, tumour necrosis factor-alpha, and a nuclear factor kappa-B mechanism in the rat brain. Exp Neurol 2012;236:215–227 [DOI] [PubMed] [Google Scholar]
- 12.Ryder J, Su Y, Ni B. Akt/GSK3beta serine/threonine kinases: Evidence for a signalling pathway mediated by familial Alzheimer's disease mutations. Cell Signal 2004;16:187–200 [DOI] [PubMed] [Google Scholar]
- 13.Xian YF, Lin ZX, Mao QQ, Chen JN, Su ZR, Lai XP, Ip PS. Isorhynchophylline protects PC12 cells against beta-amyloid-induced apoptosis via PI3K/Akt signaling pathway. Evid Based Complement Alternat Med 2013;2013:163057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baliga MS, Haniadka R, Pereira MM, D'Souza JJ, Pallaty PL, Bhat HP, Popuri S. Update on the chemopreventive effects of ginger and its phytochemicals. Crit Rev Food Sci Nutr 2011;51:499–523 [DOI] [PubMed] [Google Scholar]
- 15.Zeng GF, Zhang ZY, Lu L, Xiao DQ, Zong SH, He JM. Protective effects of ginger root extract on Alzheimer disease-induced behavioral dysfunction in rats. Rejuvenation Res 2013;16:124–133 [DOI] [PubMed] [Google Scholar]
- 16.Radhakrishnan EK, Bava SV, Narayanan SS, Nath LR, Thulasidasan AK, Soniya EV, Anto RJ. [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PloS One 2014;9:e104401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborty D, Mukherjee A, Sikdar S, Paul A, Ghosh S, Khuda-Bukhsh AR. [6]-Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol Lett 2012;210:34–43 [DOI] [PubMed] [Google Scholar]
- 18.Lee TY, Lee KC, Chen SY, Chang HH. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Biophys Res Commun 2009;382:134–139 [DOI] [PubMed] [Google Scholar]
- 19.Radhakrishnan EK, Bava SV, Narayanan SS, Nath LR, Thulasidasan AK, Soniya EV, Anto RJ. [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PLoS One 2014;9:e104401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee C, Park GH, Kim CY, Jang JH. [6]-Gingerol attenuates beta-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem Toxicol 2011;49:1261–1269 [DOI] [PubMed] [Google Scholar]
- 21.Tischler AS, Greene LA. Nerve growth factor-induced process formation by cultured rat pheochromocytoma cells. Nature 1975;258:341–342 [DOI] [PubMed] [Google Scholar]
- 22.Das KP, Freudenrich TM, Mundy WR. Assessment of PC12 cell differentiation and neurite growth: A comparison of morphological and neurochemical measures. Neurotoxicol Teratol 2004;26:397–406 [DOI] [PubMed] [Google Scholar]
- 23.Behl C, Davis JB, Klier FG, Schubert D. Amyloid beta peptide induces necrosis rather than apoptosis. Brain Res 1994;645:253–264 [DOI] [PubMed] [Google Scholar]
- 24.Geci C, How J, Alturaihi H, Kumar U. Beta-amyloid increases somatostatin expression in cultured cortical neurons. J Neurochem 2007;101:664–673 [DOI] [PubMed] [Google Scholar]
- 25.Hiddinga HJ, Eberhardt NL. Intracellular amyloidogenesis by human islet amyloid polypeptide induces apoptosis in COS-1 cells. Am J Pathol 1999;154:1077–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belkhelfa M, Rafa H, Medjeber O, Arroul-Lammali A, Behairi N, Abada-Bendib M, Makrelouf M, Belarbi S, Masmoudi AN, Tazir M, Touil-Boukoffa C. IFN-gamma and TNF-alpha are involved during alzheimer disease progression and correlate with nitric oxide production: A study in Algerian patients. J Interferon Cytokine Res 2014;34:839–847 [DOI] [PubMed] [Google Scholar]
- 27.Lin AJ, Liu G, Castello NA, Yeh JJ, Rahimian R, Lee G, Tsay V, Durkin AJ, Choi B, LaFerla FM, Chen Z, Green KN, Tromberg BJ. Optical imaging in an Alzheimer's mouse model reveals amyloid-dependent vascular impairment. Neurophotonics 2014;1:011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canzoniero LM, Granzotto A, Turetsky DM, Choi DW, Dugan LL, Sensi SL. nNOS(+) striatal neurons, a subpopulation spared in Huntington's Disease, possess functional NMDA receptors but fail to generate mitochondrial ROS in response to an excitotoxic challenge. Front Physiol 2013;4:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hroudova J, Singh N, Fisar Z. Mitochondrial dysfunctions in neurodegenerative diseases: Relevance to Alzheimer's disease. Biomed Res Int 2014;2014:175062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebenezer PJ, Weidner AM, LeVine H, 3rd, Markesbery WR, Murphy MP, Zhang L, Dasuri K, Fernandez-Kim SO, Bruce-Keller AJ, Gavilan E, Keller JN. Neuron specific toxicity of oligomeric amyloid-beta: Role for JUN-kinase and oxidative stress. J Alzheimers Dis 2010;22:839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001;40:959–975 [DOI] [PubMed] [Google Scholar]
- 32.Hassan HM. Biosynthesis and regulation of superoxide dismutases. Free Radic Biol Med 1988;5:377–385 [DOI] [PubMed] [Google Scholar]
- 33.Medina M, Avila J. New insights into the role of glycogen synthase kinase-3 in Alzheimer's disease. Expert Opin Ther Targets 2014;18:69–77 [DOI] [PubMed] [Google Scholar]
- 34.Deng Y, Xiong Z, Chen P, Wei J, Chen S, Yan Z. beta-amyloid impairs the regulation of N-methyl-D-aspartate receptors by glycogen synthase kinase 3. Neurobiol Aging 2014;35:449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ly PT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A, Zhang M, Yang Y, Cai F, Woodgett J, Song W. Inhibition of GSK3beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Invest 2013;123:224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem 1998;273:19929–19932 [DOI] [PubMed] [Google Scholar]
- 37.Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O'Connor R, O'Neill C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J Neurochem 2005;93:105–117 [DOI] [PubMed] [Google Scholar]
- 38.Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: Effects of FAD mutations. EMBO J 2004;23:2586–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu X, Wang S, Yu L, Yang H, Tan R, Yin K, Jin J, Zhao H, Guan D, Xu Y. TL-2 attenuates β-amyloid induced neuronal apoptosis through the AKT/GSK-3β/β-catenin pathway. Int J Neuropsychopharmacol 2014;17: 1511–1519 [DOI] [PubMed] [Google Scholar]
- 40.Kitagishi Y, Nakanishi A, Ogura Y, Matsuda S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer's disease. Alzheimers Res Ther. 2014; 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weidner MS, Sigwart K. The safety of a ginger extract in the rat. J Ethnopharmacol 2000;73:513–520 [DOI] [PubMed] [Google Scholar]
- 42.Kubra IR, Rao LJ. An impression on current developments in the technology, chemistry, and biological activities of ginger (Zingiber officinale Roscoe). Crit Rev Food Sci. 2012;52:651–688 [DOI] [PubMed] [Google Scholar]
- 43.O'Hara M, Kiefer D, Farrell K, Kemper K. A review of 12 commonly used medicinal herbs. Arch Fam Med 1998;7:523. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson JM. Effect of ginger tea on the fetal development of Sprague-Dawley rats. Reprod Toxicol 2000;14:507–512 [DOI] [PubMed] [Google Scholar]