Abstract
Host–pathogen interaction is an area of considerable interest. Intracellular parasites such as Leishmania reside inside phagocytes such as macrophages, dendritic cells and neutrophils. Macrophages can be activated by cytokines such as IFN-γ and Toll like receptor (TLR) agonists resulting in enhanced microbicidal activity. Leishmania parasites hijack the microbicidal function of macrophages, mainly by interfering with intracellular signaling initiated by IFN-γ and TLR ligands. Here we used transgenic Leishmania donovani parasites expressing the red fluorescent protein DsRed2 and imaging-flow cytometry technology to evaluate parasitic loads inside the macrophage in vitro. Further, this methodology enables us to visualize impairment in NFκB translocation to the nucleus in L. donovani infected macrophages. Additionally we show that uninfected bystander macrophages have a similar impairment in NFκB translocation as in L. donovani infected macrophages in response to the TLR4 agonist LPS. This evidence suggests a possible immunosuppressive role for infected macrophages in regulating the activation of uninfected bystander macrophages.
Keywords: Leishmania, Automated, Macrophages, Imaging, Flow cytometry, Intracellular signaling
1. Introduction
Leishmaniasis is caused by a protozoan of the genus Leishmania which is transmitted by the bite of the sandfly (Phlebotomus). The two main forms of the disease are cutaneous leishmaniasis (CL) caused by Leishmania major, Leishmania mexicana and Leishmania tropica; and visceral leishmaniasis (VL) caused by Leishmania donovani and Leishmania infantum. CL infection causes nonhealing lesions in the form of ulcers in the skin. In contrast, the spleen, liver and bone marrow are the main tissues infected during VL. VL patients can die from secondary infections or liver damage (Herwaldt, 1999). Leishmania infects and survives inside dendritic cells, neutrophils and macrophages thereby modulating their activation (Gupta et al., 2013; Terrazas et al., 2010). The interaction between parasites and host cells is critical in understanding how the parasite survives inside the phagocyte and prevents its elimination.
Lack of efficient and safe treatments have led to the study of new treatments for leishmaniasis. The study of Leishmania drugs is based first on in vitro screening studies of compounds with leishmanicidal activity mainly using infected macrophages.
Anti-leishmanicidal drugs are tested in vitro by infecting macrophages with Leishmania spp. and exposing them to different drugs. Evaluation of parasitic load can be determined by staining macrophages with Giemsa and counting parasites and cell nuclei under the microscope then estimating the infection index (Lezama-Dávila et al., 2014). The generation of transgenic parasites expressing fluorescent proteins (GFP, RFP, and DsRed) enables the use of flow cytometry to estimate parasitic loads more broadly and rapidly as the analysis is based on thousands of cells (Kram et al., 2008; Stenger and Zandbergen, 2011). Further, an indirect evidence of infection could be estimated using the mean fluorescence intensity of the infected population.
Different approaches have been used to study Leishmania–phagocyte interaction. Hijacking of intracellular signaling by Leishmania parasites has been studied by measuring phosphorylation and nuclear translocation of key molecules involved in the inflammatory pathway such as STAT1, NFκB and MAPK mainly by using Western blots (Gupta et al., 2013; Terrazas et al., 2010). However total lysates from infected and uninfected macrophages are usually used, lacking the ability to distinguish between infected or bystander macrophage responses.
Here we report the use of the new flow cytometer (Flowsight) which combines flow cytometry and imaging as a powerful tool to estimate parasitic loads in an automated manner. Additionally we report the use of Flowsight to evaluate Leishmania modulation of NFκB activation in macrophages, distinguishing activation between infected and bystander macrophages. Our data demonstrate a more accurate and automated method for intracellular parasite enumeration and these new methods described, represent a new tool to study host cell–parasite interactions.
2. Materials and methods
2.1. Bone marrow derived macrophages
All mice used were maintained in a pathogen free animal facility at The Ohio State University in accordance with NIH and institutional guidelines. Bone marrow-derived macrophages were obtained as previously published (Lezama-Dávila et al., 2014). In brief, tibia and femur of BALB/c mice were obtained and marrow wash flushed with PBS. Cells were recovered and plated at 1 × 106/ml (RPMI 1640 medium containing 10% (v/v) L199 cell supernatant, 10% FBS, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, and 1× penicillin/streptomycin) in 75 cm3 flasks. Six days after plating, floating cells were discarded and attached macrophages were scraped from the flasks and plated at 0.5 × 106/ml in 24 well plates.
2.2. Macrophage infection
Transgenic L. donovani LV82 expressing the red fluorescent protein DsRed2 has been described previously (Lezama-Davila et al., 2012). Expression of DsRed2 was confirmed in both promastigotes and intracellular amastigotes by fluorescent microscopy. Amastigotes recovered from the spleen of DsRed2 L. donovani infected BALB/c mice were cultured in M199 media and serial passages were performed until parasites reached the promastigote stationary phase, and were subsequently used for macrophage infection. Macrophages were infected with parasites (1:10 ratio) for various times as indicated in the figures. After 24 h free parasites were aspirated and macrophage culture was washed three times with PBS.
2.3. Macrophage fluorescent staining
Macrophages were stimulated for 45 min in the presence or absence of Escherichia coli lipopolysaccharide (LPS) (1 μg/ml) and recovered for analysis. Fc receptors were blocked with normal mouse serum for 15 min at 4 °C. For intracellular detection of NFκB, cells were fixed by incubation in 4% formaldehyde for 10 min at room temperature. Then cells were washed twice with permeabilization buffer (Biolegend). Macrophages were incubated with monoclonal rabbit antimouse pNFκBp65 (Cell signaling) at a 1:50 dilution in permeabilization buffer for 30 min at room temperature. Thereafter, cells were stained with Alexa 647 conjugated goat anti rabbit secondary antibody at a 1:200 dilution at room temperature for 30 min. DAPI was added to the cells for nuclear staining before acquisition.
2.4. Flowsight data acquisition and analysis
Acquisition speed was set up to low speed and the highest resolution, an automated condition provided in Flowsight. Cells were acquired based on area and aspect ratio, gating out cell debris and free parasites from the analysis. About 1000–5000 cells were acquired. Channel 3 was used to acquire DsRed2, channel 7 was used to detect DAPI and channel 11 was used to detect Alexa 647. Data were analyzed in IDEAS software after compensation of single color control samples using a compensation matrix. The number of parasites was recorded using the spot analysis application Wizard in IDEAS software. NFκB translocation to the nuclei was analyzed using the nuclear translocation application wizard for total, uninfected or infected macrophages.
3. Results
3.1. Flowsight effectively determines levels of infectivity and accurately estimates parasite load
Flow cytometry is a broadly used technology which facilitates the analysis of different characteristics in large cell populations. However it lacks the ability to determine localization of molecules within the cell. Although microscopic imaging accomplishes these goals, this method is limited in the number of cells and samples that are reasonably able to be screened and in the representative results obtained from the data analyzed. Recently, new technologies have emerged which combine the large and quick sample acquisition ability of flow cytometry with the ability to capture individual images of every cell acquired by flow cytometry. We therefore evaluated the capabilities of Flowsight in the estimation of parasitic loads and determination of macrophage infectivity levels after infection of macrophages with L. donovani. We used L. donovani DsRed2 parasites as a tool to identify parasites by fluorescence which is expressed only in live parasites (Lezama-Davila et al., 2012). This allows us to accurately discriminate between live and dead parasites.
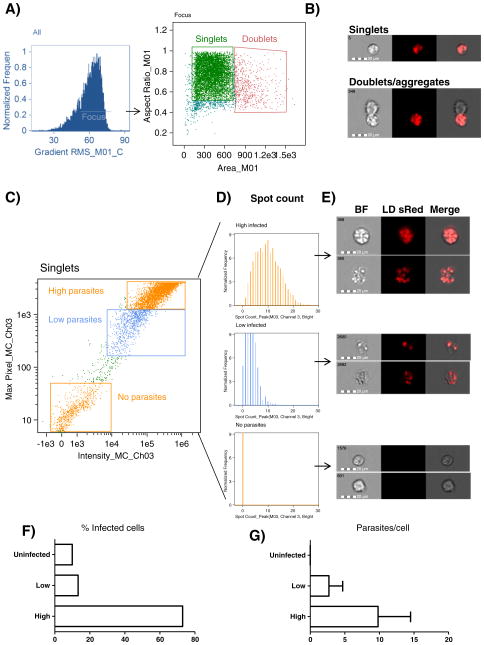
First, we determined whether Flowsight had the ability to distinguish infected from non-infected cells. For this purpose, macrophages were infected with L. donovani for 24 h in a 10:1 parasite to cell ratio. We first plotted total cells based on area vs. aspect ratio, and chose the region enclosing single cells (Fig. 1A). Based on the remarkable ability of Flowsight technology to image every single cell in this region, we confirmed our gating strategy by looking at images of the gated population to ensure they were single cells (Fig. 1B). To further investigate the infected macrophage population, we plotted Max pixel of channel 3 vs. intensity of channel 3. This combination provides an excellent means to discriminate between high, medium or negative fluorescent cells. Using this approach we were able to identify highly infected macrophages, macrophages with low parasitic load and uninfected macrophages (Fig. 1C). Highly infected macrophages were 73% of total macrophages; macrophages with low parasite loads were 13.3%, while uninfected cells were only 9.9% (Fig. 1F). Next, we determined whether fluorescence intensity correlated with parasite numbers. We analyzed our three different sub-populations with the Spot count application wizard in IDEAS software. Spot-count histograms (Fig. 1D), were correlated with fluorescence intensities showing an average of 9.8 parasites/cell in the high fluorescent region, 2.7 parasites/cell in low florescent region and 0 parasites/cell in the negative region. Further, image display showed that the numbers of L. donovani parasites inside the macrophage were representative of the spots calculated by IDEAS software (Fig. 1D, E, G).
Fig. 1.
Estimation of parasitic loads using Flowsight. A) Single cells were gated based on focus, area and aspect ratio. B) Representative image of cells gated as singlets and doublets. C) Singlets were plotted using Max pixel of channel 3 vs. intensity of channel 3. Gating of highly infected, least infected and uninfected cells. D) Histograms representing gates obtained in C) obtained using the Spot count wizard in IDEAS software. E) Representative image of regions gated in C). F) Percentage of gated populations. G) Mean and standard deviation of spots/cell calculated by IDEAS software.
3.2. NFκBp65 translocation to the nucleus is impaired in L. donovani infected macrophages
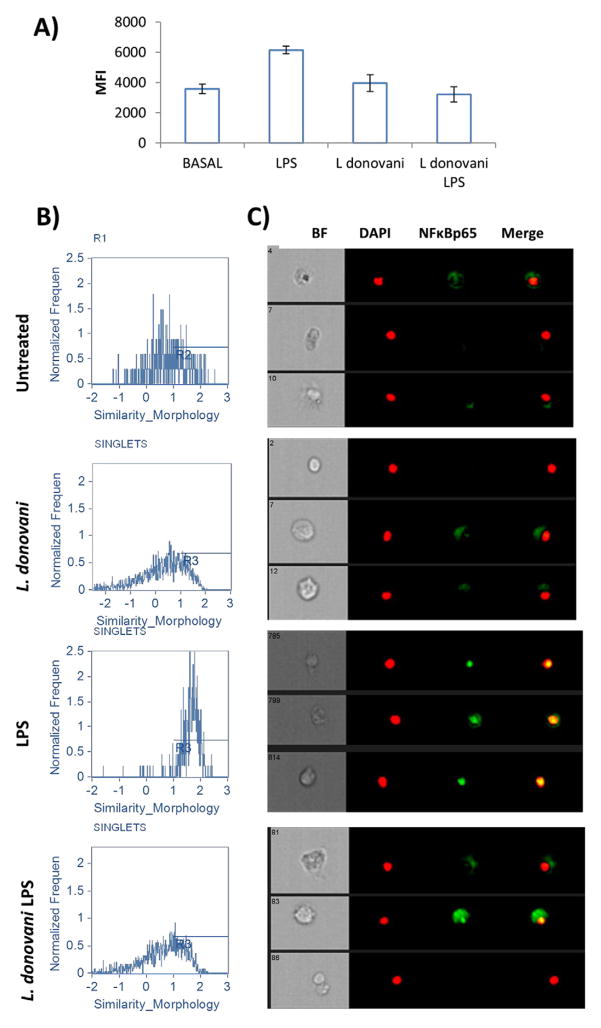
Pathogens are recognized by innate cells by pattern recognition receptors such as TLRs and C-type lectin receptors among others. TLR engagement leads to activation of the cell by triggering intracellular signaling cascades which result in NFκB translocation to the nucleus. NFκB translocation initiates the transcription of inflammatory mediators that ultimately may lead to pathogen elimination (Kawai and Akira, 2007). Leishmania parasites are known to modulate intracellular signaling triggered by TLRs impairing cytokine production (Chandra and Naik, 2008). We therefore tested whether L. donovani infection interfered with NFκBp65 translocation to the nucleus in response to stimulation by LPS, a TLR4 ligand. For this purpose we explored the ability of Flowsight flowcytometer to identify co-localization of fluorescent probes. Infected or uninfected macrophages were treated with LPS to induce NFκBp65 phosphorylation/translocation to the nuclei. After 45 min of LPS activation, macrophages were intracellularly stained with anti-pNFκBp65. First, we gated single cells to analyze the phosphorylation of NFκBp65 by measuring median fluorescence intensity (Fig. 2A). As expected LPS treated macrophages showed enhanced NFκB phosphorylation compared with untreated controls. L. donovani infected macrophages showed basal p-NFκBp65 levels. Interestingly NFκBp65 phosphorylation was impaired in L. donovani infected macrophages stimulated with LPS. Next, we evaluated the nuclear translocation of NFκB by using the nuclear localization application wizard in IDEAS software. As expected, uninfected controls and L. donovani-infected macrophages displayed low NFκBp65 nuclear translocation indices. In contrast, NFκBp65 translocation to the nucleus was enhanced in LPS-treated macrophages. Interestingly, L. donovani infected macrophages treated with LPS displayed lower NFκBp65 translocation than uninfected LPS treated macrophages (Fig. 2A, B).
Fig. 2.
Visualization of NFκB translocation to the nucleus. Macrophages were infected with L. donovani at a 10:1 ratio (parasites to macrophages) with or without LPS stimulation and nuclear translocation was analyzed in Flowsight. A) MFI of NFκBp65 was calculated in the different treatments. B) Histograms representing nuclear translocation index and C) representative images of treated cells. For improved visualization, red (DAPI) and green (NFkB) colors were assigned in IDEAS software. Brightfield (BF), co-localization (yellow).
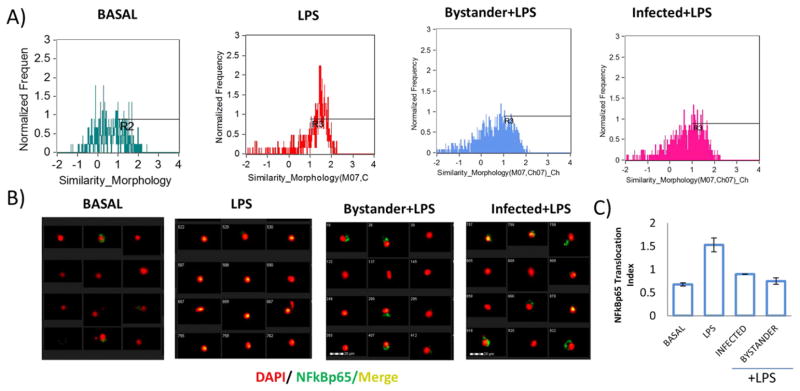
3.3. Bystander uninfected macrophages are affected by L. donovani infection and display impaired NFκBp65 translocation to the nucleus
Co-culture of L. donovani with BMDMs resulted in a 90% infection rate with the remaining 10% of co-cultured uninfected macrophages (Fig. 1C). Using the capability of Flowsight to distinguish among the infected and co-cultured uninfected macrophages we determined whether translocation of NFκBp65 in response to LPS was differentially affected depending on the infection. First, we gated on infected and uninfected macrophage populations, and then looked for NFκBp65 translocation to the nucleus using the nuclear localization application wizard in IDEAS software. We observed that co-cultured uninfected macrophages displayed low NFκBp65 translocation to the nucleus to a similar extent as L. donovani infected macrophages when macrophages were exposed to LPS (Fig. 3A, B). This effect found in the uninfected subpopulation of macrophages could be the result of parasite derived factors which exert the same effect as in parasite-infected cells. For example GP63, a protease released by L. mexicana has been shown to degrade NFκB (Isnard et al., 2012). Alternatively, this effect could be a result of infected macrophage-derived cytokines or contact dependent due to the infected–uninfected macrophage interaction in the cultures (Fig. 1B). It is possible that other transcription factors are affected in co-cultured uninfected macrophages, which makes the use of imaging-flow cytometry a valuable approach to defining the pathways and mechanisms involved. This method provides the unique advantage of distinguishing between infected and uninfected cells within a mixed population of infected macrophages, and to correlate infectivity with transcription factor activation and cell activation.
Fig. 3.
Bystander macrophages have impaired NFκB p65 translocation to the nucleus. Macrophages were cocultured with L. donovani, and stimulated with LPS. A) Nuclear translocation index was evaluated in infected or bystander macrophages. Cells were gated and analyzed independently. B) Merged images of bystander or infected macrophages exposed to LPS, as well as LPS treated uninfected macrophages. C) Bar graph comparing the nuclear translocation index among different treatments.
The standard method for determining nuclear translocation of NFκB and other molecules is cell lysis and fractionation of cell compartments by differential centrifugation, followed by Western blots of the different fractions. This method is time consuming, averaging about 3 to 4 days to get results. Further, this method has the drawback of quantifying total cells in a mixed population, including those which do not undergo nuclear translocation. Flowsight technology can accurately assess phosphorylation/translocation of NFκB or other signaling molecules in a single cell within a heterogeneous population of cells that may or may not come in contact with the infecting pathogen. This provides unique advantages over traditional Western blot.
4. Conclusions
Imaging-flow cytometry represents a new method for automated evaluation of Leishmania parasitic loads and can be used to improve high throughput drug screening in vitro. Importantly, this method has a definite advantage of distinguishing between infected and uninfected sub-populations of cells and determining the cellular localization of important signaling molecules, which could provide additional insights into the complex interplay of host–pathogen interactions.
Acknowledgments
SMB was supported by NIH AI29646. DMS received support from CONACYT Mexico.
Abbreviations
- BMDM
bone marrow derived macrophages
- LPS
lipopolysaccharide
- NFκB
nuclear factor kappa B
- CL
cutaneous leishmaniasis
- VL
visceral leishmaniasis
- TLR
toll like receptor
- IFN-γ
interferon gamma
References
- Chandra D, Naik S. Leishmania donovani infection down-regulates TLR2-stimulated IL-12p40 and activates IL-10 in cells of macrophage/monocytic lineage by modulating MAPK pathways through a contact-dependent mechanism. Clin Exp Immunol. 2008;154(2):224. doi: 10.1111/j.1365-2249.2008.03741.x. http://dx.doi.org/10.1111/j.1365-2249.2008.03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G, Oghumu S, Satoskar AR. Mechanisms of immune evasion in leishmaniasis. Adv Appl Microbiol. 2013;82:155. doi: 10.1016/B978-0-12-407679-2.00005-3. http://dx.doi.org/10.1016/B978-0-12-407679-2.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191. doi: 10.1016/S0140-6736(98)10178-2. http://dx.doi.org/10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- Isnard A, Shio MT, Olivier M. Impact of Leishmania metalloprotease GP63 on macrophage signaling. Front Cell Infect Microbiol. 2012 doi: 10.3389/fcimb.2012.00072. http://dx.doi.org/10.3389/fcimb.2012.00072. [DOI] [PMC free article] [PubMed]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460. doi: 10.1016/j.molmed.2007.09.002. http://dx.doi.org/10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kram D, Thäle T, Kolodziej H, Kiderlen A. Intracellular parasite kill: flow cytometry and NO detection for rapid discrimination between anti-leishmanial activity and macrophage activation. J Immunol Methods. 2008;333(1–2):79. doi: 10.1016/j.jim.2008.01.004. http://dx.doi.org/10.1016/j.jim.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Lezama-Davila C, Kapadia G, Marquez-Issac A, Owens KL, Oghumu S, Beverley SM, Satoskar A. Leishmanicidal activity of two naphthoquinones against Leishmania donovani. Biol Pharm Bull. 2012;35:1761. doi: 10.1248/bpb.b12-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezama-Dávila CM, Pan L, Isaac-Márquez AP, Terrazas C, Oghumu S, Isaac-Márquez R, Satoskar AR. Pentalinon andrieuxii root extract is effective in the topical treatment of cutaneous leishmaniasis caused by Leishmania mexicana. Phytother Res. 2014;28:909. doi: 10.1002/ptr.5079. http://dx.doi.org/10.1002/ptr.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger S, Zandbergen G. Measuring the killing of intracellular pathogens: Leishmania. Curr Protoc Immunol. 2011 doi: 10.1002/0471142735.im1423s93. http://dx.doi.org/10.1002/0471142735.im1423s93. [DOI] [PubMed]
- Terrazas CA, Terrazas LI, Gómez-García L. Modulation of dendritic cell responses by parasites: a common strategy to survive. J Biomed Biotechnol. 2010;2010:357106. doi: 10.1155/2010/357106. [DOI] [PMC free article] [PubMed] [Google Scholar]