Abstract
Several drugs are effective in attenuating intestinal ischemia-reperfusion injury (IRI); however little is known about the effect of montelukast. Fifty rats were randomly assigned to 3 groups: model group (operation with clamping), sham group (operation without clamping), and study group (operation with clamping and 0.2, 2 and 20 mg/kg montelukast pretreatment). Intestinal ischemia-reperfusion was performed by occlusion (clamping) of the arteria mesenterica anterior for 45 min, followed by 24 h reperfusion. Intestinal IRI in the model group led to severe damage of the intestinal mucosa, liver and kidney. The Chiu scores of the intestines from the study group (2 and 20 mg/kg) were lower than that of the model group. Intestinal IRI induced a marked increase in CysLTR1, Caspase-8 and -9 expression in intestine, liver and kidney, which were markedly reduced by preconditioning with 2 mg/kg montelukast. Preconditioning with 2 g/kg montelukast significantly attenuated hepatic tissue injury and kidney damage, and decreased plasma interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels in plasma after intestinal IRI. In conclusion, preconditioning with montelukast could attenuate intestinal IRI and the subsequent systemic inflammatory response in rats.
Intestinal ischemia is generally the result of arterial occlusion by thrombi or emboli and, more frequently, by nonocclusive processes, such as in situations of low mesenteric flow1,2. The arteries most compromised by obstruction are the celiac artery, superior mesenteric artery and inferior mesenteric artery3.
Ischemia leads to hypoxia, which initiates a series of events primarily related to the activation of platelets and the release of vasoconstrictive mediators, which further restricts blood flow to the ischemic area. The degree of tissue injury is further enhanced and accelerated by reperfusion, which is not only associated with local changes, but also with systemic changes4,5,6. Moreover, it has been demonstrated that the 5-lipoxygenase (5-LO) pathway plays a significant role in the pathophysiology of intestinal IRI7,8. Cysteinyl leukotrienes (CysLTs) are produced from arachidonic acid through the 5-LO pathway and act on the CysLT1 and CysLT2 receptors9,10,11.
Montelukast is used for the maintenance treatment of asthma and to relieve symptoms of seasonal allergies as a selective reversible CysLT1 receptor antagonist. CysLT1 receptor antagonists or biosynthesis inhibitors ameliorate ethanol-induced gastric mucosal damage12 and impaired wound healing. Recent studies have shown that montelukast has an antioxidant effect in testicular injury and also reduces renal damage13. Furthermore, the beneficial effects of montelukast have also been reported in various experimental models of inflammation14,15,16. To our knowledge, few studies have investigated the protective/therapeutic effects of montelukast in intestinal IRI and the mechanisms involved. Therefore, the current study was designed to explore the protective effects and possible mechanism of montelukast against intestinal IRI in rats.
Results
Rats preconditioned with montelukast exhibit significantly reduced damage to the intestinal mucosa
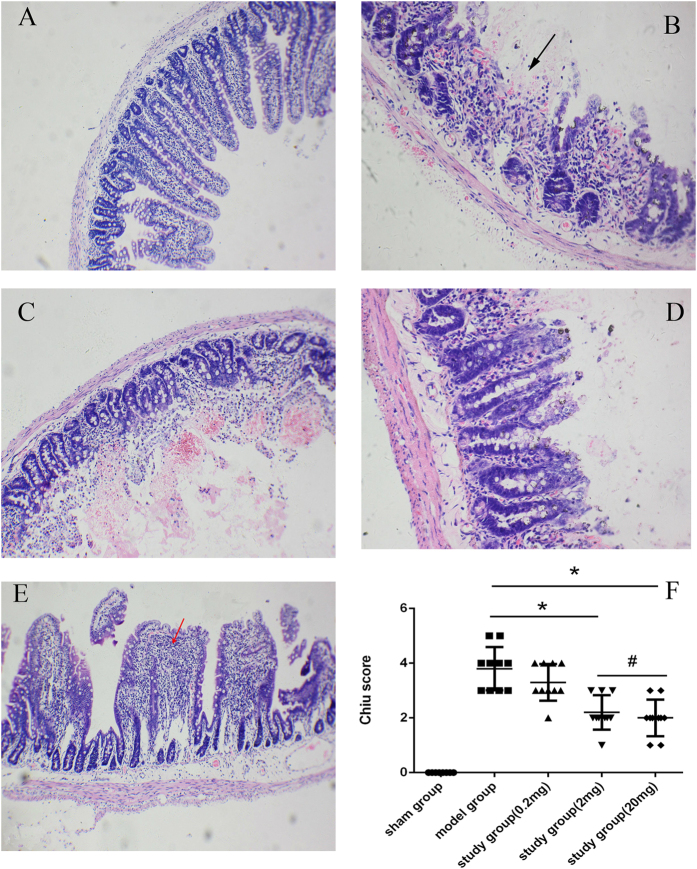
In Fig. 1, we demonstrate the protective effects of montelukast in the small intestine. Intestinal IRI in the model group led to severe damage to the intestinal mucosa (Fig. 1B). Compared with the model group, rats preconditioned with montelukast (2 and 20 mg/kg) prior to intestinal IRI had markedly reduced intestinal ischemia reperfusion injury (Fig. 1D,E) and had a significant reduction in Chiu score (Fig. 1F, P = 0.017 and 0.009). However, there was no obvious difference in Chiu score between the 2 and 20 mg/kg groups (P = 0.106).
Figure 1. Montelukast protects against intestinal injury after intestinal ischemia-reperfusion injury (IRI).
Representative photomicrographs (100×) of sham-operated group (A), model group (B), and study group with 0.2 mg/kg (C), 2 mg/kg (D), 20 mg/kg (E) montelukast. Black arrow denotes areas of bleeding and necrosis, red arrow denotes areas of neutrophil infiltration. Intestinal histology was evaluated using the Chiu score ((F); scale 0–5). *P < 0.05.
CysLTR1 protein and mRNA expression is attenuated following pretreatment with the 2 mg/kg dose of Montelukast
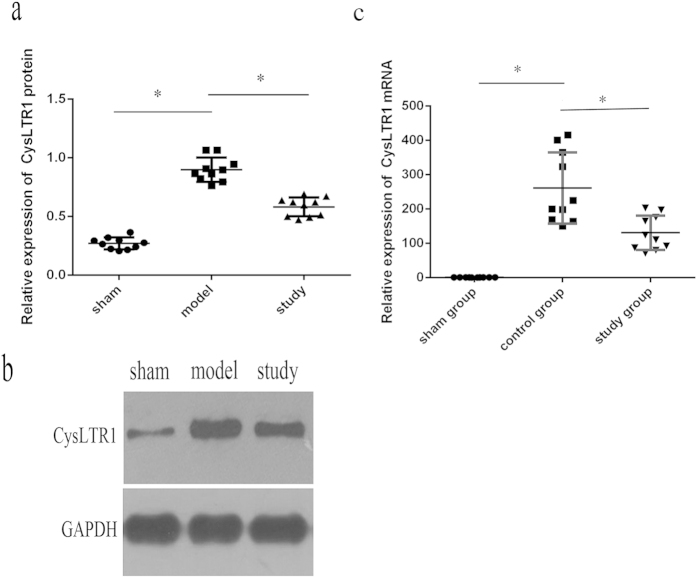
As expected, the expression of the CysLT1 receptor in the intestinal tissue of sham-operated rats was low. Intestinal IRI induced a marked increase in CysLTR1 protein expression in intestinal tissue (P = 0.006), which was dramatically reduced following preconditioning with montelukast (P = 0.014). Similar results were obtained for CysLTR1 receptor mRNA expression (Fig. 2).
Figure 2. Cysteinyl leukotrienes receptor-1(CysLTR1) protein and mRNA expression after intestinal IRI.
CysLTR1 protein expression was detected by western blotting (a,b) and mRNA expression by real-time PCR (c). *P < 0.05.
Preconditioning with montelukast (2 mg/kg) reduces intestinal apoptosis after IRI
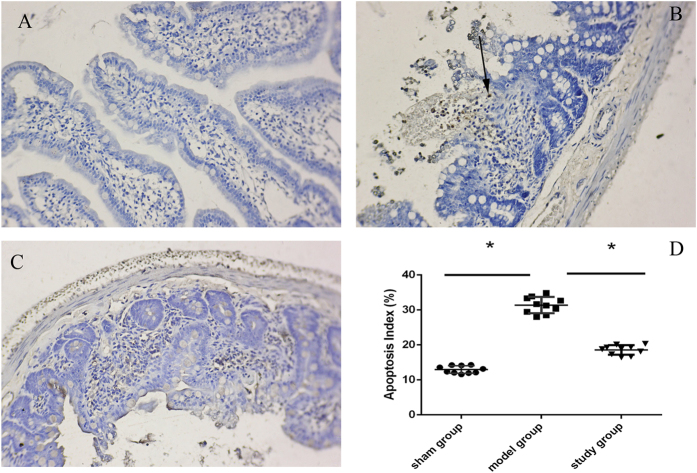
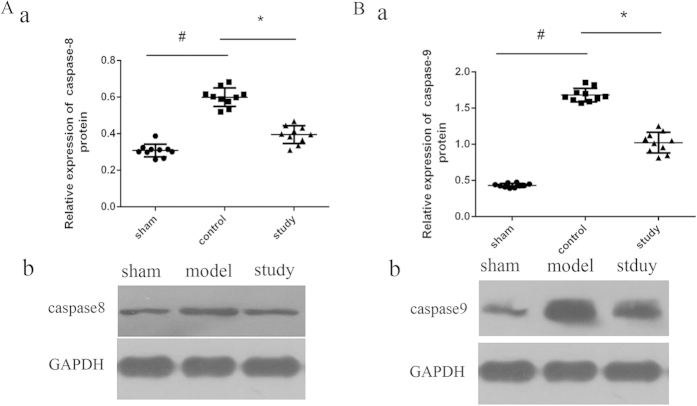
Apoptosis in intestinal tissue was detected using TRITC staining and observed under an ImageScope (Aperio, Vista, USA). Five random fields of view (400× magnification) were taken and positive cells were counted. The apoptosis index (AI) = the average number of apoptotic cells/200 × 100%. The assessor was blinded to the treatment groups. As seen in Fig. 3, the AI decreased from 33.33 ± 6.76% in the model group to 17.11 ± 5.58% with 2 mg/kg montelukast preconditioning (P = 0.019). Thus, preconditioning with montelukast reduces apoptosis in intestinal IRI. As evident in Fig. 4, the expression of caspase-8 and caspase-9 markedly decreased with montelukast preconditioning (P = 0.021 and 0.018).
Figure 3. Montelukast protects against apoptosis after intestinal IRI.
Representative photomicrographs illustrating apoptotic nuclei (TUNEL fluorescence staining) in the intestine of the sham group (A), model group (B), and study group ((C), montelukast, 2 mg/kg) (representative of four experiments, 100× magnifications). (D) Histogram of the AI in the ileum of rats. *P < 0.05. Rats after intestinal IRI showed many TUNEL-positive cells in the distal tips of villi in the small intestine (Fig. 3B, arrow denotes TUNEL-positive cell).
Figure 4. Caspase-8 and caspase-9 protein expression after intestinal IRI.
Protein expression was detected by western blotting. *P < 0.05.
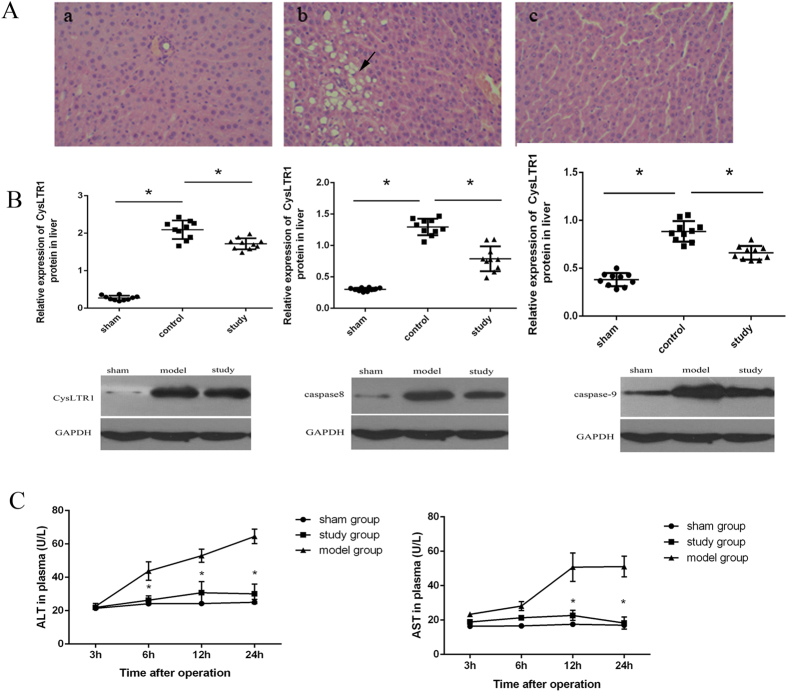
Montelukast (2 mg/kg) protects against acute hepatic and kidney injury after intestinal IRI in rats
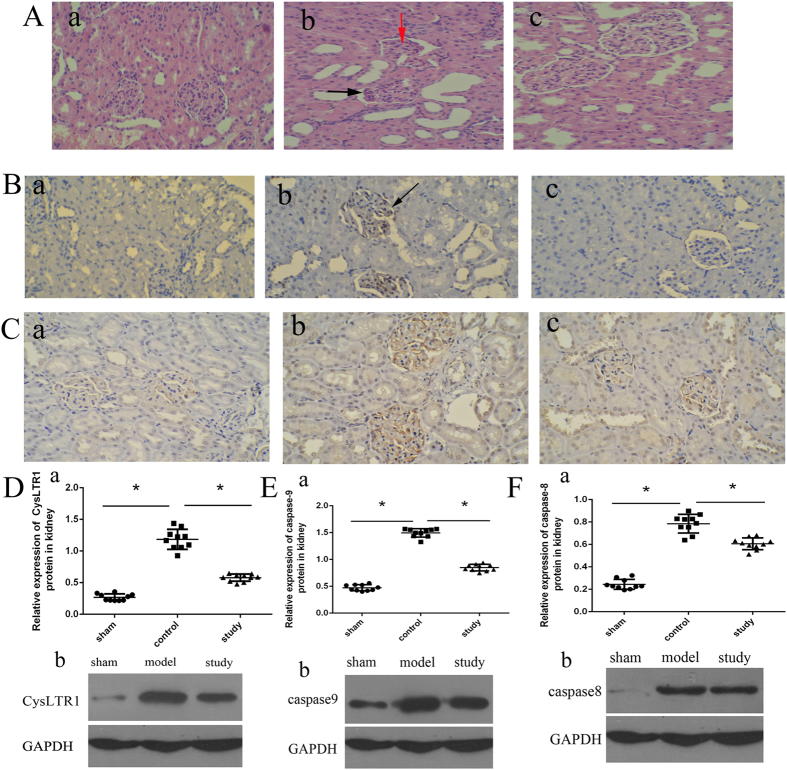
Twenty-four hours after intestinal IRI, rats developed significant hepatic and renal dysfunction as indicated by a rise in plasma ALT (P = 0.000) and AST levels (P = 0.000) (Fig. 5C), and the expression of NGAL in renal tissue (Fig. 6B-b) was above sham values. In the liver, intestinal IRI resulted in marked vacuolisation and leukocyte infiltration (Fig. 5A-b, left arrows denote vacuolisation area) compared with sham-operated rats (Fig. 5A-a). With montelukast preconditioning, there was an obvious decrease in vacuolisation and leukocyte infiltration after intestinal IRI (Fig. 5A-c). The expression of CysLT1, caspase-8 and caspase-9 also decreased with montelukast preconditioning (P = 0.034, 0.015 and 0.039 respectively). In the kidney, there was increased proximal tubule simplification (Fig. 6A-b, right black arrows), proximal tubular hypereosinophilia/single cell necrosis, and glomerular atrophy (Fig. 6A-b, down red arrows). The expression of CysLT1 decreased with montelukast preconditioning (Fig. 6C,D), whereas caspase-8 and -9 expression were similar (Fig. 6E,F).
Figure 5. Montelukast reduces liver injury after intestinal IRI.
Representative photomicrographs of livers from rats in different treatment groups ((A); H&E staining, 200× magnification. A-a, sham group; A-b, control group; A-c, study group). Arrows indicate vacuolisation. CysLTR1, caspase-8 and caspase-9 protein expression in liver from different groups. Plasma ALT and AST levels were measured in rats at various times after intestinal IRI (C).
Figure 6. Montelukast reduces kidney injury after intestinal IRI.
Representative photomicrographs of kidneys from rats in different groups (A-a, sham group; A-b, control group; A-c, study group. H&E, 200× magnification). highlighting tubular simplification, hypereosinophilia and glomerular atrophy (A-b, down and red arrows). Expression of NGAL in renal tissues in different treatment groups ((B), B-a, sham group; B-b, control group; B-c, study group). CysLTR1 protein expression in the kidney from different treatment groups by immunohistochemistry and western blotting ((C,D). C-a, sham group; C-b, control group; C-c, study group). Protein expression of caspase-8 and caspase-9 in the kidney (E,F). *P < 0.05.
The induction of IL-6 and TNF-α are attenuated by pretreatment with with the 2 mg/kg dose of Montelukast
As expected, the levels of IL-6 and TNF-α in plasma from sham-operated rats was low and intestinal IRI induced a marked increase in plasma IL-6 (to 782 pg/mL, approximately 5-fold) and TNF-α (to 2.248 μg/L, approximately 9-fold), which were markedly reduced (282 pg/mL and 0.701 μg/L, respectively, P = 0.000) by preconditioning with montelukast (Fig. 7).
Figure 7. Plasma interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels after intestinal IRI in rats.
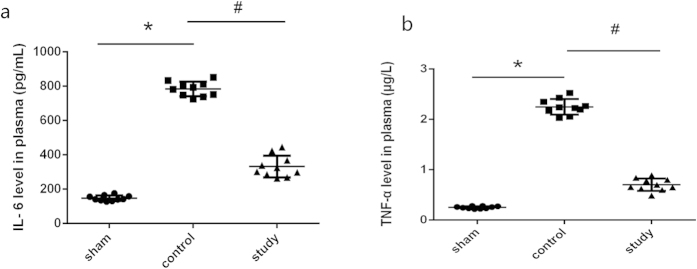
Blood was obtained immediately after serum creatinine reperfusion, and IL-6 and TNF-α levels were assessed by ELISA. *P < 0.05.
Discussion
Intestinal IRI is a troublesome clinical disease with a high mortality rate, despite surgical intervention17. Ischemia and reperfusion injury has different pathophysiological features with the major mucosal injury happening during the reperfusion phase18,19. Thus, using pharmacological agents is an important strategy for preventing intestinal ischemic injury during intestinal IRI19,20,21,22,23.
Montelukast has been shown to have a neuroprotective and antiapoptotic effect in an IRI mouse model by acting as a CysLT1 receptor antagonist at concentrations of 0.1–1.0 mg/kg24,25,26, and these effects are related to the inhibition of neutrophil accumulation, lipid peroxidation and pro-inflammatory cytokine release. Genovese et al.27 also showed that montelukast can reduce spinal cord inflammation, neutrophil infiltration and tissue injury. Recently, Erdem et al. from Turkey reported on the protective effects of montelukast and hypericum perforatum against intestinal IRI in rats28, and this effect may be due to its anti-apoptotic properties and the increased expression of malondialdehyde, myeloperoxidase, glutathione, and cardiotrophin-129. However, the detailed signalling mechanism involved in the preventive effects of montelukast against intestinal IRI remains unclear. In addition, how montelukast prevents subsequent systemic inflammatory responses and distant organ damage, such as to the liver and kidney, during intestinal IRI in rats has not previously been established. Our study aimed to explore the role of montelukast on reducing intestinal IRI, including injury to the liver and kidney, in rats, and the possible signalling pathway involved.
We used three doses of montelukast (0.2, 2 and 20 mg/kg) to explore the effect on intestinal IRI. Intestinal morphological changes were observed by microscopy. Submucosa villi shrinkage, haemorrhagic necrosis, obvious inflammatory cell infiltration, muscle layer thickness changes, and bleeding were observed in rats from the model group, demonstrating that intestinal IRI could lead to the destruction of intestinal mucosa structure. The results demonstrated that 2 mg/kg montelukast could markedly alleviate the degree of damage in intestinal lesions. Further protection was not observed by increasing the dose. Thus, we explored the mechanism of action of 2 mg/kg montelukast against intestinal IRI. Montelukast is used for the maintenance treatment of asthma at a recommended dose of one 10-mg tablet/day for adults (approximately 0.2 mg/kg). In our study, the effective dose was 2 mg/kg, which is 10 times greater than that used regularly in humans. However, our basic study was conducted in rats, and a further suitable dose for preventing intestinal IRI, and the possible side effects associated with such a high dose requires further research.
Several studies have shown that the protective role of montelukast in IRI is related to the inhibition of apoptosis29,30,31. Our data also revealed that IRI can significantly increase intestinal cell apoptosis, suggesting that CysLTs/CysLTsR1 can promote intestinal apoptosis, and that apoptosis is involved in the mechanism of intestinal IRI31,32. With the application of montelukast, apoptosis was reduced in tissue and the expression of caspases-8 and -9 decreased, indicating that montelukast inhibits caspase-8 and -9 mediated pathways of apoptosis in intestinal IRI.
Intestinal IRI not only leads to injury in the intestine, but affects distant organs such as the liver and kidney6. In our study, montelukast reduced injury in both the liver and kidneys after intestinal IRI, as evidenced by improved histological findings and markers of organ function (plasma ALT, AST and NGAL levels). Preventive strategies are most effective when started before oliguria and elevated serum creatinine are evident, both of which are delayed and unreliable markers of kidney damage. A new promising, early biomarker is NGAL, which is believed to participate in the regeneration process. NGAL is an important biomarker of acute kidney injury (AKI) and acute tubular necrosis and correlates well with the degree of renal dysfunction6.
Inflammation is another important contributor to the exacerbation of IRI18, and we detected IL-6 and TNF-α as markers of the inflammatory response20. The role of montelukast to reduce IL-6 and TNF-α has not been previously reported in intestinal IRI. We found that montelukast markedly reduced systemic IL-6 and TNF-α after intestinal IRI, indicating that montelukast may protect not only the organ affected by the ischemic insult, but also blunt the systemic inflammatory response. Thus, montelukast may be of potential benefit in attenuating the systemic consequences of intestinal injury32.
Based on this evidence, we postulate that montelukast can directly protect the liver and kidneys after intestinal IRI by reducing the production and release of IL-6 in the small intestine, thereby reducing the systemic inflammatory effects in distant organs such as the liver and kidney. In addition, we found that montelukast could inhibit the expression of CysLTR1 in the liver and kidney. However, it is difficult to determine in an in vivo study whether the protective effect is mediated via direct inhibition of CysLTR1 signalling or via systemic effects such as modulation of IL-6 and TNF-α release. Based on the available evidence, it is likely that montelukast has multiple effects, including both direct cytoprotective effects in the intestine with inhibition of caspase pathways, as well as systemic anti-inflammatory mechanisms.
In conclusion, we demonstrated that montelukast could reduce intestinal mucosal injury and apoptosis after intestinal IRI, as well as damage to the liver and kidneys. Further elucidation of the mechanisms of protection may lead to advancements in the treatment of intestinal and multi-organ dysfunction following intestinal IRI.
Methods
Ethics Statements
This study was approved by the Animal Experimental Committee of Wenzhou Medical College. Animal care and all procedures were performed according to the guidelines for the care and use of laboratory animals.
Animals
Male Sprague–Dawley rats (n = 50), weighing between 250–350 g, were used for this study. Rats were randomly divided into sham, control and study groups. In the sham group (n = 10), laparotomy was performed, but without aortic occlusion. In the control group (n = 10), after laparotomy the arteria mesenterica anterior was clamped for 45 min, followed by reperfusion for 24 h. In the study group, montelukast (0.2, 2 and 20 mg/kg) was administered intragastrically before the procedure (each group n = 10). The anaesthesia was maintained with intermittent delivery of hydral, and intraperitoneal cephalosporin (10 mg/kg) was administered before skin incision. The abdominal aorta was explored through a transperitoneal approach, retracting the intestines after surface cleaning of the surgical area and standard midline laparotomy. Intestinal ischemia was induced by clamping the arteria mesenterica anterior for 45 min. After surgery, the abdominal wall was sutured with 5/0 polypropylene suture thread. After 24 h, all animals were anaesthetised and sacrificed. Some distal ileum tissue and blood were extracted and stored at −80 °C until needed for further detection.
Drug
Montelukast was purchased from the Simvastatin pharmacy company. The soluble form was prepared from oral tablets because we could not obtain the commercial soluble form. Ten tablets of montelukast (10 mg) were dissolved in 10 mL of ethanol. The solution was centrifuged for 5 min, and the supernatant was collected and filtered with a 0.2-mM filter. The resulting solution was concentrated by evaporation and reduced to a volume of 3 mL. The concentration of montelukast sodium was approximately 30 mg/mL.
Histopathological Assessment
Paraffin sections (4-μm thickness) of intestinal tissue were prepared and stained with haematoxylin and eosin (H&E). Histopathological assessment was performed by a researcher blinded to the treatment groups. The slides were observed by light microscopy at 100× magnification, and the severity of mucosal injury was evaluated by using the scoring system published by Chiu et al.26. Grade 0 represented normal villi and grades 1–5 indicated increments of severity of injury (grade 1: mild development of subepithelial Gruenhagen spaces; grade 2 or 3: moderate (grade 2) or severe (grade 3) progressive lifting of the epithelial layer from the lamina propria; grade 4: completely denuded villi; grade 5: disintegration of the lamina propria). Kidney and liver tissue also underwent H&E examination.
Real-time quantitative PCR
Real-time PCR was performed with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and an ABI Prism 7500 Sequence Detector (Applied Biosystems). Briefly, total RNA was isolated from intestinal tissue with Trizol reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA), and complementary DNA was produced according to the manufacturer’s instructions for the transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, USA). All samples were detected in triplicate and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The expression level of CysLTR1 mRNA was normalised to that of GAPDH and was expressed as a ratio relative to GAPDH.
Western blot analysis
The protein expression of CysLTR1, Caspase-8 and Caspase-9 was detected by western blotting. Briefly, total protein was extracted, and the concentration of supernatants was measured using the BCA protein assay (Pierce). Aliquots of supernatants containing 100 μg of protein were electrophoresed on 5% (w/v) sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene difluoride membrane. After blocking for 1 h with 5% (w/v) non-fat milk, the blots were incubated with primary antibody at 4 °C overnight. The membranes were washed in tris-buffered saline and incubated with the secondary antibody. After additional washing, the blots were exposed to films in a dark room. Protein bands were analysed with image analysis software (Quantity one, Bio-rad, Hercules, CA, USA). GAPDH served as the internal control, and the results were expressed as a ratio relative to GAPDH.
Apoptosis detection using terminal dexynucleotidyl transferase(TdT)-mediated dUTP nick end labeling (TUNEL)
Apoptosis detection in tissue was performed using the Tetramethylrhodamine (TRITC) staining Apoptosis Detection Kit (Keygen Biotech, China) and fluorescence microscopy (BD Biosciences, San Jose, CA, USA). Briefly, cells were trypsinised, washed with Phosphate Buffered Saline (PBS), centrifuged, fixed for 20 min at room temperature, incubated with blocking solution containing 3% (v/v) H2O2 in methanol for 10 min at room temperature, and incubated in permeabilisation solution for 2 min on ice. Fifty microliters of TUNEL Reaction Mixture was added to samples and incubated for 60 min at 37 °C in the dark according to the manufacturer’s instructions. Last, samples were assessed using a fluorescence microscope at an excitation wavelength of 543 nm and an emission wavelength of 571 nm within 1 h.
Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels after intestinal IRI
ALT and AST levels in rat plasma were measured at 3, 6, 12 and 24 h using the assay kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA).
Neutrophil gelatinase associated lipocalin (NGAL) expression in the kidney
NGAL expression in the kidney was measured immunohistochemically. Paraffin sections were prepared, and tissue sections were deparaffinised, and hydrated in xylene and graded alcohol. The polyclonal anti-rabbit NGAL antibody (Invitrogen, Life Technologies, Carlsbad, CA, USA) was used for the immunohistochemical detection according to standard procedures. Briefly, paraffin sections were deparaffinized and heat treated with citrate buffer, and incubated with NGAL antibody for 1 h (dilution 1:100) and mixed with skimmed milk powder at 2% again to reduce unspecific staining. Then they were reacted with biotinylated secondary antibody (dilution 1:500) for 30 min. Negative controls were performed by omitting the primary antibody.
Determination of IL-6 and TNF-α in plasma
After reperfusion, blood was collected into heparinised tubes and plasma was isolated via centrifugation (300 × g for 10 min). Plasma was snap frozen in liquid nitrogen and stored at −20 °C until assayed. Plasma IL-6 was measured by ELISA using the rat IL-6 ELISA kit (BD Biosciences), and Plasma TNF-α level was also measured by rat kit (Boster, WuHan, China).
Data analysis
According to the relative study of intestinal IRI18with a standard of 0.05, and avoiding unnecessary waste, we used 10 rats in each group. Statistical comparisons were performed with the software package SPSS 16.0 (IBM Corp., Armonk, NY, USA). The distribution of data was analysed first, followed by a two-tailed Student’s t-test for comparisons between two groups, and one-way ANOVA plus Tukey’s post hoc multiple comparison test to compare multiple groups when data was normally distributed. The ordinal values of the Chiu scores were analysed by the Mann–Whitney nonparametric test. A P < 0.05 was considered statistically significant. All data are expressed as the mean ± SEM.
Additional Information
How to cite this article: Wu, S. et al. The protective role of montelukast against intestinal ischemia-reperfusion injury in rats. Sci. Rep. 5, 15787; doi: 10.1038/srep15787 (2015).
Acknowledgments
This study was supported by the Clinical Studies Foundation of the Institute of Medicine of Zhejiang province (Title of project: “The protection role of montelukast in intestinal ischemia-reperfusion injury”, Project no. 2012zyc-a87).
Footnotes
Author Contributions S.B.W. and W.D.S. performed the experiments, analysed the data, interpreted the results, prepared the figures, and drafted the manuscript. X.X.Z., Z.H.J. and X.P.T. performed the experiments and analysed the data. L.Q.Z. and X.F.H. interpreted the results, and edited and revised the manuscript. X.F.Z. and P.F.L. conceived and designed the research, and interpreted the results. All authors read and approved the final manuscript.
References
- Huang X. J. et al. Activation of CysLT receptors induces astrocyte proliferation and death after oxygen-glucose deprivation. Glia 56, 27–37 (2008). [DOI] [PubMed] [Google Scholar]
- Pergel A. et al. Anti-inflammatory and antioxidant effects of infliximab in a rat model of intestinal ischemia/reperfusion injury. Toxicol Ind Health 28, 923–932 (2012). [DOI] [PubMed] [Google Scholar]
- Matsuo S. et al. Cyclic arginine-glycine-aspartate attenuates acute lung injury in mice after intestinal ischemia/reperfusion. Crit Care 17, R19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar H. H. & Abd El Tawab R. Cysteinyl leukotriene receptor antagonism alleviates renal injury induced by ischemia-reperfusion in rats. J Surg Res 178, e25–34 (2012). [DOI] [PubMed] [Google Scholar]
- Eaton A. et al. Cysteinyl leukotriene signaling through perinuclear CysLT(1) receptors on vascular smooth muscle cells transduces nuclear calcium signaling and alterations of gene expression. J Mol Med (Berl) 90, 1223–1231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala A. & Folco G. Neutrophils, endothelial cells, and cysteinyl leukotrienes: a new approach to neutrophil-dependent inflammation? Biochem Biophys Res Commun 283, 1003–1006 (2001). [DOI] [PubMed] [Google Scholar]
- Pontell L. et al. Damaging effects of ischemia/reperfusion on intestinal muscle. Cell Tissue Res 343, 411–419 (2011). [DOI] [PubMed] [Google Scholar]
- Lau W. K., Chow A. W., Au S. C. & Ko W. H. Differential inhibitory effects of CysLT(1) receptor antagonists on P2Y(6) receptor-mediated signaling and ion transport in human bronchial epithelia. PLoS One 6, e22363 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D. et al. Differential signaling of cysteinyl leukotrienes and a novel cysteinyl leukotriene receptor 2 (CysLT(2)) agonist, N-methyl-leukotriene C(4), in calcium reporter and beta arrestin assays. Mol Pharmacol 79, 270–278 (2011). [DOI] [PubMed] [Google Scholar]
- Isikdemir F. et al. Effects of montelukast and zileuton on testicular torsion/detorsion injury in rats. Andrologia 3, 10.1111/and.12042 (2012). [DOI] [PubMed] [Google Scholar]
- Corrigan C. et al. Expression of the cysteinyl leukotriene receptors cysLT(1) and cysLT(2) in aspirin-sensitive and aspirin-tolerant chronic rhinosinusitis. J Allergy Clin Immunol 115, 316–322 (2005). [DOI] [PubMed] [Google Scholar]
- Dengiz G. O. et al. Gastroprotective and antioxidant effects of amiodarone on indomethacin-induced gastric ulcers in rats. Arch Pharm Res 30, 1426–1434 (2007). [DOI] [PubMed] [Google Scholar]
- Piper H. M., Meuter K. & Schafer C. Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg 75, S644–648 (2003). [DOI] [PubMed] [Google Scholar]
- Ohshima N. et al. A functional study on CysLT(1) receptors in human eosinophils. Int Arch Allergy Immunol 129, 67–75 (2002). [DOI] [PubMed] [Google Scholar]
- Vollmar B. & Menger M. D. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch Surg 396, 13–29 (2011). [DOI] [PubMed] [Google Scholar]
- Rossi A., Cuzzocrea S. & Sautebin L. Involvement of leukotriene pathway in the pathogenesis of ischemia-reperfusion injury and septic and non-septic shock. Curr Vasc Pharmacol 7, 185–197 (2009). [DOI] [PubMed] [Google Scholar]
- Young C. M., Kingma S. D. & Neu J. Ischemia-reperfusion and neonatal intestinal injury. J Pediatr 158, e25–28 (2011). [DOI] [PubMed] [Google Scholar]
- Thomaz Neto F. J. et al. Ischemic preconditioning attenuates remote pulmonary inflammatory infiltration of diabetic rats with an intestinal and hepatic ischemia-reperfusion injury. Acta Cir Bras 28, 174–178 (2013). [DOI] [PubMed] [Google Scholar]
- Deng Z. H. et al. Leptin relieves intestinal ischemia/reperfusion injury by promoting ERK1/2 phosphorylation and the NO signaling pathway. J Trauma Acute Care Surg 72, 143–149 (2012). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Leukotrienes mediate 5-hydroxytryptamine-induced plasma extravasation in the rat knee joint via CysLT-type receptors. Inflamm Res 53, 66–71 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang Y. J., Zhang L., Wang S. B., Shen H. H. & Wei E. Q. Montelukast modulates lung CysLT(1) receptor expression and eosinophilic inflammation in asthmatic mice. Acta Pharmacol Sin 25, 1341–1346 (2004). [PubMed] [Google Scholar]
- Lenaerts K. et al. New insights in intestinal ischemia-reperfusion injury: implications for intestinal transplantation. Curr Opin Organ Transplant 18, 298–303 (2013). [DOI] [PubMed] [Google Scholar]
- Okudan N., Belviranli M., Gokbel H., Oz M. & Kumak A. Protective effects of curcumin supplementation on intestinal ischemia reperfusion injury. Phytomedicine 20, 844–848 (2013). [DOI] [PubMed] [Google Scholar]
- Ozkan E. et al. Protective potential of montelukast against hepatic ischemia/reperfusion injury in rats. J Surg Res 159, 588–594 (2010). [DOI] [PubMed] [Google Scholar]
- Lafci G. et al. Efficacy of iloprost and montelukast combination on spinal cord ischemia/reperfusion injury in a rat model. J Cardiothorac Surg 8, 64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersahin M. et al. Montelukast inhibits caspase-3 activity and ameliorates oxidative damage in the spinal cord and urinary bladder of rats with spinal cord injury. Prostaglandins Other Lipid Mediat 99, 131–139 (2012). [DOI] [PubMed] [Google Scholar]
- Genovese T. et al. Effects of zileuton and montelukast in mouse experimental spinal cord injury. Br J Pharmacol 153, 568–582 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik A. et al. Effects of montelukast on the healing of ischemic colon anastomoses. Am J Surg 206(4), 502–8 (2013). [DOI] [PubMed] [Google Scholar]
- Duran A. et al. Protective effect of montelukast, a cysteinyl leukotriene receptor-1 antagonist, against intestinal ischemia-reperfusion injury in the rat. Acta Chir Belg 113(6), 401–405 (2013). [PubMed] [Google Scholar]
- Cho S. et al. Remifentanil ameliorates intestinal ischemia-reperfusion injury. BMC Gastroenterol 13, 69, 10.1186/1471-230X-13-69 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M. O. et al. Role of adenosine on intestinal ischemia-reperfusion injury in rabbits. Transplant Proc 42, 454–456 (2010). [DOI] [PubMed] [Google Scholar]
- Matsuyama M. et al. The role of cysteinyl-LT(1)receptor (CysLT(1)R) in renal ischemia-reperfusion injury. Transplant Proc 41, 73–75 (2009). [DOI] [PubMed] [Google Scholar]