Abstract
In this issue of Science Translational Medicine, El-Khatib et al. describe a “closed-loop” bi-hormonal artificial pancreas, designed to avert episodes of low blood sugar in patients with insulin-dependent diabetes. We discuss the benefits and challenges of therapy directed at tight control of blood glucose and ask whether this and similar technological breakthroughs can address as yet unanswered questions in the biology of diabetes.
THE PANCREAS: ORCHESTRATING GLUCOSE HOMEOSTASIS
“I look upon the diabetic as charioteer and his chariot as drawn by three steeds named Diet, Insulin, and Exercise. It takes skill to drive one horse, intelligence to manage a team of two, but a man must be a very good teamster who can get all three to pull together.” E. P. Joslin, 1933 (1)
Years before insulin became the standard of therapy, Elliott Joslin’s enlightened care significantly increased the quality of life and survival of children with diabetes through careful implementation of controlled diet and exercise (2). His innovations extended a 6-month mean survival to more than two years. The advent of insulin was clearly a crucial advance, but it may have pushed the role of diet and exercise in controlling diabetes somewhat into the background. Joslin incorporated insulin into his complete care profile, careful not to neglect any aspect of the disease. Joslin's three steeds remind the diabetic to use every available tool to maintain blood glucose concentrations as close to normal as possible. Numerous clinical trials have established that the maintenance of near-normal blood glucose levels mitigates the progression of a number of secondary complications associated with diabetes, including retinopathy, nephropathy, neuropathy, microvascular, and cardiovascular sequelae (3, 4). However, euglycemia can result in hyperinsulinemia, which increases the risk of hypoglycemia. Furthermore, clinical trials in intensively treated diabetics have documented increased weight gain, blood pressure, cholesterol and lipid profiles (5, 6). Other studies have documented decreased insulin action (7). In this issue of Science Translational Medicine, El-Khatib et al. describe a “closed-loop” artificial pancreas that uses intravenous blood glucose measurements to titrate the delivery of insulin and glucagon, with the goal of maintaining normoglycemia while minimizing hypoglycemic episodes (8). Their findings suggest that the safe use of a bi-hormonal artificial endocrine pancreas to control blood glucose concentrations is possible.
In 1869, while still a student, Paul Langerhans described clear islands of cells throughout the pancreas that stained differently from surrounding cells (9). Some 24 years later, the French pathologist Edouard Laguesse proposed a secretory function to these small clusters of cells and named them the “Islets of Langerhans” in honor of Paul Langerhans. The association of morphological changes in islets with the diabetic state was noted soon thereafter by Eugene Opie, also at the time a student (10). Today, in the 21st century, we well appreciate that pancreatic islets are multicellular, with a defined higher-order structure and vast secretory capacity (Fig. 1). In human pancreatic islets, insulin- and amylin-secreting beta cells dominate in number (11, 12) and these cells are juxtaposed to glucagon-secreting alpha cells and in close proximity to three additional cells that exist in far lesser densities: (i) delta cells, which secrete somatostatin, which regulates alpha and beta cell production of glucagon and insulin in the pancreas; (ii) PP cells, which produce pancreatic polypeptide, an agent that regulates pancreatic secretory functions; and (iii) scattered epsilon cells, which make ghrelin, a hormone that stimulates hunger. The spatial distribution of these cells, their proximity to blood vessels, and their complex biochemistry provides for a sophisticated cell-signaling network in which the cell sending the signal is in close proximity to the cell that receives the signal. Thus, somatostatin regulates alpha and beta cell production of glucagon and insulin, respectively, glucagon regulates alpha cell activation and subsequent stimulation of beta and delta cells, and insulin promotes further beta cell activation and alpha cell inhibition. The means of using multiple hormones is, therefore, a logical extension of insulin as replacement therapy. The need for computer-controlled infusion recognizes the complexity of the physiological system and provides a means to recapitulate autocrine and paracrine feedback control.
Fig. 1. The pancreas' glucose regulatory pathways.
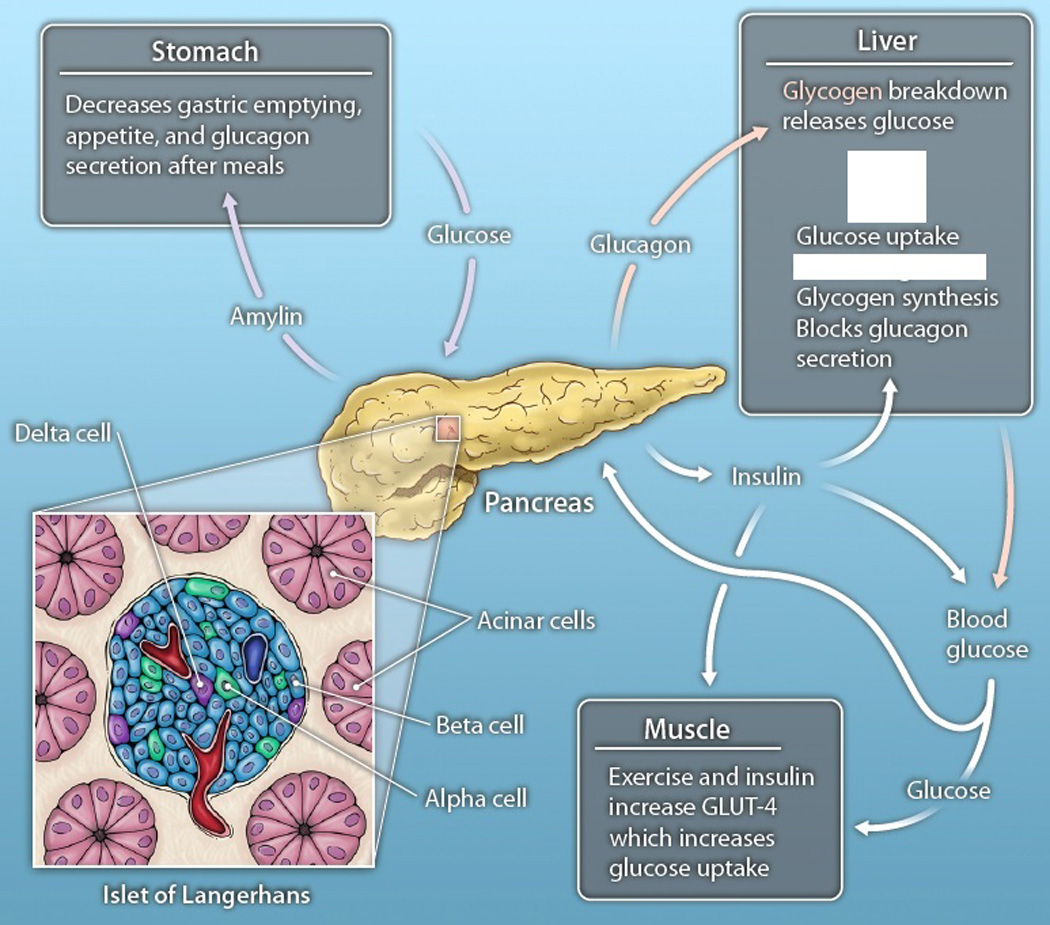
Insulin secretion from the beta cells of the pancreas results in glucose uptake and gluconeogenesis by the liver, upregulation of the GLUT-4 glucose transporter in muscle, and attenuation of glucagon secretion from the islets. Glucagon secreted by the alpha cells stimulates glycogen breakdown by the liver, thereby releasing glucose in times of need. Somatostatin secreted from the delta cells attenuates both insulin and glucagon secretion. Amylin secreted by beta cells delays gastric emptying, decreases appetite, and suppresses glucagon secretion after a meal. Cells within the islets are in close proximity with one another. CREDIT: CHRIS BICKEL/SCIENCE TRANSLATIONAL MEDICINE.
GLUCAGON: INSULIN'S HIDDEN PARTNER
The history of the discovery of insulin is well documented, having reached epic poetic levels. If we set 1922 as the date of definitive isolation of therapeutic insulin preparations, most would be surprised to learn that Kimball and Murlin isolated a hyperglycemic compound they termed glucagon from pancreatic extracts only one year later, using techniques designed to aid in insulin purification (13). Difficulties in defining glucagon structure and biology delayed its potential therapeutic use, but by the 1970s myriad manuscripts appeared discussing glucagon's potentiation of insulin therapy. It is not surprising, then, that many have since advocated use of multiple pancreatic hormones in the treatment of diabetes mellitus and the culminating technology of beta cell or islet transplantation.
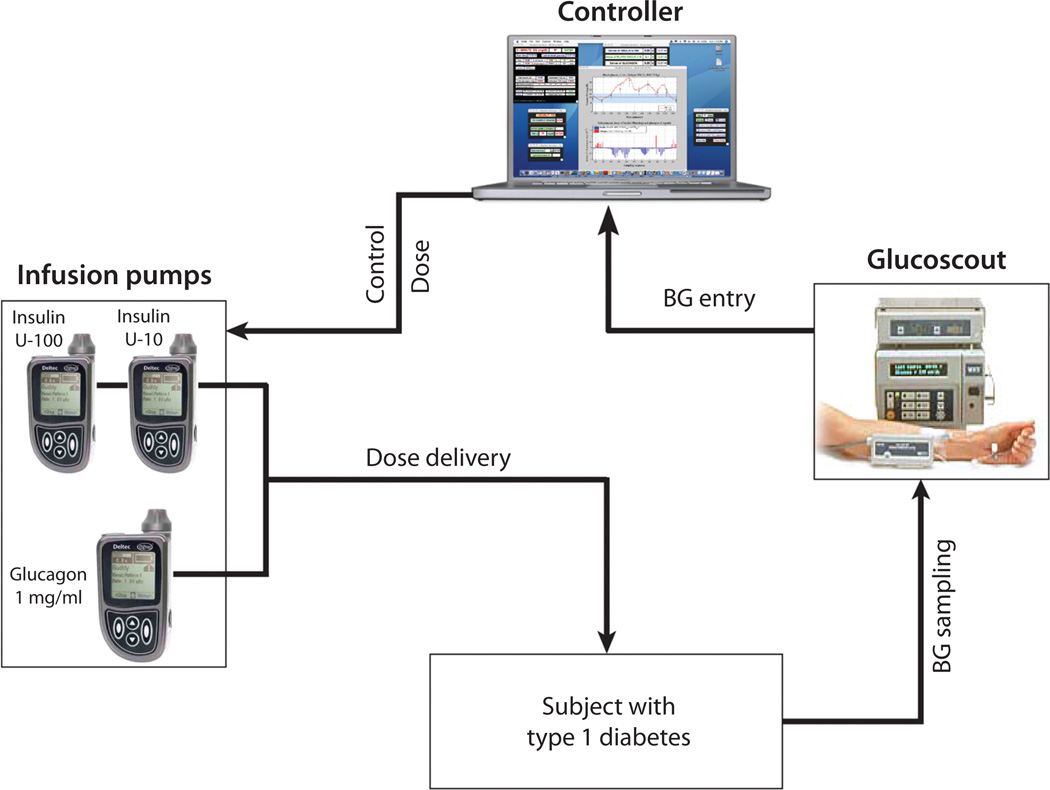
The approach by El-Khatib et al.—to infuse glucagon into diabetic patients via a continuous insulin-delivery pump as a counter-regulatory hormone to protect patients from severe hypoglycemia—is not in and of itself new, but their implementation is exciting and their findings are worthy of note (8). The closed-loop system employed by Khatib et al., comprised of four basic components (Fig. 2): (i) Intravenous blood glucose samples were collected from the subject every 5 minutes and analyzed by the GlucoScout monitor. (ii) The glucose measurements were then communicated to a computer running a customized model-predictive-control algorithm that directed Deltec Cozmo subcutaneous infusion pumps to deliver insulin, to lower blood glucose, or glucagon, to raise blood sugar. The authors observed that, of the 11 subjects treated with both hormones, six exhibited near normal blood glucose concentrations without apparent instances of hypoglycemia. The remaining five subjects did experience hypoglycemia, but exhibited slower insulin pharmacokinetics and delayed absorption relative to the other six patients. The authors then adjusted their algorithms’ pharmacokinetic inputs to prevent hypoglycemia, albeit at the expense higher average plasma glucose. Over a 27-hour test period, their system and algorithm achieved near-normal glucose control with an aggregate mean blood glucose of 164 mg/dL.
Fig. 2.
Bi-hormonal closed-loop insulin and glucagon delivery system. Developed by El-Khatib et al. (8). Closed-loop insulin delivery is designed to maintain near normal glycemia. Intravenous glucose is measured every five minutes using the GlucoScout device, and these data are used to guide a computer-controlled algorithm that titrates the delivery of two hormones. An increase in blood glucose is countered by insulin administration. Glucagon is administered to increase blood glucose by stimulating liver breakdown of glycogen. From (8) (Supplemental Fig. 1), used by permission.
The complex paracrine feedback of the multitude of pancreatic hormones (Fig. 1) maintains blood glucose and other metabolic parameters within a narrow range without dramatic shifts in the circulating hormones or metabolites (12). Optimized algorithm-controlled infusion of glucagon with insulin enabled avoidance of hypoglycemia. Prevention or reversal of hypoglycemia generally requires the delivery of tens of grams of glucose. In contrast, El-Khatib’s studies showed that their closed-loop artificial pancreas required less than 1 milligram of glucagon during the 27-hour study to treat hypoglycemia. One could pragmatically envision that these relatively small doses of glucagon aimed at preventing hypoglycemia could be delivered from a single device independently of insulin through a dual lumen catheter. However, the glucagon-dosing regimen employed may have unveiled a rebound pharmacological effect that could result in excess insulin infusion.
In humans with intact insulin metabolism, basal and bolus insulin release each accounts for roughly half of the daily insulin requirement, with low-level basal release occurring continuously and higher-level bolus release occurring in response to food intake (14). A similar 50:50% ratio of basal-to-bolus infusion appears optimal in pump-treated Type 1 diabetic patients given insulin alone (15, 16). Indeed, early studies of continuous subcutaneous infusion pumps show a decreased incidence of hypoglycemia compared to multiple daily insulin injections (15, 17, 18). The algorithm used in the El-Khatib study shifted this ratio—only 26% of the daily insulin was delivered basally and 74% as boluses. This significant increase of intermittent, bolus insulin delivery is presumably in response to the carbohydrate-rich meals given in this study and has been observed before. However, this shift may also have arisen as result of a need to counteract the effect of increased glucagon-induced glycogen breakdown (19).
TRUE BLOOD GLUCOSE
The reliable measurement of blood glucose is perhaps the single most important parameter to ensure normoglycemia and avoidance of hypoglycemic events. Some have attributed the success of Banting, Macleod, Best and Collip—the team that enabled the therapeutic use of insulin—to their ability to track and avoid the extreme lows of blood sugar with accurate assays, and the frustrations of their predecessors to the absence of such metrics. The gold standard for continuous blood glucose measurement has been the Biostator, which measures glucose amounts quickly and, within 2 minutes, infuses insulin or glucose intravenously to clamp glucose concentrations (20). Such a system minimizes the 10- to 45-minute lag, analytical errors associated with continuous subcutaneous glucose monitoring of interstitial fluid, and the delay in response from subcutaneous insulin uptake (21–25). Most continuous monitoring studies, however, measure glucose and deliver insulin subcutaneously (15, 17,18,21–25). El Khatib et al. (8) employed a hybrid approach, using the GlucoScout to measure intravenous glucose as a more real-time measurement, but infusing insulin and glucagon by the subcutaneous route.
THE COMPLETE ARTIFICIAL PANCREAS
Might other counter-regulatory hormones also be considered? The normal pancreas controls blood glucose through the balanced and coordinated release of insulin, glucagon, amylin, somatostatin, and other pancreatic hormones (Fig. 1) (12). Amylin, which is found in pancreatic beta cells, slows gastric emptying, modulates appetite, and suppresses postprandial glucagon secretion. The 37-amino acid synthetic amylin analog pramlintide acetate has been approved for use by the U.S. Food and Drug Administration since 2005. Numerous clinical studies show that pramlintide acetate significantly reduces post-prandial hyperglycemia, thus decreasing insulin requirements by 30 to 50% without concomitant weight gain (26, 27). Continuous delivery of somatostatin or its analogs also lowers the insulin requirement for diabetic subjects and virtually eliminates hypoglycemia (28). Preclinical and clinical studies have repeatedly shown concomitant insulin and somatostatin administration to be effective in treating diabetic acidosis or in simply decreasing the insulin dose required to maintain normoglycemia (29, 30). Perhaps an optimized algorithm for the co-administration of somatostatin and/or amylin with reduced insulin dosing might also be used to decrease hypoglycemic risk.
Is the co-administration of insulin and glucagon a pragmatic approach to developing a truly feedback-controlled artificial pancreas? The complete sensing and control of hypoglycemia, which involves the intact central and peripheral nervous systems and extrapancreatic organs such as the hepato-portal system, would be challenging to emulate in any electro-mechanical artificial pancreas (31). Intensive insulin therapy with multiple daily injections or by continuous subcutaneous insulin pumps also achieves near-normalization of blood glucose, but with increased hypoglycemic risk in Type 1 diabetes and a substantially increased complexity of execution (3, 32). Hypoglycemia may be partially attributed to the comparatively high insulin concentrations delivered by the peripheral subcutaneous route (33, 34), from latent absorption of insulin from subcutaneous injection depots (even with fast-acting insulin analogs), or from variable insulin absorption and metabolism, as seen in the current study (8).
There are additional practical challenges that also must be considered. Glucagon is inherently chemically and physically unstable and presents significant pharmaceutical formulation challenges (35–37). The tendency of glucagon to fibrillate in solution also could induce an untoward immunogenic response in patients (38). The present study only tested glucagon in solution for a 27-hour period. Today’s insulin pumps provide a 3-day supply of insulin. A usable glucagon infusion system would require demonstrable drug stability of greater than 3 days under worst-case temperature storage and agitation conditions.
Successful closed-loop delivery will require accurate measurement of blood glucose, combined with optimized delivery of insulin, glucagon, or other compounds, and a mathematical algorithm capable of analyzing data and regulating delivery in near real-time and under all means of environmental and physiological stresses (23). Increasingly sophisticated blood glucose control algorithms continue to be developed (21–25). Yet, not all of the variables that influence blood glucose control can be predicted or programmed. Diet, exercise, residual insulin production and extent of insulin dose, site of insulin delivery, and metabolic state are all critical to instantaneous glucose control and cannot be foreseen and foreprogrammed. Technical limitations cannot be underestimated. Analytical errors in glucose measurement, catheter blockage preventing insulin delivery, and extended strenuous exercise or lack of access to carbohydrates may all adversely affect delivery systems.
SMART SYSTEMS, SMARTER CELLS?
As we move to such smart delivery systems, including ones that might employ multiple pancreatic peptide hormones, we must reconsider islet encapsulation and transplantation (39–42). Islets cells can be collected and embedded within a semi-permeable membrane. Glucose and other signals diffuse into the membrane and stimulate insulin release from the beta cell islets within. The membrane carrier enhances the logistics of implantation and unit dosing, and offers a means of protection from the immune system. Islet cells encapsulated in alginate microcapsules or polysulphone hollow fibers are designed to eliminate immune reactivity and reduce the need for immune suppression (39).
Unlike mechanical closed-loop delivery systems, islet cell transplants are generally placed in the portal vein of the recipient (43, 44). The portal vein is the ideal site for such cells, as it is the vessel into which the endocrine pancreas and liver work together to control glucose homeostasis. The liver is central to glucose homeostasis, buffering wide glucose variations as it removes glucose from the bloodstream in times of plenty, and degrades glycogen for energy as needed (45). The liver also detects changes in energy needs by monitoring blood insulin and glucose concentrations. Portal vein glucose detection and direct insulin secretion are the key parameters missing from closed-loop insulin delivery systems.
The physiological attractiveness of beta cell transplantation is none-the-less beset by significant challenges. Mature islet cells do not readily divide, so that the sources of insulin-producing beta cells have traditionally been allograft donors. Additional sources of beta cells are being investigated, including embryonic and adult stem cells, as well as xenografts. Allotransplantation of islet cells has allowed patients to remain independent of exogenous insulin injections for years after the transplant procedure (42, 43). However, beta cell transplants are disadvantaged by the need for immunosuppressive agents, the limited viability of the islets, or, in the case of encapsulated cells, the inevitable confinement of the devices by fibrotic tissue. The optimal glycemic control of beta cell implants is clearly more effective in maintaining normoglycemia compared to closed-loop mechanical devices. However, the paucity of islets, the need for immunosupression, and long-term effectiveness of this approach remain significant challenges.
The quest for greater control of blood glucose has resulted in other important unwanted side effects other than hypoglycemia. Several studies have confirmed detrimental side effects in patients on intensive insulin therapy, includin increased weight gain and blood pressure; elevated blood triglyceride, cholesterol, and low-density lipoprotein (LDL)–C concentrations; lower-than-normal blood concentrations of high-density lipoprotein; and greater-than-normal cholesterol distribution in very low–density lipoprotein (VLDL), intermediate–density lipoprotein (IDL), and dense LDL particles (5, 6). These observations should be of concern to patients with insulin-dependent diabetes given modern society’s lack of attention to Joslin’s first steed, diet. Studies have confirmed the value of improved blood glucose control without an increase in body weight (5).
Exercise—Joslin’s third steed—and insulin exhibit a marked synergistic effect on glucose transport in rodents and people (46, 47). The GLUT-4 glucose transport protein located in skeletal muscle and adipose tissue increases glucose transport from the blood to the muscle (Fig. 1). Over relatively short periods of exercise, GLUT-4 is translocated from an intracellular site to the cell surface. In fact, the GLUT-4 protein is present in increased amounts in endurance-trained individuals compared to sedentary subjects (48, 49). The development of any closed-loop technologies along with means to improve blood glucose control must be balanced with insulin administration, additional medications, diet, and exercise so as not to expose the diabetic patient to glucose concentration extremes or the increased risks of obesity, atherosclerosis, and hypertension (50).
The studies by El-Khatib and others describe unique advanced technologies that can now be used to augment studies of disease biology. Subsequent scientific findings can then drive further technological development. Multi-hormonal infusion may allow investigators to address critical questions regarding the pathobiology of diabetes mellitus and optimization of its therapy. One could well examine studies of the same patients treated with insulin alone or with glucagon, amylin, and somatostatin dispensed according to tight-control or loose-control algorithms, which would allow investigators to ask a series of questions that heretofore could not be addressed. What, for example, is the relation between cardiovascular risk factors and blood glucose control, blood insulin concentrations, or diurnal variations in hormonal and glucose metabolism? Delineation of optimal set points that balance secreted hormone levels and metabolic set points could then be integrated with additional variables, such as diet and physical activity, to enable the development of new algorithm guidelines that provide patients with insulin-dependent diabetes better health and greater independence.
Until there is a cure for insulin-dependent diabetes, the influence of the patient on controlling his or her diabetes will remain significant. As Joslin noted in 1933, people are the primary controller of their diabetic fate. “Insulin, the second horse of the diabetic’s chariot, is a clever steed, practically never fails to do what he is asked, but unless understood, may run away with the driver. One needs a good many lessons and much practice to ride a horse, and this Insulin horse is no exception” (1). We have learned a great deal since Joslin wrote this piece in the Joslin Manual. We must continue to investigate intelligent and flexible methods to translate these lessons to computer-controlled closed-loop insulin delivery.
Acknowledgments
E.R.E. is supported in part by grants from the U.S. National Institutes of Health (R01 GM 49039).
REFERENCES AND NOTES
- 1.Joslin EP. Diabetic Manual for the Mutual Use of Doctor and Patient. ed. 5th. Philadelphia, PA: Lea & Febiger; 1933. The treatment of diabetes with diet and exercise; pp. 100–120. [Google Scholar]
- 2.Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. ed. 1st. Philadelphia, PA: Lea & Febinger; 1918. [Google Scholar]
- 3.Cryer PE, Gerich JE. Glucose counterregulation, hypoglycemia, and intensive insulin therapy in diabetes mellitus. N Engl. J. Med. 1985;313:232–241. doi: 10.1056/NEJM198507253130405. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N/ Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Palmer AJ, Roze S, Valentine WJ, Minshall ME, Lammert M, Nicklasson L, Spinas GA. Deleterious effects of increased body weight associated with intensive insulin therapy for type 1 diabetes: increased blood pressure and worsened lipid profile partially negate improvements in life expectancy. Curr. Med. Res. Opin. 2004;20(Suppl 1):S67–S73. doi: 10.1185/030079904X2033. [DOI] [PubMed] [Google Scholar]
- 6.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of Type 1 diabetes on lipid levels and blood pressure: Results from the DCCT. JAMA. 1998;280:140–146. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuinness OP, Myers SR, Neal D, Cherrington AD. Chronic hyperinsulinemia decreases insulin action but not insulin sensitivity. Metabolism. 1990;39:931–937. doi: 10.1016/0026-0495(90)90303-t. [DOI] [PubMed] [Google Scholar]
- 8.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano EDR. A Bi-hormonal closed-loop artificial pancreas for Type 1 diabetes. Sci. Transl. Med. 2010;2:27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langerhans P. Beiträge zur mikroskopischen Anatomie der Bauchspeicheldrüse, Doctoral thesis [Berlin Pathological Institute (1869)], reprinted in English in Bull. Inst. Hist. Med. 1933;5:259–297. [Google Scholar]
- 10.Opie EL. On the histology of the islands of Langerhans of the pancreas. BJHH. 1900;11:205–209. [Google Scholar]
- 11.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thaler JP, Cummings DE. Food alert. Nature. 2008;452:941–942. doi: 10.1038/452941a. [DOI] [PubMed] [Google Scholar]
- 13.Kimball C, Murlin J. Aqueous extracts of pancreas III. Some precipitation reactions of insulin. J Biol. Chem. 1923;58:337–348. [Google Scholar]
- 14.Polonsky KS, Given BD, Cauter EV. twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin. Invest. 1998;81:442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle (Boland) EA, Weinzimer SA, Steffen AT, Ahern JAH, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care. 2004;27:1554–1558. doi: 10.2337/diacare.27.7.1554. [DOI] [PubMed] [Google Scholar]
- 16.Ahern JAH, Boland EA, Doane R, Ahern JJ, Rose P, Vincent M, Tamborlane WV. Insulin pump therapy in pediatrics: a therapeutic alternative to safely lower HbA1c levels across all age groups. Pediatric Diabetes. 2002;3:10–15. doi: 10.1034/j.1399-5448.2002.30103.x. [DOI] [PubMed] [Google Scholar]
- 17.Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber J-P. The study group for the development of pump therapy in diabetes: Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment. Diabetes Care. 2000;23:1232–1235. doi: 10.2337/diacare.23.9.1232. [DOI] [PubMed] [Google Scholar]
- 18.Renard E. Intensive insulin therapy today: ‘Basal-bolus’ using multiple daily injectionsor CSII? Diabetes Metab. 2005;31:4S40–44S44. doi: 10.1016/s1262-3636(05)88266-7. [DOI] [PubMed] [Google Scholar]
- 19.Bondia J, Dassau E, Zisser H, Calm R, Vehí J, Jovanovič L, III FJD. Coordinated basal-bolus infusion for tighter postprandial glucose control in insulin pump therapy. J Diabetes Sci. Technol. 2009;3:89–97. doi: 10.1177/193229680900300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogt EJ, Dodd LM, Jenning EM, Clemens AH. Development and evaluation of a glucose analyzer for a glucose-controlled insulin infusion system (Biostator®) Clin. Chem. 1978;24:1366–1372. [PubMed] [Google Scholar]
- 21.Hovorka R. Continuous glucose monitoring and closed loop systems. Diabetic Medicine. 2005;23:1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 22.Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AMF, Nodale M, Palma AD, Wilinska ME, Acerini CL, Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. The Lancet. 2010;135:743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 23.Rebrin K, Steil GM, Antwerp WPv, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am. J. Physiol. Endocrinol. Metab. 1999;277:561–571. doi: 10.1152/ajpendo.1999.277.3.E561. [DOI] [PubMed] [Google Scholar]
- 24.Steil G, Rebrin K, Mastrototaro JJ. Metabolic modeling and the closed-loop insulin delivery problem. Diabetes Res. Clin. Practice. 2006;74:S183–S186. doi: 10.1016/S0168-8227(06)70028-6. [DOI] [PubMed] [Google Scholar]
- 25.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 26.Ratner R, Whitehouse F, Fineman MS, Strobel S, Shen L, Maggs DG, Kolterman OG, Weyer C. Adjunctive therapy with pramlintide lowers HbA1c without concomitant weight gain and increased risk of severe hypoglycemia in patients with type 1 diabetes approaching glycemic targets. Exp. Clin. Endocrinol. Diabetes. 2005;113:199–204. doi: 10.1055/s-2005-837662. [DOI] [PubMed] [Google Scholar]
- 27.Weyer C, Gottlieb A, Kim DD, Lutz K, Schwartz S, Gutierrez M, Wang Y, Ruggles JA, Kolterman OG, Maggs DG. Pramlintide reduces postprandial glucose excursions when added to regular insulin or insulin lispro in subjects with type 1 diabetes. Diabetes Care. 2003;26:3074–3079. doi: 10.2337/diacare.26.11.3074. [DOI] [PubMed] [Google Scholar]
- 28.Bruttomesso D, Fongher C, Silvestri B, Barberio S, Marescotti MC, Iori E, Valerio A, Crazzolara D, Pianta A, Tiengo A, Prato SD. Combination of continuous subcutaneous infusion of insulin and octreotide in Type 1 diabetic patients. Diabetes Res. Clin. Practice. 2001;51:97–105. doi: 10.1016/s0168-8227(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 29.Bosnak M, Dikici B, Dogru O, Davutoglu M, Haspolat K. Somatostatin therapy in the management of resistant diabetic ketoacidosis. Diabetes Care. 2002;25:629–630. doi: 10.2337/diacare.25.3.629. [DOI] [PubMed] [Google Scholar]
- 30.Edelman ER, Brown L, Langer R. Quantification of insulin release from implantable polymer-based delivery systems and augmentation of therapeutic effect with simultaneous release of somatostatin. J Pharm. Sci. 1996;85:1271–1275. doi: 10.1021/js9601694. [DOI] [PubMed] [Google Scholar]
- 31.Cherrington AD. Central versus peripheral glucose sensing and the response to hypoglycemia. Diabetes. 2008;57:1158–1159. doi: 10.2337/db08-0315. [DOI] [PubMed] [Google Scholar]
- 32.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Schichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complicaions in Japanese patients with non-insulin dependent diabetes mellitus: a randomized propespective 6-year study. Diab. Res. Clin. Prac. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 33.Bolli GB, Gottesman IS, Cryer PE, Gerich JE. Glucose counterregulation during prolonged hypoglycemia in normal humans. Am. J. Physiol. Endocrinol. Metab. 1984;247:E206–E214. doi: 10.1152/ajpendo.1984.247.2.E206. [DOI] [PubMed] [Google Scholar]
- 34.Kruszynska YT, Home RD, Alberti KGMM. Comparison of portal and peripheral insulin delivery on carbohydrate metabolism in streptozotocin-diabetic rats. Diabetologia. 1985;28:161–171. doi: 10.1007/BF00273866. [DOI] [PubMed] [Google Scholar]
- 35.Matilainen L, Larsen KL, Wimmer R, Keski-Rahkonen P, Auriola S, Järvinen T, Jarho P. The Effect of Cyclodextrins on Chemical and Physical Stability of Glucagon and Characterization of Glucagon/γ-CD Inclusion Complexes. J Pharm. Sci. 2008;97:2720–2729. doi: 10.1002/jps.21209. [DOI] [PubMed] [Google Scholar]
- 36.Onoue S, Iwasa S, Kojima T, Katoh F, Debari K, Koh K, Matsuda Y, Yajima T. Structural transition of glucagon in the concentrated solution observed by electrophoretic and spectroscopic techniques. J Chromatogr. A. 2006;1109:167–173. doi: 10.1016/j.chroma.2005.11.130. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen JS, Dikov D, Flink JL, Hjuler HA, Christiansen G, Otzen DE. The changing face of glucagon fibrillation: Structural polymorphism and conformational imprinting. J Mol. Biol. 2006;355:501–523. doi: 10.1016/j.jmb.2005.09.100. [DOI] [PubMed] [Google Scholar]
- 38.Frokjaer S, Otzen DE. Protein drug stability: A formulation challenge. Nat. Rev. Drug Disc. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 39.Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. Islet encapsulation: Strategies to enhance islet cell functions. Tissue Engineering. 2007;13:589–599. doi: 10.1089/ten.2006.0183. [DOI] [PubMed] [Google Scholar]
- 40.Bellin MD, Kandaswamy R, Parkeyb J, Zhang HJ, Liu B, Ihm SH, Ansite JD, Witsonb J, Bansal-Pakala P, Balamuruganb AN, Papas K, Sutherland DER, Moran A, Hering BJ. Prolonged insulin independence after islet allotransplants in recipients with Type 1 diabetes. Am. J. Transplant. 8:263–2470. doi: 10.1111/j.1600-6143.2008.02404.x. 2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gimi B, Kwon J, Kuznetsov A, Vachha B, Magin RL, Philipson LH, Lee J-B. A Nanoporous, Transparent microcontainer for encapsulated islet therapy. J Diabetes Sci.Technol. 2009;3:297–303. doi: 10.1901/jaba.2009.3-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nature Biotechnology. 1996;14:1107–1111. doi: 10.1038/nbt0996-1107. [DOI] [PubMed] [Google Scholar]
- 43.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JRT, Shapiro AMJ. Five-year fllow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 44.Stock PG, Bluestone JA. Beta-cell replacement for Type 1 diabetes. Annu. Rev. Med. 2004;55:133–156. doi: 10.1146/annurev.med.55.091902.103539. [DOI] [PubMed] [Google Scholar]
- 45.Gribble FM. A higher power for insulin. Nature. 2005;434:965–966. doi: 10.1038/434965a. [DOI] [PubMed] [Google Scholar]
- 46.Goodyear LJ. Exercise, glucose transport, and insulin sensitivity. Annu. Rev. Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 47.Thorell A, Hirshman MF, Nygren J, Jorfeldt L, Wojtaszewski JFP, Dufresne SD, Horton ES, Ljungqvist O, Goodyear LJ. Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 1999;277:E733–E741. doi: 10.1152/ajpendo.1999.277.4.E733. [DOI] [PubMed] [Google Scholar]
- 48.Andersen PH, Lund S, Schmitz O, Junker S, Kahn BB, Pedersen O. Increased insulin-stimulated glucose uptake in athletes: The importance of GLUT4 mRNA, GLUT4 protein and fibre type composition of skeletal muscle. Acta Physiol. Scand. 1993;149:393–404. doi: 10.1111/j.1748-1716.1993.tb09635.x. [DOI] [PubMed] [Google Scholar]
- 49.Daugaard JR, Richter EA. Relationship between muscle fibre composition, glucose transporter protein 4 and exercise training: possible consequences in non-insulin-dependent diabetes mellitus. Acta Physiol. Scand. 2001;171:267–276. doi: 10.1046/j.1365-201x.2001.00829.x. [DOI] [PubMed] [Google Scholar]
- 50.Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children. Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]