Abstract
Purpose
Sampling of arterial blood for metabolite correction is often required to define a true radiotracer input function in quantitative modeling of PET data. However, arterial puncture for blood sampling is often undesirable. To establish whether venous blood could substitute for arterial blood in metabolite analysis for quantitative PET studies with 1-[11C]acetate and 1-[11C]palmitate, we compared the results of [11C]CO2-metabolite analyses performed on simultaneously collected arterial and venous blood samples.
Methods
Paired arterial and venous blood samples were drawn from anesthetized pigs at 1, 3, 6, 8, 10, 15, 20, 25 and 30 minutes after i.v. administration of 1-[11C]acetate and 1[11C]palmitate. Blood radioactivity present as [11C]CO2 was determined employing a validated 10-minutes gas-purge method. Briefly, total blood 11C radioactivity was counted in base-treated [11C]-blood samples, and non-[11C]CO2 radioactivity was counted after the [11C]-blood was acidified using 6N HCl and bubbled with air for 10 minutes to quantitatively remove [11C]CO2.
Results
An excellent correlation was found between concurrent arterial and venous [11C]CO2 levels. For the [11C]acetate study, the regression equation derived to estimate the venous [11C]CO2 from the arterial values was:y = 0.994x + 0.004 (r2=0.97), and for the[11C]palmitate: y = 0.964x -0.001 (r2=0.9).Over the 1-30 minute period, the fraction of total blood 11C present as [11C]CO2 rose from 4% to 64% for acetate, and 0% to 24% for palmitate. The rate of [11C]CO2 appearance in venous blood appears similar for the pig model and humans following i.v. [11C]-acetate administration.
Conclusion
Venous blood [11C]CO2 values appear suitable as substitutes for arterial blood samples in [11C]CO2 metabolite analysis after administration of [11C]acetate or [11C]palmitate.
Advances in Knowledge and Implications for Patient Care
Quantitative PET studies employing 1-[11C]acetate and 1-[11C]palmitate can employ venous blood samples for metabolite correction of an image-derived tracer arterial input function, thereby avoiding the risks of direct arterial blood sampling.
Keywords: arterial, venous, 11CO2, metabolite, acetate, palmitate
1. Introduction
Simple carboxylic acids radiolabeled with carbon-11 (t1/2: 20.4 min), especially [11C]acetate and [11C]palmitate, have been used widely as tools for imaging with positron emission tomography (PET) to evaluate the rate at which their endogenous counterparts are being oxidatively metabolized by myocardium under various physiological conditions. One of the major radiolabeled metabolites produced by these compounds is [11C]CO2, a product of the tricarboxylic acid cycle. Models for quantification of myocardial metabolic rate from the resulting PET data require knowledge of both the concentration of radioactivity in myocardium as a function of time, as well as knowledge of the concentration of radiopharmaceutical in the arterial blood supplying that tissue. While the PET images readily quantify radioactivity in the myocardium, as well as the arterial blood radioactivity (based on a region of interest in the cavity of the left ventricle or left atrium), those PET data simply define regional radionuclide concentrations without information on the chemical form of the radionuclide. Thus, for tracer kinetic modeling to quantify metabolic rates, it is necessary to independently measure the fraction of total blood 11C activity present in blood as [11C]CO2 to generate a correct radiopharmaceutical arterial input function.
The technique most commonly used to determine the radiolabeled CO2 concentration of blood is simplein vitro acidification of a blood sample, followed by purging with an inert gas to drive off CO2. The fraction of total blood 11C activity that remains after the gas purge represents 11C species other than 11CO2. This method has been used in a number of preclinical and clinical PET studies yielding important insights into myocardial metabolism. Here we report experimental validation of the selectivity of the gas-purge technique for removing 11CO2 from blood. Since direct arterial cannulation for blood sampling is invasive, painful, and can result in undesirable complications, we have then applied this analytical method to assess whether arterial blood [11C]CO2 concentrations following administration of [11C]acetate and [11C]palmitate can be accurately, and less invasively, estimated by the [11C]CO2 levels of venous blood.
2. Materials and Methods
2.1 General
The [11C]CO2, 1-[11C]acetate, and 1-[11C]palmitate radiotracers were obtained from the Radiochemistry Core of the Indiana Institute for Biomedical Imaging Sciences (IIBIS). The 1-[11C]acetate and 1-[11C]palmitate tracers were prepared following the published methods. The [11C]CO2 in aqueous solution was obtained by dissolving the no-carrier-added [11C]CO2 gas in a 2 mL sodium hydroxide (100mM) solution. The resultant sodium 11C-bicarbonate solution was then mixed with 3 mL of saline. The final solution contained 10-15 mCi/mL (370-555 MBq/mL). Theheparanized blood samples used for the air purge validation study were taken from the arterial line of swineprior to initiation of planned PET studies. Heparinized blood samples for the arterial-venous comparison study were obtained from four mature miniature pigs (male; ∼25kg) at specified times after sequential injection of 1-[11C]acetate and 1-[11C]palmitate(allowing ∼90-minutes between injections for decay of the prior dose). Arterial blood samples were drawn from the carotid artery, while venous blood was sampled from the jugular vein. The pigs were anesthetized with 1-3% isoflurane and mechanically ventilated throughout the period of the study. The protocol for the animal studies was approved by the Indiana University Animal Care and Use Committee. Radioactivity measurements were made by dose calibrator or NaI(Tl) automatic gamma counter (Beckman Gamma 8000).
2.2 Validation of protocol for the determination of [11C]CO2 in blood
Radioactive sample handling was carried out behind lead-shielding in a chemical fume hood. A 50-100 μCi sample (20-100 μL) of aqueous [11C]CO2 or [11C]acetate or [11C]palmitate/HSA was added to 3 mL of blood in vitro and gently mixed. A 1 mL aliquot of the blood was then transferred to a counting vial containing 1 mL sodium hydroxide(0.1 N) and 3 mL isopropanol, which was then immediately capped to retain any 11CO2 as bicarbonate. This base-treated blood contained all 11C radioactivity (vial A). A second 1 mL aliquot of the blood containing radiotracer was added to a counting vial containing 1 mL sodium bicarbonate (0.5 N) and 3 mL isopropanol, and the mixture was vortex mixed. Then, 1 mL 6N hydrochloric acid was added followed by 10-20 secondsof vortex mixing. Next, the acid-treated blood sample was purged with air,bubbled into the blood via a 4-inch flexible filter straw (B Braun Medical, Inc., Bethlehem, PA), for 10 and 15 minutes to drive off the 11CO2 (vial B). The activity remaining in “vial B” represents for total non-11CO2 radioactivity. After decay correction of the radioactivity measurements to a common time point, the percentage of total radioactivity released as 11CO2 was calculated as:
2.3 Determination of [11C]CO2 in paired arterial and venous blood samples afterintravenous administration of [11C]acetate and [11C]palmitate
Paired arterial and venous blood samples (each 2-3 mL) were drawn simultaneously from anesthetized swine into heparinized syringes at 1, 3, 6, 8, 10, 15, 20, 25 and 30 min after i.v. administration of 1-[11C]acetate (32.2±3.5 mCi; 1.19±0.1 GBq; n=4) or 1-[11C]palmitate (27.5±5.4 mCi;1.0±0.2 GBq; n=4). For each time point, a 1 mL aliquot of the blood was transferred to a counting vial containing 1 mL 0.1 N sodium hydroxide and 3 mL isopropanol, and then immediately capped to retain any 11CO2 as bicarbonate (vial A). A second 1 mL aliquot of each blood sample was added to a counting vial containing 1 mL of 0.5 N sodium bicarbonate and 3 mL isopropanol. Then, 1 mL of 6N hydrochloric acid was added, and the acid-treated blood was vortex mixed.The mixture was then bubbled with air for 10 minutes to release 11CO2 (vial B). The 11C radioactivity in each vial was then measured with an automatic gamma counter, and after decay correction to a common time point, the fraction of total blood 11C present as [11C]CO2 was calculated as:
2.4 Analysis of blood 11CO2 after [11C] acetate administration to humans
In conjunction with an IRB-approved study of [11C]acetate PET imaging in humans, the levels of [11C]CO2 were also determined in peripheral venous human blood samples. The blood was drawn from a forearm vein into heparinized syringes at 1, 3, 6, 8, 10, 15, 20, 25 and 30 min (2-3 mL each) following i.v. administration of 1-[11C]acetate (876.9±140.6 MBq;3 men and 3 women) and immediately analyzed following the procedures described above.
3. Results and Discussion
To verify the suitability of the gas purge method for the determining the level of blood-borne [11C]CO2, analysis was carried out after directly mixing [11C]CO2 with whole blood in vitro. After acidification and air bubbling through the sample for 10-minutes, more than 99% of the 11C was released from the [11C]CO2-spiked whole blood (Table 1). Continuation of the gas purge for an additional 5-minutes did not appreciably alter the residual blood 11C level. As expected, blood samples similarly spiked with [11C]acetate or [11C]palmitate showed no loss of radioactivity, even after 15 min of air purging (Table 1). These results are similar to previous reported findings, where 95% of [11C]acetate was retained, and 98% of [11C]-labeled bicarbonate was eliminated, in acid-treated blood samples after 10-minutes of inert gas bubbling. This validation study confirmed that the gas purge method was quite effective for selectively releasing [11C]CO2 from acid-treated whole blood. Additionally, the analysis method is technically straightforward, simple to implement, and can readily be completed on a time frame compatible with the 20-minute 11C half-life.
Table 1.
Effectiveness of the gas-purge method for selectively removing [11C]CO2 from acidified whole blood.
| Radiopharmaceutical Added to Blood | Percentage of initial 11C lost from blood sample after 10-minute air purge |
|---|---|
| 11CO2 | 99.8 ± 0.3% (n = 4) |
| 1-[11C]acetate | -0.1 ± 0.5% (n = 3) * |
| 1-[11C]palmitate | -2.4 ± 1.9% (n = 3) * |
All results were reported as the mean ± SD for the number of experiments performed (n)
15 min of air purge
To evaluate whether venous blood sampling might reliably substitute for arterial blood sampling as this method is translated to support quantitative PET studies of acetate and palmitate metabolism in humans, we wanted to examine the correlation between arterial and venous blood [11C]CO2 concentrations after i.v. administration of [11C]acetate or [11C]palmitate. The kinetic models for quantification of tissue metabolism with [11C]acetate and [11C]palmitate require knowledge of the true tracer arterial blood time-activity curve, but direct arterial cannulation for blood sampling is undesirably invasive in human studies. Reliance solely on the PET data for quantification of the total arterial blood level of radioactivity, coupled with venous blood sampling for metabolite analysis, is far more practical to implement in clinical studies. But, this latter approach is only valid if venous blood can be demonstrated to be a reasonable surrogate for quantifying the relative levels of parent tracer and metabolite(s) in arterial blood.
Paired arterial and venous blood samples were concurrently collected at 1, 3, 6, 8, 10, 15, 20, 25 and 30 minutes after i.v. administration of either 1-[11C]acetate and 1-[11C]palmitate in four mini pigs at rest. (The length of each imaging session was 30-minutes, setting the final blood sampling time.) Each of the collected blood samples was analyzed immediately to determine the fraction of total blood radioactivity present as [11C]CO2.
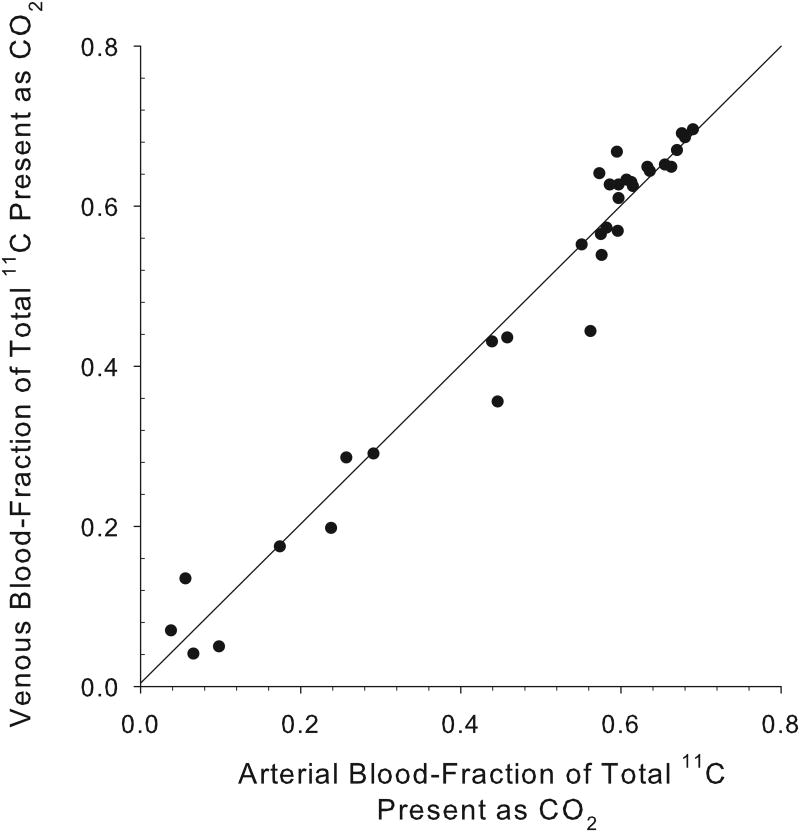
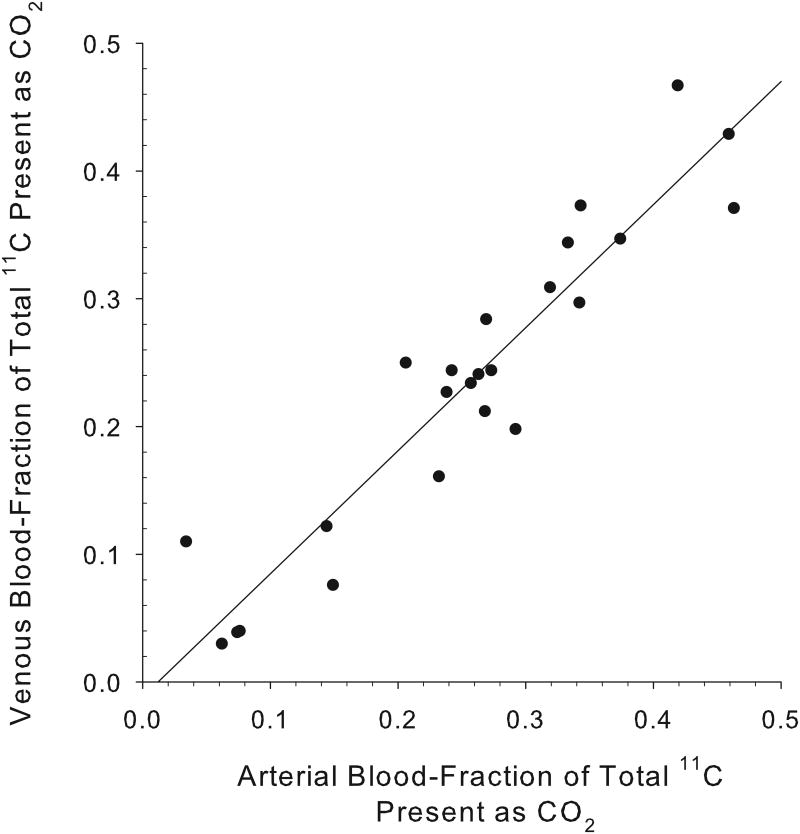
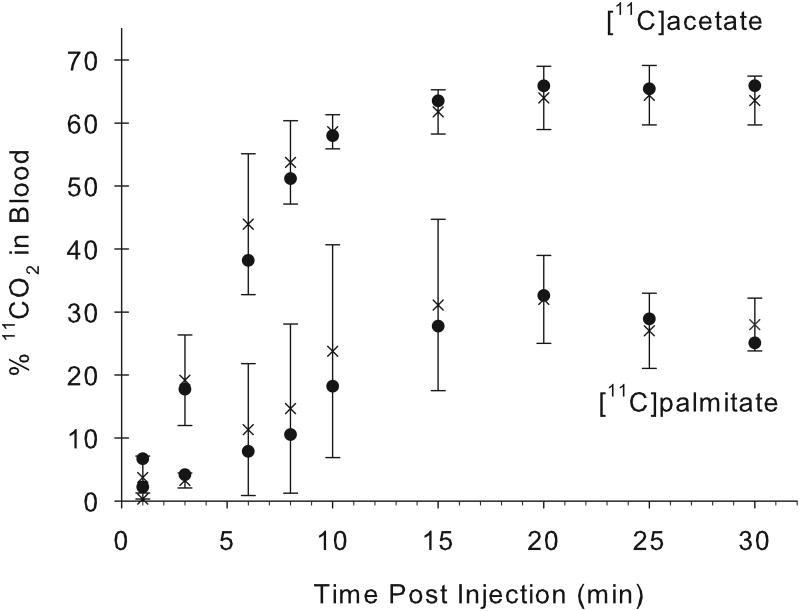
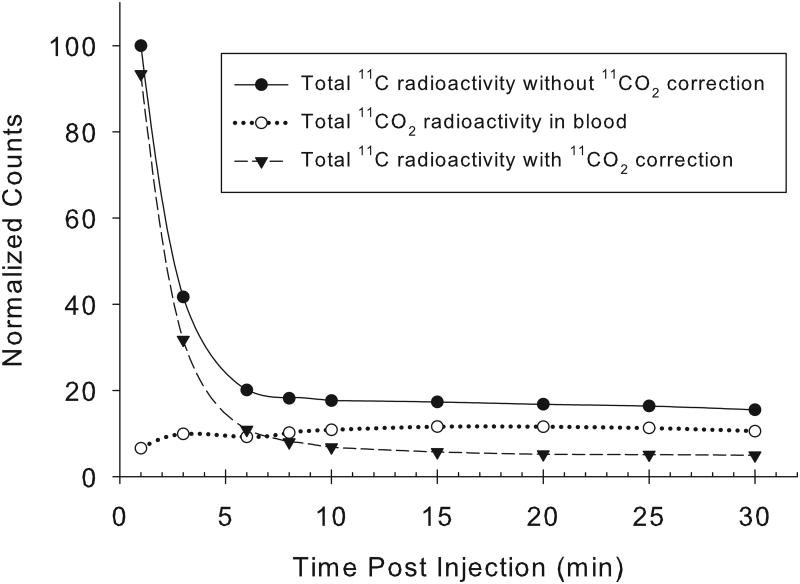
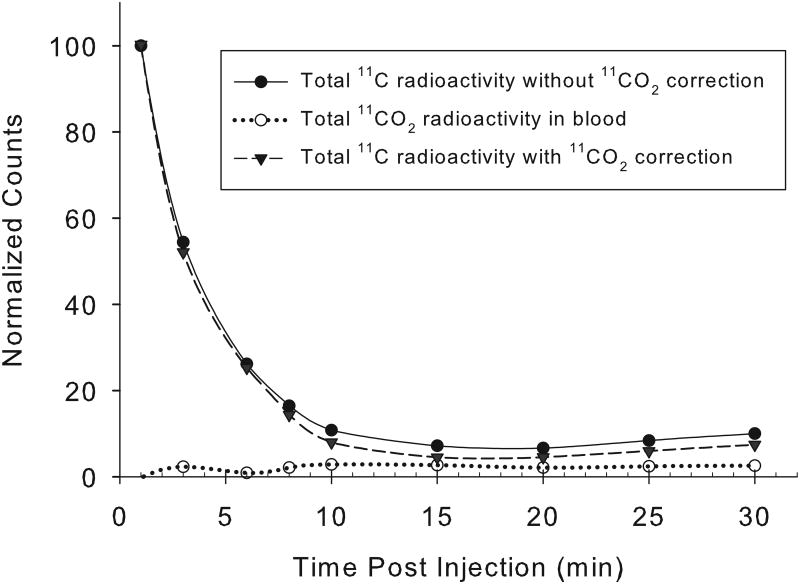
An excellent correlation was found between the arterial and venous blood [11C]CO2 concentrations at all times following [11C]acetate injection, with a correlation coefficient, r2=0.97 (n=34 pairs; Fig. 1A). For 11C-palmitate, there is also a good correlation between the measured fractions of radioactivity present as [11C]CO2 in arterial and venous blood (r2= 0.9;n=26 pairs; Fig. 1B). Over the 1-30 minute period, the fraction of total blood 11C present as 11CO2rose from 4% to 64% for acetate, and from 0% to 24% for palmitate (Fig. 2). However, comparing [11C]acetate to [11C]palmitate, there is a marked difference in the rates at which total blood radioactivity comes to be dominated by the [11C]CO2 metabolite.
Fig. 1.
Concordance of measured arterial and venous [11C]CO2 levels. For [11C]acetate (A), the data shown represent a total of 34 paired swine arterial and venous blood samples collected at times: 1, 3, 6, 8,10, 15, 20, 25 and 30 minutes post- i.v injection (y = 0.994x + 0.004; r2= 0.97). At all time points from 1-30 minutes post-injection, venous and arterialblood concentrations of [11C]CO2 appear essentially identical.For [11C]palmitate (B), the data shown represent 26 paired blood samples collected at 6, 8, 10, 15, 20, 25 and 30 minutes post-i.v injection (y = 0.964x - 0.011; r2=0.9). Data from one and three minutes post injection of [11C]palmitate are omitted due to the absence of 11CO2 in blood.
Fig. 2.
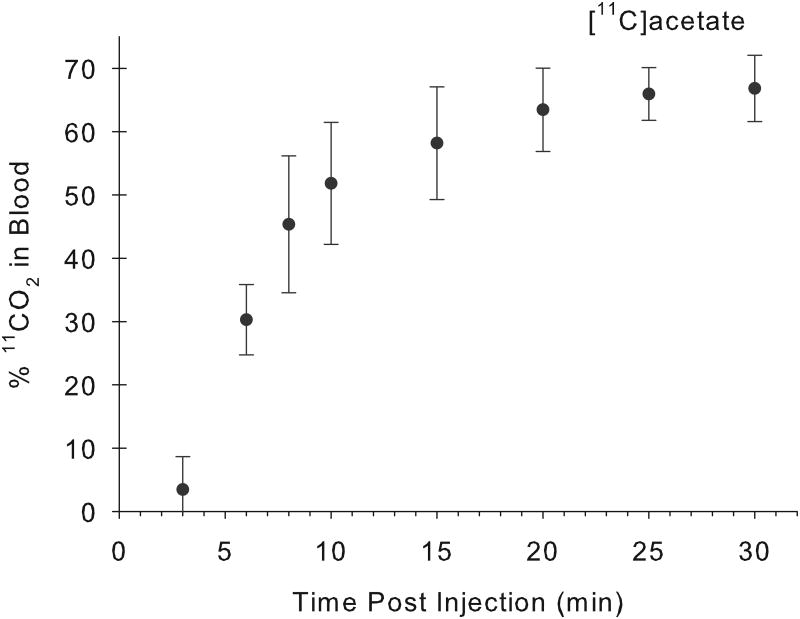
Fractions of arterial and venous total blood radioactivity present as 11CO2 following i.v. administration of [11C]acetate and [11C]palmitate to miniaturepig (A; n =4), and human (B; n = 6).In pig, the percentage of 11CO2 rose from 4% to 64% for acetate, and 0% to 24% for palmitate, over a 30-minute period following the i.v administration of the indicated tracer. The rate of 11CO2 appearance in human blood following[11C]acetate administration is very similar to the finding in the pig model. Values given are% 11CO2 in blood (mean±S.D.)[x : arterial blood; ● : venous blood].
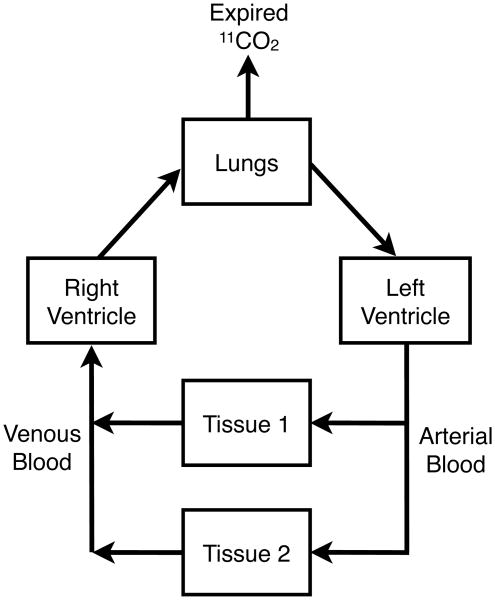
The concordance of the metabolite data for these paired venous and arterial blood samples has a significant impact on imaging study design. For PET studies to quantify the rates of tissue metabolism of [11C]acetate and/or [11C]palmitate, venous blood sampling will be adequate for determining the [11C]CO2 metabolite correction required in an image-derived arterial blood 11C time-activity curve. Since 11CO2 is exhaled, the simple mass balance obviously requires that arterial blood have a lower 11CO2 concentration than the venous blood entering the lungs (Fig.3). Our results, however, indicate the fractional loss of 11CO2 from the labeled blood pool of CO2/H2CO3 is sufficiently small that blood samples from the vein remain a reasonable surrogate for defining the arterial blood level of the [11C]CO2 metabolite after administration of 1-[11C]acetate and 1-[11C]palmitate.
Fig. 3.
While exhaled 11CO2 precludes arterial and venous blood from having absolutely identical concentrations of the [11C]CO2 metabolite of [11C]acetate and [11C]palmitate, the data from this study indicates that venous blood samples remain an excellent surrogate for quantifying the fraction of blood 11C radioactivity present as [11C]CO2 after i.v. administration of these radiopharmaceuticals.
Arterial blood time-radioactivity curves showed similarly rapid clearance of both 1-[11C]acetate and 1-[11C]palmitate (Fig. 4). In the myocardium, [11C]acetate is converted to acetyl-CoA, and immediately enters the tricarboxylic acid cycle, where radioactive CO2 is produced as its end product. The fraction of blood 11C present as [11C]CO2 in arterial/venous increased over time steadily, with the absolute [11C]CO2 concentration reaching a peak at about 10-15 min post injection, and then remaining stable until the end of imaging at 30 minutes. As shown in Figure 2A, by 15 minutes after [11C]acetate administration, approximately 60-70% of the blood radioactivity was labeled bicarbonate, with the radioactivity of 11CO2/HCO3- contributing significantly to the blood radioactivity at even a few minutes post-radiopharmaceutical administration. These results are consistent with previous studies.
Fig. 4.
Representative normalized decay-corrected arterial blood time-radioactivity curves obtained from a single pig by direct measurement of 11C radioactivity in sampled arterial blood. The [11C]-acetate (A) and [11C]-palmitate (B) are both fairly cleared rapidly from blood, with the [11C]CO2 metabolite representing a significant fraction of the total radioactivity beyond a few minutes post-injection.
In the case of [11C]palmitate, approximately 25-30% of the blood radioactivity was present as 11CO2 at 20 min post-administration (Fig. 2A). This result is also similar to previously reported findings. Unlike 1-[11C]acetate, the 16-carbon 1-[11C]palmitate has a more complicated biochemical fate. Some of the 1-[11C]palmitate undergoes β-oxidation, where a 2-C fragment is released as acetyl-CoA and enters the citric acid cycle for complete oxidation. On the other hand, some of the 1-[11C]palmitate administrated may not undergo immediate oxidation, but instead be stored as triglyceride.
For comparison with the findings in the pig model, the levels of 11CO2 in venous blood were also measured in six human subjects after intravenous administration of 1-[11C]acetate(Fig. 2B). The rate at which human blood radioactivity becomes predominantly 11CO2 is very similar to the findings in the pig model.
4 Conclusions
The 10-minute gas purge method is quite reliable for removing 11CO2 from blood. In modeling to quantify the rate of tissue oxidative metabolism using [11C]acetate and [11C]palmitate, [11C]CO2 levels measured in venous blood appear suitable as a surrogate for quantifying the metabolite contribution to total 11C-radioactivity in arterial blood.
Acknowledgments
Support for this work came from Eli Lilly, NIH (grants NHLBI 092245 and HL 092799), as well as the Indiana Institute for Biomedical Imaging Sciences (IIBIS) core resource of the Department of Radiology, Indiana University School of Medicine. These tracer development and validation efforts were also partially underwritten by the Department of Radiology and Imaging Sciences through the Indiana Institute for Biomedical Imaging Sciences (IIBIS). YN gratefully acknowledges the fellowship support provided by Public Service Department of Malaysia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fox KA, Abendschein DR, Ambos HD, Sobel BE, Bergmann SR. Efflux of metabolized and nonmetabolized fatty acid from canine myocardium. Implications for quantifying myocardial metabolism tomographically. Circ Res. 1985;57(2):232–43. doi: 10.1161/01.res.57.2.232. [DOI] [PubMed] [Google Scholar]
- 2.Kisrieva-Ware Z, Coggan AR, Sharp TL, Dence CS, Gropler RJ, Herrero P. Assessment of myocardial triglyceride oxidation with PET and C-11-palmitate. J Nucl Cardiol. 2009;16(3):411–21. doi: 10.1007/s12350-009-9051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown MA, Myears DW, Bergmann SR. Validity of estimates of myocardial oxidative-metabolism with C-11 acetate and positron emission tomography despite altered patterns of substrate utilization. J Nucl Med. 1989;30(2):187–93. [PubMed] [Google Scholar]
- 4.Walsh MN, Geltman EM, Brown MA, Henes CG, Weinheimer CJ, Sobel BE, et al. Noninvasive estimation of regional myocardial oxygen-consumption by positron emission tomography with C-11 acetate in patients with myocardial-infarction. J Nucl Med. 1989;30(11):1798–808. [PubMed] [Google Scholar]
- 5.Clark VL, Kruse JA. Arterial catherization. Crit Care Clin. 1992;8:687–97. [PubMed] [Google Scholar]
- 6.Mock BH, Brown-Proctor C, Green MA, Steele B, Glick-Wilson BE, Zheng QH. An automated SPE-based high-yield synthesis of [C-11]acetate and [C-11]palmitate: no liquid-liquid extraction, solvent evaporation or distillation required. Nucl Med Biol. 2011;38(8):1135–42. doi: 10.1016/j.nucmedbio.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Brown MA, Myears DW, Bergmann SR. Noninvasive assessment of canine myocardial oxidative-metabolism with C-11 acetate and positron emission tomography. J Am Coll Cardiol. 1988;12(4):1054–63. doi: 10.1016/0735-1097(88)90476-7. [DOI] [PubMed] [Google Scholar]
- 8.Shields AF, Graham MM, Kozawa SM, Kozell LB, Link JM, Swenson ER, et al. Contribution of labeled carbon-dioxide to PET imaging of carbon-11-labeled compounds. J Nucl Med. 1992;33(4):581–4. [PubMed] [Google Scholar]
- 9.Collier TL, Hwang Y, Ramasamy R, Sciacca RR, Hickey KT, Simpson NR, et al. Synthesis and initial evaluation of 17-C-11-heptadecanoic acid for measurement of myocardial fatty acid metabolism. J Nucl Med. 2002;43(12):1707–14. [PubMed] [Google Scholar]