Abstract
The development of a reagent for the efficient synthesis of 5- and 6-membered azoles at room temperature is proposed. A variety of substituted 2-aminobenzimidazoles are synthesized in good to excellent yields. The ability to incorporate various protecting groups makes the imidoyl dichloride reagent amenable to a large number of syntheses. The reagent is applied to the total synthesis of the 2-aminobenzimidazole containing carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), from 2-chloro-3-nitropyridine in >60 % yield in 6 steps.
Keywords: 2-aminobenzimidazole, Imidoyl dichloride, Azole synthesis, PhIP, Benzimidazole
Graphical Abstract
1. Introduction
The 2-aminobenzimidazole core is an integral component of many compounds having interesting biological activity. This core is found in antagonists against glucagon receptor,1 G9a histone methyltransferase,2 IL-1 receptor-associated kinase-4,3 inducible T-cell kinase,4 and H3-receptor.5 Additionally, drugs with this core have shown antimicrobial and antifungal activity including inhibition of the growth of gram-negative bacteria and inhibition of biofilm formation.6 Furthermore, 2-aminobenzimidazoles have been reported to have antiproliferative and cytotoxic activity against a variety of cancer cells.7 Because of the diverse biological functions of this class of compounds, a synthetic route that is applicable to multiple substrates, easy to perform and high yielding would facilitate exploration of the immense opportunities for useful bioactivity that is afforded by this core.
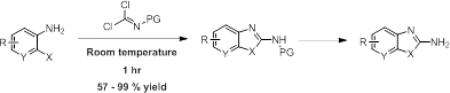
Common methods for generating 2-aminobenzimidazoles include cyclization of o-phenylenediamines with cyanogen bromide, reaction of 2-chlorobenzimidazoles with amines at high temperatures, activation of o-phenylenediamine as a thiourea followed by cyclization and desulfurization using mercury(II) oxide, or reflux of an aryl isothiocyanate with a o-phenylenediamine using a carbodiimide reagent.8 However, because of high temperatures, long reaction times and/or low yields involved in these methods, a rapid and efficient production of 2-aminobenzimidazoles amenable to a variety of functionality is desired.
2. Results and Discussion
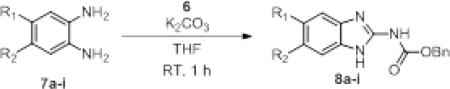
We were inspired by a paper9 utilizing a carbonimidodithioic ester reagent that selectively reacted with anilines in the presence of a free carboxylic acid. However, the reaction necessitated heating in DMF at 150 °C for 9h. We hypothesized that replacement of the thioethers with chlorides would allow for a more rapid reaction, perhaps even at room temperature. The reagent, carboxybenzyl protected imidoyl dichloride (6), was synthesized (Scheme 1) beginning with carbon disulfide and ammonia. The thioethers were installed by reaction with methyl iodide, and the CBZ-protecting group was easily attached under normal conditions. Lastly, the thioethers were substituted using chlorine condensed in CCl4. 6 is an oil that is stable for months in the freezer. The reaction of 6 with 1,2-phenylenediamine proceeded mildly within 1 hr at room temperature in either tetrahydrofuran or dichloromethane with potassium carbonate as the base (Table 1, entry 1). The use of triethylamine resulted in longer reaction times and produced impurities as identified by 1H NMR of the crude product (data not shown).
Scheme 1.
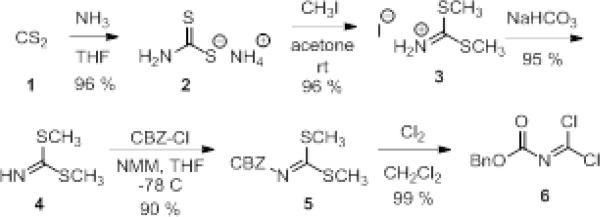
Synthesis of CBZ-protected imidoyl dichloride, 6.
Table 1.
Scope of reaction with aryldiamines.a
| ||||
|---|---|---|---|---|
| entry | R1 | R2 | product | Yield (%) |
| 1b | H | H | 8a | 75 |
| 2 | Br | H | 8b | 76 |
| 3 | F | H | 8c | 71 |
| 4 | Cl | H | 8d | 78 |
| 5 | Cl | Cl | 8e | 97 |
| 6 | CN | H | 8f | 70 |
| 7 | OCH3 | H | 8g | 70 |
| 8 | NO2 | H | 8h | 81 |
| 9 | CO2H | H | 8i | 70 |
Reaction conditions: 7 (0.1 mmol) and K2CO3 (0.25 mmol) were suspended in THF (1 mL). 6 (0.12 mmol) in THF (1 mL) was added and the reaction stirred for 1 hr at RT.
Reaction performed on 1 mmol scale.
We tested the scope of our reagent by reacting 6 with a variety of commercially available o-phenylenediamines (Table 1, entries 2-9) for 1 hr at room temperature with potassium carbonate in tetrahydrofuran. Each reaction proceeded in good to excellent yield under mild conditions. The reaction is tolerant of electron-releasing (entry 7) and electron-withdrawing groups (entries 2, 3, 4, 5, 6, 8) substituents. Of note, only the 2-aminobenzimidazole was formed even in the presence of a free carboxylic acid (entry 9).
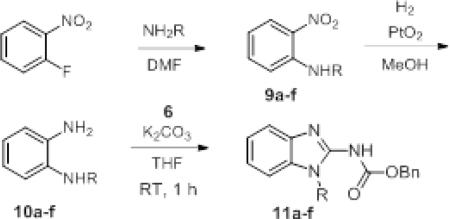
Next, we expanded the screen to include N1-substituted 1,2-phenylenediamines (Table 2). These were synthesized by a traditional sequence starting from 1-fluoro-2-nitrobenzene. The nucleophilic aromatic substitution proceeded in good to excellent yields to produce 9a-f and reduction of the nitro group to 10a-f through hydrogenation using platinum oxide was essentially quantitative. When these compounds were subjected to 6 at room temperature for 1 hr, the reaction proceeded very well (11a-f). Even with more sterically hindered substituents like t-butyl and phenyl (11d, 11f, respectively) the reaction proceeded in good yield.
Table 2.
Scope of reaction with substituted o-phenylenediamines.
| ||||
|---|---|---|---|---|
| entry | R | Yield 9 (%) | Yield 10 (%) | Yield 11 (%) |
| a | Ethyl | 72 | 98 | 89 |
| b | n-Propyl | 85 | 99 | 93 |
| c | n-Butyl | 85 | 99 | 73 |
| d | t-Butyl | 98 | 98 | 70 |
| e | Benzyl | 85 | 99 | 97 |
| f | Phenyl | 98 | 99 | 70 |
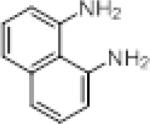
We investigated the synthetic utility of our new reagent by expanding the scope of the starting material. As illustrated in Table 3, the reaction with 1,8-diaminonaphthalene (entry 1, 12) proceeded in a good yield within 1 hr at room temperature. When 2-aminophenol (entry 2, 13) or 2-aminothiophenol (entry 3, 14) were used, the reaction proceeded favorably to produce the corresponding benzoxazole and benzothiazole, respectively.
Table 3.
Scope of reaction with alternative scaffolds.
| entry | sm | product | Yield (%) |
|---|---|---|---|
| 1 |
|
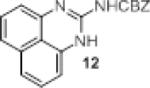
|
78 |
| 2 |
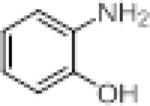
|
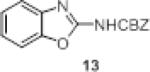
|
85 |
| 3 |
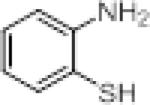
|
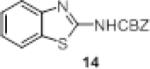
|
65 |
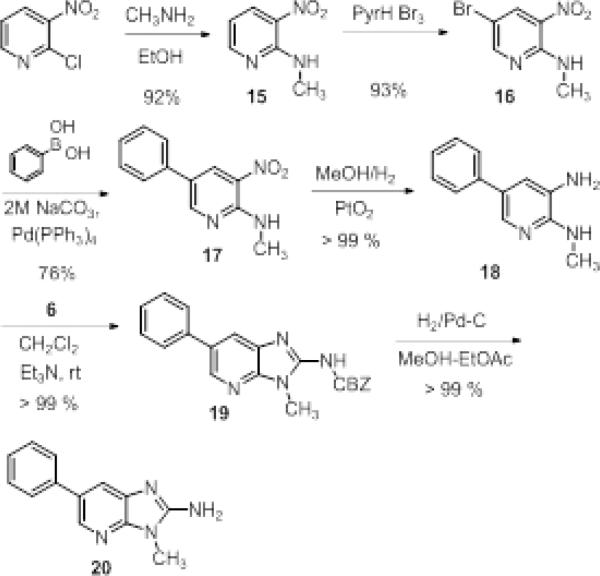
2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP, 20) is a known carcinogen produced amino acid condensations during high temperature cooking of meat and fish.10 Previous syntheses of 20 have required steps at high temperatures and proceeding in low yields.11 We applied our CBZ-protected imidoyl dichloride reagent 6 as the penultimate step (Scheme 2) and were able to produce 20 from the inexpensive 2-chloro-3-nitropyridine in >60 % yield over 6 steps.
Scheme 2.
Synthesis of PhIP.
Initially, we chose a carboxybenzyl protecting group for our imidoyl dichloride reagent for ease of monitoring the reactions by TLC and removal by hydrogenation. However, we predicted the chemistry could be amenable to additional protecting groups. Indeed, we were able to prepare a t-butyloxycarbonyl protected imidoyl dichloride (22) through reaction of the S,S’-dimethyliminiothiocarbonate (4) with phosgene followed by t-butanol and finally the displacement of the thioethers with chlorine. Similar to 6, reaction of 22 with 1,2-phenylenediamines afforded the 2-aminobenzimidazoles (Table 4) rapidly and in good yields regardless of the electronic nature of the ring substituents (entries b-c) and the N-substitution (entry d). In addition, reaction of 22 with 2-aminophenol afforded the corresponding benzoxazole in good yield (entry e). We predict that the imidoyl dichoride reagents would be amenable, as well, to alternative amine protecting groups, allowing for the rapid production of 2-aminobenzimidazoles and related azoles that could be further derivatized as desired.
Table 4.
Scope of reaction with 22.
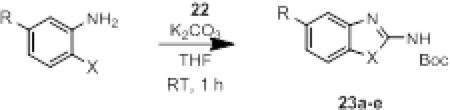
| |||
|---|---|---|---|
| entry | R | X | Yield (%) |
| a | H | NH | 81 |
| b | OCH3 | NH | 70 |
| c | NO2 | NH | 93 |
| d | H | N(CH2CH2CH3) | 88 |
| e | H | O | 57 |
3. Conclusions
Overall, we have developed a robust reagent tolerant of multiple functional groups that allows for the rapid formation of benzimidazole or alternative azole rings at room temperature.
Supplementary Material
Scheme 3.
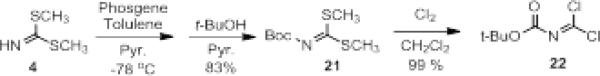
Synthesis of Boc-protected Imidoyl Dichloride, 22.
Acknowledgments
Research support is granted from the National Institutes of Health (PHS 5R37DK015556 to J.A.K). J.A.P. was supported by training fellowship from the National Institutes of Health (T32ES007326).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data (experimental procedures and spectral data for all new compounds) associated with this article can be found in the online version.
References and notes
- 1.Kim RM, Chang J, Lins AR, Brady E, Candelore MR, Dallas-Yang Q, Ding V, Dragovic J, Iliff S, Jiang GQ, Mock S, Qureshi S, Saperstein R, Szalkowski D, Tamvakopoulos C, Tota L, Wright M, Yang XD, Tata JR, Chapman K, Zhang BB, Parmee ER. Bioorg. Med. Chem. Lett. 2008;18:3701. doi: 10.1016/j.bmcl.2008.05.072. [DOI] [PubMed] [Google Scholar]
- 2.Kubicek S, O'Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro, Miguel L, Rea S, Mechtler K, Kowalski JA, Homon CA, Kelly TA, Jenuwein T. Mol. Cell. 2007;25:473. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Powers JP, Li S, Jaen JC, Liu J, Walker NPC, Wang Z, Wesche H. Bioorg. Med. Chem. Lett. 2006;16:2842. doi: 10.1016/j.bmcl.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 4.a Moriarty KJ, Takahashi H, Pullen SS, Khine HH, Sallati RH, Raymond EL, Woska JR, Jeanfavre DD, Roth GP, Winters MP, Qiao L, Ryan D, DesJarlais R, Robinson D, Wilson M, Bobko M, Cook BN, Lo HY, Nemoto PA, Kashem MA, Wolak JP, White A, Magolda RL, Tomczuk B. Bioorg. Med. Chem. Lett. 2008;18:5545. doi: 10.1016/j.bmcl.2008.09.015. [DOI] [PubMed] [Google Scholar]; b Winters MP, Robinson DJ, Khine HH, Pullen SS, Woska JR, Raymond EL, Sellati R, Cywin CL, Snow RJ, Kashem MA, Wolak JP, King J, Kaplita PV, Liu LH, Farrell TM, DesJarlais R, Roth GP, Takahashi H, Moriarty KJ. Bioorg. Med. Chem. Lett. 2008;18:5541. doi: 10.1016/j.bmcl.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Rivara M, Zuliani V, Cocconcelli G, Morini G, Comini M, Rivara S, Mor M, Bordi F, Barocelli E, Ballabeni V, Bertoni S, Plazzi PV. Bioorg. Med. Chem. 2006;14:1413. doi: 10.1016/j.bmc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 6.a Kus C, Goker H, Altanlar N. Arch. Pharm. 2001;334:361. doi: 10.1002/1521-4184(200112)334:11<361::aid-ardp361>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]; b Taher AT, Khalil NA, Ahmed EM, Ragab YM. Chem. Pharm. Bull. 2012;60:778. doi: 10.1248/cpb.60.778. [DOI] [PubMed] [Google Scholar]; c Rogers SA, Huigens RW, Melander C. J. Am. Chem. Soc. 2009;131:9868. doi: 10.1021/ja9024676. [DOI] [PubMed] [Google Scholar]; d Frei R, Breitbach AS, Blackwell HE. Angew. Chem. Int. Ed. 2012;51:5226. doi: 10.1002/anie.201109258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nawrocka W, Sztuba B, Kowalska MW, Liszkiewicz H, Wietrzyk J, Nasulewicz A, Pełczy ska M, Opolski A. Il. Farmaco. 2004;59:83. doi: 10.1016/j.farmac.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 8.a Turteltaub KW, Knize MG, Healy SK, Tucker JD, Felton JS. Fd. Chem. Toxic. 1989;27:667. doi: 10.1016/0278-6915(89)90121-x. [DOI] [PubMed] [Google Scholar]; b Perkins JJ, Zartman AE, Meissner RS. Tet. Lett. 1999;40:1103. [Google Scholar]; c Carpenter RD, Deberdt PB, Lam KS, Kurth MJ. J. Comb. Chem. 2006;8:907–914. doi: 10.1021/cc060106b. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, An MH, Choi EH, Choo HYP, Han G. Heterocycles. 2006;70:571. [Google Scholar]
- 10.a Felton JS, Knize MG, Shen NH, Lewis PR, Andresen BD, Happe J, Hatch FT. Carcinogenesis. 1986;7:1081. doi: 10.1093/carcin/7.7.1081. [DOI] [PubMed] [Google Scholar]; b Felton JS, Knize MG, Shen NH, Lewis PR, Andresen BD, Bjeldanes LF, Hatch FT. Environ. Health Perspect. 1986;67:17. doi: 10.1289/ehp.866717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a Knize MG, Felton JS. Heterocycles. 1986;24:1815. [Google Scholar]; b Choshi T, Tonari A, Yoshioka H, Harada K, Sugino E, Hibino S. J. Org. Chem. 1993;58:7952. [Google Scholar]; c Nguyen TM, Novak M. J. Org. Chem. 2007;72:4698. doi: 10.1021/jo070306p. [DOI] [PubMed] [Google Scholar]; d Chrisman W, Knize MG, Tanga MJ. J. Heterocycl. Chem. 2008;45:1641. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.