Abstract
Mild traumatic brain injury patients (mTBI) frequently report symptoms of increased distractability and sensory disturbances during mutisensory stimulation. These common post‐concussive symptoms could putatively result from dysfunction within the cognitive control network (CCN; top‐down) or from unisensory cortex (bottom‐up) itself. Functional magnetic resonance imaging (fMRI) and high‐resolution structural data were therefore prospectively collected during a multisensory (audio‐visual) cognitive control task from 46 mTBI patients within 3 weeks of injury and 46 matched healthy controls (HC), with a subset of participants returning at 4 months. Multisensory stimuli were presented at two frequencies to manipulate cognitive and perceptual load. Patients self‐reported more cognitive, emotional, somatic, vestibular and visual symptoms relative to HC, which improved, but did not entirely resolve, over the 4 month follow‐up period. There were no group differences in behavior or functional activation during cognitive control (incongruent – congruent trials). In contrast, patients exhibited abnormal activation within different regions of visual cortex that depended on whether attention was focused on auditory or visual information streams. Patients also exhibited increased activation within bilateral inferior parietal lobules during higher cognitive/perceptual loads, suggesting a compensatory mechanism to achieve similar levels of behavioral performance. Functional abnormalities within the visual cortex and inferior parietal lobules were only partially resolved at 4 months post‐injury, suggesting that neural abnormalities may take longer to resolve than behavioral measures used in most clinical settings. In summary, current results indicate that abnormalities within unisensory cortex (particularly visual areas) following mTBI, which likely contribute to deficits commonly reported during multisensory stimulation. Hum Brain Mapp 36:4394–4406, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: cognitive control, multisensory, auditory, visual, functional magnetic resonance imaging
Abbreviations
- A1
primary auditory cortex
- A2
secondary auditory cortex
- ARM
attention related modulations
- BDI‐II
Beck Depression Inventory – Second Edition
- CCN
cognitive control network
- dMFC
dorsal medial prefrontal cortex
- HC
healthy control
- IPL
inferior parietal lobe
- lPFC
dorsolateral and ventrolateral prefrontal cortex
- mTBI
mild traumatic brain injury
- NSI
Neurobehavioral Symptom Inventory
- PSC
percent signal change
- STAI
State‐Trait Anxiety Inventory
- TOMM
Test of Memory Malingering
- V1
primary visual cortex
- V2
secondary visual cortex
- WTAR
Wechsler Test of Adult Reading
INTRODUCTION
Mild traumatic brain injury (mTBI) represents a major health concern, accounting for a majority of the 1.7 million head injuries sustained each year (Faul et al., 2010). Importantly, this number is likely underestimated given that many mTBI, and sports‐related concussions in particular, go unreported (McCrea et al., 2013 ). Common patient complaints during the acute to sub‐acute injury phase include increased distractibility and sensory overstimulation, which could arise from difficulties in processing conflicting information (i.e., one form of cognitive control) or from neurosensory impairments (Fischer et al., 2014; Halterman et al., 2006; Hoffer, 2015). Few studies have prospectively examined (e.g., semi‐acute to chronic injury stage) abnormalities within the cognitive control network (CCN) or within unisensory cortex (e.g., primary or secondary auditory and visual cortices) in homogeneous samples of mTBI patients, a necessary first step for understanding potential physiological basis of self‐reported symptoms.
As demonstrated by Hubel et al. (1959), there are multifaceted interactions between top‐down cognitive control and unisensory cortex (Talsma et al., 2010). The dorsal medial prefrontal cortex (dMFC), dorsolateral and ventrolateral prefrontal cortex (lPFC), anterior insula, and the inferior parietal lobes (IPL) of the CCN exhibit increased activation during the processing of conflicting stimuli (Ridderinkhof et al., 2004; Roberts and Hall, 2008), orchestrating changes in neural representations that ultimately affect behavior (Shenhav et al., 2013). One of these neural changes include the appearance of attention related modulations (ARM) within unisensory cortex, including enhanced neural responses (i.e., up‐regulation) for attended stimuli and suppressed responses (i.e., inhibition) for ignored stimuli (Baier et al., 2006; Mayer et al., 2009a; Weissman et al., 2004).
Studies examining cognitive control/working memory following mTBI have reported both hyper‐activation and hypo‐activation of the CCN, with mixed findings likely driven by the types of mTBI patients studied (e.g., acute vs. chronic, symptomatic vs. asymptomatic) and differences in task requirements (reviewed in Mayer et al., 2014). To date no studies have examined the neural basis (i.e., structural or functional) of neurosensory deficits (e.g., visual, auditory or vestibular symptoms) during the acute to semi‐acute phase of mTBI despite suggestions that these deficits represent a major contributing factor for chronic post‐concussive symptoms (Hoffer, 2015; Pogoda et al., 2012). Early non‐invasive imaging work examining unisensory cortical functioning suggests gating deficits across the TBI spectrum (Arciniegas et al., 2000; Arciniegas and Topkoff, 2004), and that post‐concussive symptoms result in part from sensory gating abnormalities (Kumar et al., 2005). SPECT (Stamatakis et al., 2002), PET (Kato et al., 2007; Nakashima et al., 2007) and fMRI (Kim et al., 2012) studies also indicate hypometabolism or hypoperfusion in unisensory cortex in severe TBI patients, providing additional evidence that cortical dysfunction may contribute to self‐reported neurosensory symptoms. Finally, incoming information from sensory organs as well as top‐down modulatory signals from heteromodal cortex are often routed through the thalamus and/or brainstem prior to reaching unisensory cortical regions. Midbrain regions may be particularly susceptible to injury due to the accumulation of shear stresses (Zhang et al., 2004), with previous studies implicating structural and functional abnormalities in these regions following mTBI (Lui et al., 2014 ; Mayer et al., 2009b ).
The aims of the current study were therefore (1) to examine the relationship between cognitive control and unisensory cortical functioning during the semi‐acute stage of mTBI, (2) determine how these relate to self‐reported neurosensory deficits and (3) to track neurosensory symptoms/neural abnormalities as a function of recovery over a four month period. We hypothesized that there would be functional abnormalities in the CCN as well as in unisensory cortex (i.e., decreased ARM) following mTBI. We also predicted that decreased ARM would be associated with neurosensory deficits in patients, both of which would normalize as a function of recovery.
METHODS
Participants
Forty‐eight mTBI patients and 48 age and education matched controls were included in the current study. Inclusion criteria included age of 18–55 years, a closed head injury with a self‐reported alteration in mental status, Glasgow Coma Score of 13–15 upon initial presentation to medical staff, a maximum of 30 minutes for loss of consciousness (if experienced) and a maximum of 24 hours of post‐traumatic amnesia (if experienced). Patients and controls were excluded from the current study based on self‐reported history of other neurological disease, recent alcohol or other drug abuse, history of Axis I disorder, prior closed head injuries with more than 5 minutes loss of consciousness, additional closed head injury in last year, strong preference for the left hand (score of less than −20 on Edinburgh handedness scale), learning disorder, or Attention Deficit Hyperactivity Disorder. Two patients were identified as outliers on frame‐wise displacement (greater than three times the interquartile range on two or more of six motion parameters) relative to their cohort, and were excluded from further analyses along with their healthy matches. All remaining participants performed at acceptable levels (above 70% accuracy) on the functional task, resulting in a total of 46 mTBI patients (24 males, 28.9 ± 9.8 years old, 13.2 ± 2.6 years of education) and 46 matched controls (24 males, 28.4 ± 9.9 years old, 13.8 ± 2.3 years of education). Informed consent was obtained according to institutional guidelines at the University of New Mexico Health Science Center.
Patients and healthy controls (HC) completed identical procedures. Patients were assessed with neuropsychological (mean day post‐injury = 13.7 ± 5.0) and neuroimaging (mean day post‐injury = 13.8 ± 5.0) measures within 21 days of injury (Table 1), with the imaging and clinical sessions typically conducted within a few days of each other (mean difference between assessments = 1.2 ± 1.5 days). At the time of assessment, seven of the mTBI participants were prescribed medications for pain and other conditions associated with injury. Data from a subset of participants on the same task has previously been reported (Mayer et al., 2012).
Table 1.
Neuropsychological and clinical summary measures
| mTBI | HC | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | Cohen's d (mTBI – HC) | |
| Demographic | |||
| Age | 28.9 (9.8) | 28.4 (9.9) | 0.05 |
| Education | 13.2 (2.6) | 13.8 (2.3) | −0.24 |
| HQ | 86.1 (27.2) | 92.2 (16.5) | −0.27 |
| Neuropsych | |||
| WTAR | 49.4 (9.3) | 54.8 (8.0) | −0.61 |
| TOMM | 54.6 (6.0) | 50.3 (12.4) | 0.45 |
| Attentiona | 52.6 (4.4) | 52.7 (5.8) | −0.01 |
| Memorya | 50.9 (7.6) | 51.7 (6.9) | −0.11 |
| WMa | 51.7 (5.6) | 50.5 (6.2) | 0.21 |
| PSa | 45.0 (6.2) | 47.8 (7.5) | −0.41 |
| EFa | 48.7 (6.0) | 48.4 (5.3) | 0.06 |
| Self‐report | |||
| NBSI‐Emot | 8.6 (5.7) | 2.8 (3.3) | 1.24 |
| NBSI‐Som | 8.6 (6.7) | 1.9 (2.9) | 1.30 |
| NBSI‐Cog | 4.7 (3.4) | 1.4 (2.3) | 1.12 |
| NBSI‐Ves | 3.0 (2.5) | 0.6 (1.0) | 1.29 |
| NBSI‐Aud | 0.2 (0.5) | 0.1 (0.4) | 0.33 |
| NBSI‐Vis | 0.9 (1.2) | 0.2 (0.5) | 0.84 |
| BDI‐II and STAI | 49.6 (8.5) | 42.9 (6.4) | 0.90 |
| Days post injury | |||
| Imaging | 13.8 (5.0) | N/A | N/A |
| Neuropsych | 13.7 (5.0) | N/A | N/A |
Means, standard deviations and effect sizes for neuropsychological indices reported following correction for WTAR as covariate.
Abbreviations: mTBI = mild traumatic brain injury patients; HC = healthy controls; HQ = handedness quotient; WM = working memory; PS = processing speed; EF = executive function; WTAR = Wechsler Test of Adult Reading; TOMM = Test of Memory Malingering; NBSI‐Emot = Neurobehavioral Symptom Inventory emotional complaints (Cog = cognitive complaints; Som = somatic complaints; Ves = vestibular complaints; Aud = auditory complaints; Vis = visual complaints); BDI‐II = Beck Depression Inventory—Second Edition; STAI = State‐Trait Anxiety Inventory; SD = standard deviation; N/A = not applicable.
Neuropsychological Assessment
Composite indices were calculated for the following cognitive domains to reduce redundancy amongst similar neuropsychological measures: attention (Trails A, Paced Auditory Serial Addition Test, Stroop [color‐word and interference scores], and Wechsler Adult Intelligence Scale‐Third Edition [WAIS‐III] digit span); working memory (WAIS‐III letter number sequence, arithmetic, and digits backward); processing speed (grooved pegboard [dominant and non‐dominant hand] and WAIS‐III digit symbol coding); executive function (Wisconsin Card Sort [errors and perseverative errors], Trails B, and Controlled Oral Word Association FAS Test); and memory (California Verbal Learning Test‐Second Edition: immediate recall, short‐delay free recall, long‐delay free recall). Emotional, somatic and cognitive complaints were assessed using the Neurobehavioral Symptom Inventory (NSI), the Beck Depression Inventory‐Second Edition (BDI‐II) and the State‐Trait Anxiety Inventory (STAI). The NSI was also used to assess vestibular, visual and auditory symptoms of neurological deficits based on previously published guidelines (Lew et al., 2011). The Wechsler Test of Adult Reading (WTAR) provided an estimate of overall premorbid intellectual functioning and the Test of Memory Malingering (TOMM) assessed effort.
One patient and one control did not complete the full neuropsychological battery. Additionally, one control's TOMM data was eliminated due to poor performance (t‐score < 0) in conjunction with normal performance on more demanding neuropsychological measures. Univariate tests were conducted to compare estimates of effort and premorbid intelligence, whereas multivariate tests were used to compare composite neuropsychological indices and self‐reported symptoms given the known moderate correlational structures. Chi‐square tests assessed for group differences in the occurrence of neurosensory deficits.
Task
Congruent or incongruent multisensory (visual and auditory) numeric stimuli (Fig. 1A,B) were simultaneously presented at either low (0.33 Hz; three trials/block) or high (0.66 Hz; six trials/block) rates of stimulation in ten‐second blocks. The target (200 ms duration) number stream (one, two, or three) was preceded by a multisensory cue (175 ms duration) word (“HEAR,” “LOOK” or “NONE”) indicating the modality for focused attention. During “HEAR” trials (attend‐auditory) participants responded to aurally presented target stimuli via a right‐handed button press while ignoring simultaneously presented visual numbers. During “LOOK” trials (attend‐visual) participants responded to the visual targets (English numerals “ONE,” “TWO” or “THREE”) while ignoring the auditory stream (spoken number words). “NONE” trials are not reported in the current manuscript. The inter‐block intervals varied between 8, 10, and 12 seconds to decrease temporal expectations and permit modeling of the baseline response. Block‐order was pseudorandom, with a total of 432 trials presented across six separate imaging runs. As response time data has a tendency towards positive skew, the median reaction time was used as a measure of central tendency. Two 2 × 2 × 2 [Group (mTBI vs. HC) × Congruency (Congruent vs. Incongruent) × Frequency (0.33 Hz vs. 0.66 Hz)] mixed‐measures ANCOVAs were conducted separately on attend‐auditory and attend‐visual conditions.
Figure 1.
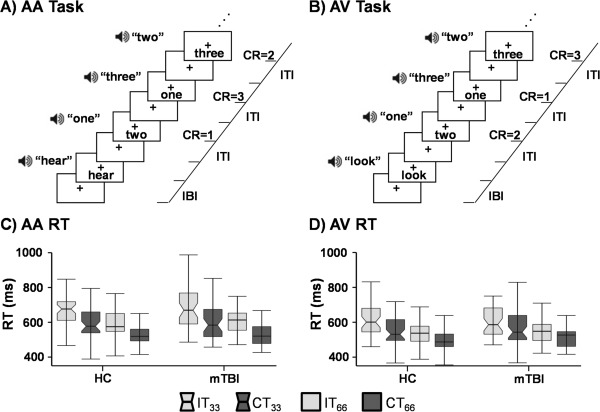
Representations of incongruent trials for A) attend‐auditory (AA) and B) attend‐visual (AV) tasks as determined by the initial cue (AA = “hear”; AV = “look”). Expected correct responses (CR), inter‐trial interval (ITI) and inter‐block interval (IBI) are represented at the right of the timeline. The bottom row presents box‐and‐whisker plots for reaction times (RT) in AA (Panel C) and AV (Panel D). Data are presented separately for HC and mTBI, with incongruent (IT; light gray) and congruent (CT; dark gray) trials at the two stimulation frequencies (0.33 Hz = notched boxes; 0.66 Hz = un‐notched boxes) used in the experiment.
MR Imaging
High‐resolution multi‐echo MPRAGE T1 [TR (repetition time) = 2.53 s, 7° flip angle, number of excitations (NEX) = 1, slice thickness = 1 mm, FOV (field of view) = 256 mm, resolution = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm] and T2 [echo time = 77.0 ms, TR = 1.55 s, flip angle 152°, NEX = 1, slice thickness = 1.5 mm, FOV = 220 mm, matrix = 192 × 192, voxel size = 1.15 × 1.15 × 1.5 mm] sequences were collected at 3 Tesla Siemens TIM Trio scanner with a 12‐channel head coil. Susceptibility weighted images were collected with a gradient echo sequence [TR = 28 ms; TE = 20 ms; flip angle 15°; bandwidth = 120 Hz/Px; FOV = 180 × 240 mm; matrix = 177 × 256; slice thickness = 1.5 mm] to better characterize petechial hemorrhages on a subset of patients (N = 21). A single‐shot, gradient‐echo echoplanar pulse sequence [TR = 2,000 ms; TE = 29 ms; flip angle = 75°; FOV = 240 mm; matrix size = 64 × 64; voxel size = 3.75 × 3.75 × 4.55 mm] was collected during the multisensory task.
Image Processing and Statistical Analyses
The first three images of each run were eliminated to account for T1 equilibrium effects, resulting in a total of 966 images for the final analyses. Anomalous time‐series data were first identified and replaced based on values from the previous and subsequent image using AFNI's despiking protocol (Cox, 1996). All time‐series data were then spatially registered in two‐ and three‐dimensional space to the second EPI image of the first run to reduce the effects of head motion, and were temporally interpolated to the first slice to account for differences in slice acquisition. Data were spatially blurred using a 6 mm Gaussian full‐width half‐maximum filter and converted to standard stereotaxic coordinate space (Talairach and Tournoux, 1988). A voxel‐wise deconvolution analysis generated a single hemodynamic response function for each trial‐type, which was derived relative to the baseline state (visual fixation plus gradient noise) based on the first 22 seconds post‐stimulus onset. Error trials were modelled separately for each trial‐type to eliminate error variance (Mayer et al., 2011). Percent signal change (PSC) for correct trials was calculated by summing the beta coefficients for images occurring six to fourteen seconds post‐cue onset (peak of the hemodynamic response function) and dividing by the average model intercept (β0) from each run.
Two voxel‐wise, 2 × 2 × 2 [Group (mTBI vs. HC) × Congruency (Congruent vs. Incongruent) × Frequency (0.33 Hz vs. 0.66 Hz)] mixed‐measures ANCOVAs were then performed on the spatially normalized percent signal change measure for the auditory and visual modality separately. In this analytic framework, our predictions of increased patient abnormalities during CCN were specifically tested by the Group × Congruency interaction. All voxel‐wise results were corrected for false positives at P < 0.05 based on 10,000 Monte‐Carlo simulations (P < 0.005 and minimum cluster size = 1,280 microliters).
Unisensory Cortical Analyses
Subject‐specific ROI were also defined by standard labels for primary and secondary unisensory cortex using the FreeSurfer reconstruction pipeline. Primary (Heschl's gyrus; A1) and secondary (planum temporale, Heschl's sulcus, planum polare, and superior temporal gyrus; A2) auditory cortex were defined using previously published labels (Destrieux et al., 2010). Secondary visual cortex (V2) was identified using Fischl's label (Fischl et al., 2008) whereas primary visual cortex (V1) was defined based on the Hinds' label (Hinds et al., 2008) with areas shared by the V2 label removed. To calculate how unisensory cortex was attentionally modulated in the presence of identical sensory stimulation (ARM), PSC data were subtracted in the expected direction of positive modulation for auditory (attend‐auditory trials minus attend‐visual trials) and visual (attend‐visual trials minus attend‐auditory trials) cortex for incongruent and congruent high frequency trials only based on our previous results (Mayer et al., 2009a). Basic sensory integrity during increased task demands was assessed by subtracting low frequency trials from high frequency trials.
Following outlier analyses, a series of 2 × 2 (Group × Congruency) ANCOVAs were conducted to evaluate hypothesis regarding group differences in ARM and basic neurovascular coupling in unisensory cortex. Single‐sample t‐tests were also conducted to evaluate the robustness of the response to the null distribution. MANCOVAs were also performed to examine for structural changes in unisensory cortices (i.e., volume) separately for auditory and visual cortex using estimated intracranial volume as a covariate.
RESULTS
Neuropsychological and Clinical Measures
There were no significant group differences (P > 0.10) on major demographic variables (Table 1). Independent samples t‐tests indicated that HC both scored lower on the TOMM and had increased variability relative to mTBI patients (t 61.5 = −2.09, P = 0.041), although performance was typically in the normal range for both groups. mTBI patients also had lower estimated premorbid intellectual functioning (t 88 = 2.90, P = 0.005). WTAR was therefore used as a covariate in neuropsychological, behavioral and imaging analyses for Visit 1 data. A MANCOVA comparing group differences in composite neuropsychological scores was not significant for main effect of Group (P > 0.10). The univariate effect of processing speed was a statistical trend (F 1,87 = 3.54, P = 0.063), with medium effect sizes suggesting reduced processing speed for mTBI patients.
A MANOVA examining self‐reported concussion symptoms was significant for the main effect of Group (F 3,87 = 14.4, P < 0.001), with univariate results indicating increased cognitive (F 1,89 = 28.53, P < 0.001), somatic (F 1,89 = 38.43, P < 0.001) and emotional (F 1,89 = 34.98, P < 0.001) complaints for mTBI patients relative to HC. An additional measure of emotional distress indicated that mTBI patients self‐reported significantly (t 89 = −4.28, P < 0.001) greater symptoms of depression (BDI‐II) and anxiety (STAI). Chi‐Square tests indicated that mTBI patients reported significantly more vestibular (Χ 2 1,N=90 = 10.67, P = 0.001; mTBI = 40.91%, HC = 10.87%) and visual (Χ 2 1,N=90 = 6.97, P = 0.008; mTBI = 27.27%, HC = 6.52%) symptoms compared to HC. In contrast, there were no differences between the groups in terms of reported auditory symptoms (mTBI: 2.27%; HC: 2.17%; P > 0.10).
Multisensory Cognitive Control Task: Behavioral Data
Accuracy data were non‐normally distributed, but there were no significant differences across patients and controls (P > 0.10) for incongruent (mTBI = 95.0%; HC = 93.9%) or congruent (mTBI = 98.1%; HC = 97.7%) trials. Reaction times for attend‐auditory trials (Fig. 1C) were significant for the main effects of Congruency (F 1,89 = 165.18, P < 0.001) and Frequency (F 1,89 =197.98, P < 0.001), with faster response times for congruent (mean = 564.40 ± 74.71) compared to incongruent (mean = 644.38 ± 90.37) trials and for higher (0.66 Hz; mean = 565.29 ± 63.60) relative to lower (0.33 Hz; mean = 643.47 ± 96.67) frequency trials. Neither the main effect of Group nor any of interactions were significant (P > 0.10).
The main effects of Congruency (F 1,89 = 88.14, P < 0.001) and Frequency (F 1,89 = 175.74, P < 0.001) were also significant for the attend‐visual trials (Fig. 1D), with participants responding more quickly to congruent (mean = 535.74 ± 70.99) compared to incongruent (mean = 578.08 ± 76.01) trials, as well as to high (0.66 Hz; mean = 527.49 ± 64.24) compared to low (0.33 Hz; mean = 586.35 ± 81.80) frequency trials. No other main effects or interaction effects approached significance (P > 0.10).
Radiological Findings
Thirty‐five mTBI patients had a CT scan as part of routine emergency care. Of those patients with brain CT, three had positive findings. A board certified radiologist blinded to participant diagnosis also reviewed all baseline structural MRI sequences for both mTBI and HC. There were no positive MRI findings among HC, but four mTBI patients had positive findings on MRI scans. Thus, there were seven mTBI patients with either a positive CT or a positive MRI scan in total. None of the patients with a positive CT finding also had a positive MRI finding.
Functional Imaging Data
Two MANOVAs were first conducted to investigate any potential group differences in head motion (both rotational and translational framewise displacements in image space), which could confound the interpretation of fMRI data. However, results indicated that the multivariate effect of Group was not significant (P > 0.10) for either of the MANOVAs, with small to medium effect sizes for all six parameters (range from 0.23 to 0.46).
Functional Results: Patient Comparisons
Voxel‐wise, 2 × 2 × 2 (Group × Congruency × Frequency) mixed‐measures ANCOVAs were performed separately for data in the attend‐auditory and attend‐visual conditions. For both attend‐auditory and attend‐visual trials, the Group × Congruency interaction was not significant following appropriate correction for false positives. During attend‐auditory trials, mTBI patients exhibited decreased activation relative to HC within the right lingual gyrus (BAs 17/18) extending into the declive of the cerebellum (Fig. 2A; µl = 1,353). In addition, the Group × Frequency interaction (Fig. 2C) was significant in the left (BAs 5/40; µl = 2,998) and right (BA 40; µl = 1,661) IPL during attend‐visual trials. Simple effects tests indicated increased activation in the right IPL for high relative to low frequency trials in mTBI patients (P < 0.05) but not HC. In the left IPL, the interaction was driven by a greater difference in activation between high and low frequency trials in mTBI relative to HC (P < 0.05). The Group × Frequency interaction was not significant in the attend‐auditory trials.
Figure 2.
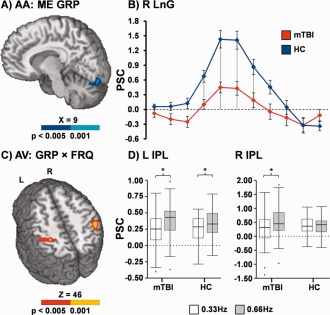
Decreased activation (Panel A; blue coloring = P < 0.005; cyan = P < 0.001) within the right lingual gyrus (LnG) was observed for mild traumatic brain injury patients (mTBI; red trace) relative to healthy controls (HC; blue trace) during the attend‐auditory (AA) trials (main effect [ME] of group). Panel B plots the mean percent signal change (PSC) at each image post‐stimulus onset for both groups separately, representing the hemodynamic response function. The drop lines in Panel B indicate images that were used in between group comparisons. Panels C and D display the right and left inferior parietal lobule (IPL), which was associated with a significant interaction (red coloring = P < 0.005; yellow coloring = P < 0.001) between group (GRP) and stimulus frequency (FRQ) during attend‐visual (AV) trials. Box‐and‐whisker plots of the average PSC data are presented in Panel D, with asterisks denoting significant differences (P < 0.05) as a function of frequency (0.66 Hz [gray] > 0.33 Hz [white]). Slice locations are given according to the Talairach atlas in Panels A and C.
Functional Results: Task Comparisons
Functional results from the identical task have been presented previously in a subset of mTBI patients (Mayer et al., 2012). Thus, the main effects associated with Congruency and Frequency are only briefly presented here to establish that the task evoked expected patterns of activation across both groups of participants. In both the attend‐auditory and attend‐visual conditions, incongruent trials resulted in increased activation within the anterior insula, lPFC, the dMFC, posterior temporal sulcus, IPL, thalamus and sub‐thalamic nuclei and cerebellum relative to congruent trials (Fig. 3). Activation was typically greater in volume and more bilateral for incongruent trials during the attend‐visual condition, whereas increased activation was observed in bilateral secondary/associative visual cortex for congruent trials during the attend‐auditory condition.
Figure 3.
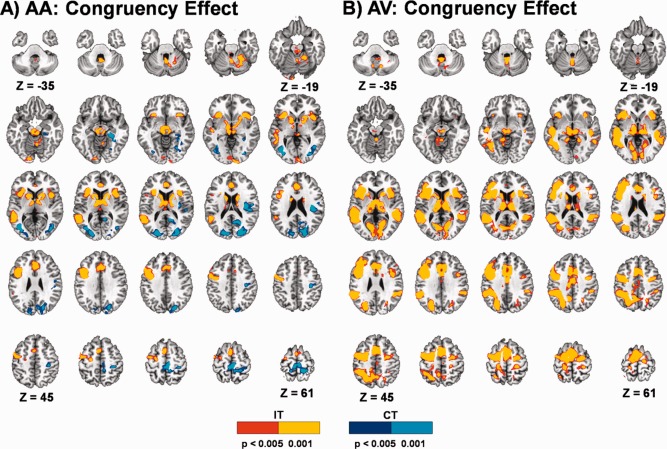
This figure presents clusters with significant activation differences between incongruent (IT) and congruent (CT) trials in attend‐auditory (AA) and attend‐visual (AV) conditions collapsed across both groups of participants. Axial slices are displayed according to the Talairach atlas. Clusters are shown in red (P < 0.005) and yellow (P < 0.001) where activation is greater in incongruent relative to congruent trials and in blue (P < 0.005) and cyan (P < 0.001) where activation is greater in congruent relative to incongruent trials.
The main effects of frequency were in the expected direction (0.66 Hz > 0.33 Hz) and similar for both the attend‐auditory and attend‐visual trials. Specifically, increased activation was observed bilaterally for higher frequency trials within primary and secondary auditory cortex, pre‐SMA and SMA, DLPFC, posterior parietal cortex, basal ganglia and cerebellum (Lobules IV‐VII) across both the attend‐visual and attend‐auditory conditions. More lateralized motor‐related activity was also observed in the left sensori‐motor cortex. In addition, increased deactivation during the high relative to low frequency trials was observed within default‐mode network for both attend‐auditory and attend‐visual conditions.
Unisensory Cortex Analyses: Patient Comparisons
Results from two MANCOVAs (intracranial volume as a covariate) indicated that there were no significant differences in either primary/secondary auditory or visual cortical volume between the two groups (P > 0.10). Four 2 × 2 (Group × Congruency) ANCOVAs examined for ARM during high frequency trials in primary and secondary visual (V1; V2) and auditory (A1; A2) cortices.
There was a non‐significant effect of group in A1 (F 1,89 = 3.11, P = 0.081), as well as non‐significant Group × Congruency interactions within A1 (F 1,89 = 2.85, P = 0.095) and A2 (F 1,89 = 3.40, P = 0.069). Simple effects tests indicated that these trends resulted from increased differential activation (attend‐auditory minus attend‐visual) for HC relative to mTBI during congruent trials only in both A1 and A2 (Fig. 4; P < 0.05). One‐sample t‐tests indicated that mTBI patients exhibited the opposite pattern of expected modulation (attend‐visual > attend‐auditory) in both A1 and A2 during congruent trials (P < 0.05).
Figure 4.
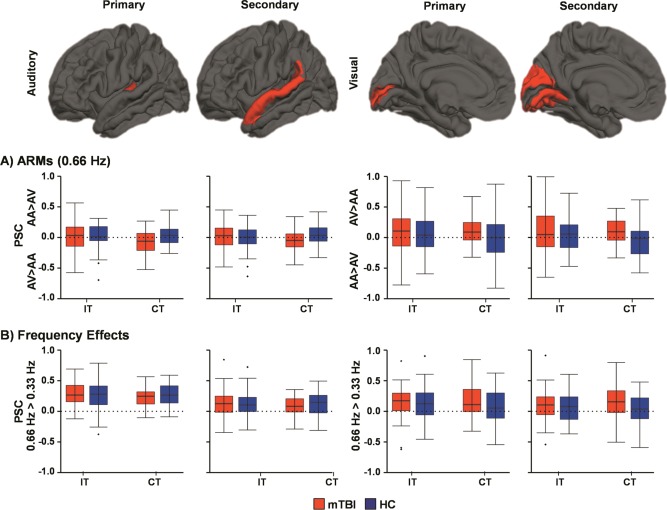
This figure presents percent signal change (PSC) values for ROI in the primary/secondary auditory and visual cortex. Data are presented for attention related modulations (ARM; Panel A) by subtracting PSC data in the expected direction of positive modulation for both auditory (attend‐auditory [AA] trials minus attend‐visual [AV] trials) and visual (AV trials minus AA trials) cortex. Thus, values above zero (dashed line) indicate the direction of expected modulation for attention whereas values at zero are equivalent to no attentional modulation. Sensory integrity was assessed by subtracting PSC data in low frequency (0.33 Hz) trials from high frequency (0.66 Hz) trials in Panel B. Box‐and‐whisker plots are used to demonstrate the direction of effect.
In visual cortices, a significant effect of group was present in V2 (F 1,88 = 5.47, P = 0.022), as well as trend interactions for V1 (F 1,88 = 2.84, P = 0.095) and V2 (F 1,88 = 3.69, P = 0.058) during ARM analyses. Simple effects tests indicated these interactions were driven by increased differential activation (attend minus ignore) in mTBI relative to HC during congruent trials in both V1 and V2 (P < 0.05). Similar to the main effect of group observed during voxel‐wise analyses, these results were partially driven by decreased activation in visual cortex on attend‐auditory trials for mTBI patients. One‐sample t‐tests indicated that robust ARM were present for mTBI patients (attend‐visual minus attend‐auditory > 0; P < 0.05), but not HC (P > 0.05), during congruent trials.
To test basic sensory cortex integrity, 2 × 2 (Group × Congruency) ANCOVAs were performed on frequency‐subtracted (0.66 Hz minus 0.33 Hz) PSC data in each a priori ROI. Null results (P > 0.10) were observed for all effects in A1 and A2. For visual cortices, the main effect of Group was a trend in V1 (F 1,89 = 3.11, P = 0.081) and significant in V2 (F 1,89 = 6.25, P = 0.014), with mTBI showing a greater hemodynamic response for high frequency trials relative to HC. No other main effects or interaction effects approached significance (P > 0.10).
Finally, binary logistic regression was performed to determine whether ARM in visual cortex during congruent trials or basic sensory integrity across both trials was associated with the higher incidence of self‐reported visual deficits following mTBI. However, the results from this analysis were not significant (P > 0.10).
Longitudinal Data Analyses
A total of 22/46 (neuroimaging mean day post‐injury = 121.6 ± 14.4; clinical mean day post‐injury = 120.7 ± 14.2) mTBI patients and 34/46 HC returned for a second visit. Returning HC were rematched to patients, retaining the original match whenever possible. There were no outliers on motion parameters for either group at Visit 2, but one patient was eliminated for poor performance along with their respective control, resulting in 21 participants for each group. There were no differences between returning patients and controls on estimates of premorbid intelligence (P > 0.10). Only significant or trend findings from the semi‐acute injury phase measures were re‐evaluated at follow‐up.
A 2 × 2 (Group × Visit) ANOVA examining differences in processing speed was not significant for any main effects or interactions. Identical analyses examining self‐reported post‐concussive symptoms indicated main effects of Group (somatic F 1,40 = 17.81, P < 0.001; cognitive F 1,40 = 11.49, P = 0.002; emotional F 1,40 = 13.91, p = 0.001) and Visit (somatic F 1,40 = 5.44, P = 0.025; cognitive F 1,40 = 3.41, P = 0.072). The Group × Visit interaction was also significant or at trend levels (somatic F 1,40 = 5.11, P = 0.029; cognitive F 1,40 = 3.87, P = 0.056; emotional F 1,40 = 3.16, P = 0.083), with simple effects testing suggesting reductions in somatic (P = 0.016), cognitive (P = 0.022) and emotional (P = 0.074) symptoms for mTBI and no change for HC (P > 0.10). Self‐reported somatic (P = 0.003), cognitive (P = 0.058) and emotional (P = 0.081) symptoms still remained elevated for mTBI relative to HC at Visit 2. Similarly, self‐reported visual symptoms remained elevated for patients at a trend level at Visit 2 (Χ 2 1,N=42 = 3.23, P = 0.072; mTBI = 14.29%, HC = 0.00%), while groups were no longer statistically different on self‐reported vestibular symptoms (Χ 2 1,N=42 = 2.04, P = 0.153; mTBI = 19.05%, HC = 4.76%).
Two 2 × 2 (Group × Visit) ANOVAs were performed on the difference between high and low frequency trials to follow‐up on the significant interactions in the bilateral posterior parietal cortex during attend‐visual trials. The main effect of group remained significant for both left (F 1,40 = 8.33, P = 0.006) and right (F 1,40 = 5.62, P = 0.023) parietal regions, with mTBI patients continuing to exhibit a greater difference in activation between high and low frequency trials relative to HC. An additional 2 × 2 (Group × Visit) ANOVA was performed on PSC data in the right lingual gyrus during attend‐auditory trials. The main effect of group remained at a trend level (F 1,39 = 4.00, P = 0.053), with mTBI still exhibiting decreased activation relative to HC. Neither the main effect of visit nor the interaction were significant.
Four 2 × 2 (Group × Visit) ANOVAs were performed on congruent trials to examine for changes in ARM within primary/secondary auditory and visual cortex. The main effect of Visit was a trend for primary (F 1,40 = 2.96, P = 0.093) and significant for secondary (F 1,40 = 6.77, P = 0.013) auditory cortex, whereas the Group × Visit interaction was significant for both primary (F 1,40 = 4.71, P = 0.036) and secondary (F 1,40 = 4.61, P = 0.038) auditory cortex. Simple effect testing indicated that the differences in auditory cortical ARM between mTBI and HC at Visit 1 (P's < 0.05) were no longer significant at Visit 2 (P's > 0.05).
Analysis of visual cortices returned trends for the main effect of Visit in both primary (F 1,40 = 3.60, P = 0.065) and secondary (F 1,40 = 4.04, P = 0.051) cortices, with ARM being higher at the second visit. In addition, a non‐significant trend for the main effect of group was also present in secondary visual cortex (F 1,40 = 3.30, P = 0.077), with increased ARM for mTBI patients relative to HC.
Finally, two 2 × 2 (Group × Time) ANOVAs were performed on frequency‐subtracted (0.66 Hz minus 0.33 Hz) PSC data to examine whether mTBI continued to exhibit a greater hemodynamic response for high frequency congruent and incongruent trials in visual cortices. Null results (P > 0.10) were observed for both tests.
DISCUSSION
Previous data suggests deficits in both cognitive control (Fischer et al., 2014; Halterman et al., 2006; Scheibel et al., 2012) and neurosensory functioning (Lew et al., 2009; Lew et al., 2010; Lew et al., 2011; Pogoda et al., 2012) following mTBI. The current investigation examined whether these deficits were driven by neural dysfunction within the CCN (top‐down), within unisensory cortical regions (bottom‐up) or potentially within the thalamus during a multisensory cognitive control task. Behavioral results indicated the successful induction of cognitive control (RT incongruent > RT congruent) and parametric variation of cognitive/perceptual load (RT low frequency > RT high frequency). As expected, incongruent trials resulted in increased activation within the CCN relative to congruent trials (Ridderinkhof et al., 2004; Roberts and Hall, 2008), which was more robust in the attend‐visual condition. However, there was no evidence of behavioral or functional deficits specific to cognitive control (i.e., processing of conflicting stimuli) for recently injured mTBI patients. In contrast, evidence of neuronal abnormalities was present in the inferior parietal and unisensory cortex, particularly within visual cortical regions, which showed only partial evidence of normalization four months post‐injury.
Clinically, mTBI patients self‐reported more cognitive, somatic, and emotional (depression and anxiety) symptoms relative to HC during the semi‐acute phase, with evidence of symptom improvement but not complete resolution at four months post‐injury. There was also evidence of mild processing speed deficits approximately two weeks post‐injury, which resolved at the second visit. Similar to previous results in a subset of patients (Mayer et al., 2012), there were no significant group differences in behavior across experimental measures of cognitive control (incongruent minus congruent trials) during the semi‐acute injury phase. The mostly null findings observed on both traditional neuropsychological testing and on laboratory measures of cognitive control are not likely to have resulted from low power given the current sample size. Rather, current null findings are likely to be reflective of the rapid resolution of objectively tested cognitive deficits that is known to occur for the majority of mTBI patients following injury on traditional clinical measures (Carroll et al., 2014; McCrea et al., 2013).
In contrast to previous studies and a priori hypotheses (Chen et al., 2007; Fischer et al., 2014; Scheibel et al., 2012; Smits et al., 2008), there were no group differences in functional activation during cognitive control (Group × Congruency interaction). These discrepancies may be due to the nature (multisensory stimuli, utilization of a cue to proactively allocate attentional control) or difficulty level of the task, or due to clinical differences in the current sample. For example, previous fMRI studies and meta‐analyses examining working memory function in mTBI suggest complex relationships between cognitive load and functional activation (McAllister et al., 1999; McAllister et al., 2001), as well as between continuous versus discreet working memory tasks (Bryer et al., 2013). Functional abnormalities are also affected by the presence of post‐concussive symptoms (Chen et al., 2004; Chen et al., 2007), and the current study purposefully recruited patients directly from the emergency room to avoid any potential bias with chronically symptomatic patients (Mayer et al., 2014). Moreover, other studies have not observed functional deficits in mTBI patients during cognitive control tasks (Elbin et al., 2012; Mayer et al., 2012; Stulemeijer et al., 2010), indicating that the resolution of functional abnormalities specific to cognitive control may also occur rapidly in typically recovering mTBI patients.
In contrast to the null findings for cognitive control, mTBI patients exhibited differential activation in the bilateral posterior parietal cortex during conditions of increased cognitive load for attend‐visual trials (i.e., increased activation for high versus low frequency trials), with abnormalities persisting into the four month follow‐up period. The posterior parietal cortex is commonly activated across many cognitive tasks (Macaluso and Driver, 2005; Nachev and Husain, 2006; Xing and Andersen, 2000; Zmigrod, 2014), and previous work has also indicated hyper‐activation of these regions during a visual cognitive control task in more severely injured TBI patients (Scheibel et al., 2007). Hyper‐activation in conjunction with normal behavioral performance is typically interpreted to be the result of compensation, suggesting that mTBI patients exhibited increased activation within the IPL to achieve the same level of behavioral performance. Thus, current results suggest that self‐reported cognitive deficits (e.g., increased distractibility) following mTBI may result more from cognitive load requirements (i.e., processing of more rapidly presented stimuli and/or dual tasking) rather than the processing of conflicting information per se.
Recently there has been an increased focus on neurosensory deficits (i.e., increased auditory, visual, and vestibular symptoms) following mTBI, especially in military settings following blast‐related trauma (Hoffer, 2015; Lew et al., 2009; Lew et al., 2011; Pogoda et al., 2012). The civilian mTBI patients in the current sample self‐report increased vestibular and visual symptoms relative to HC using an identical measure (NSI) previously employed in military research settings (Pogoda et al., 2012). No significant differences were observed between mTBI patients and HC for auditory symptoms in the current study, suggesting that blast in military settings may be uniquely detrimental to auditory functioning due to high levels of acoustic noise (Gallun et al., 2012; Oleksiak et al., 2012). In contrast, similar levels of visual symptoms have been reported following both blast related versus non‐blast related TBI, suggesting that the mechanism of injury may play a smaller role within this sensory modality (Goodrich et al., 2013). Visual symptoms also persisted at a trend level into the early chronic stage, similar to other self‐reported emotional and cognitive symptoms. Although self‐reported vestibular symptoms were no longer statistically different between groups, the total number of mTBI patients reporting residual vestibular and visual symptoms was similar, potentially reflecting a base‐rate issue.
Deficits across multiple sensory domains following mTBI are common (Lew et al., 2011), suggesting that central rather than peripheral mechanisms are responsible for reported multi‐sensory dysfunction. The current study therefore also examined (1) atrophic changes within unisensory cortex, (2) how unisensory cortex is modulated through top‐down attentional control (ARM) and (3) basic unisensory cortical integrity (neurovascular coupling) in response to increased perceptual load (rate of stimulus presentation). There were no differences between patients and controls in terms of unisensory cortical volume, which is not surprising given that atrophic cortical changes would be unlikely to occur within the first few weeks of mild brain injury (Ling et al., 2013; Zhou et al., 2013).
Patients failed to up‐regulate auditory cortex during the attend‐auditory condition and showed decreased activation within visual cortex (main effect of group). mTBI patients also exhibited an increased BOLD response in visual cortex during increased perceptual load (stimulus rate) as well as attentional demands (ARM). However, the latter finding was partially driven by a decreased response in visual cortex during attend‐auditory trials, which was also present as a main effect in voxel‐wise analyses. Thus, current findings indicate different visual cortical abnormalities that were partially dependent on whether attention was focused on auditory or visual information streams. Similar to symptom self‐report, there was only partial resolution of neuronal abnormalities in visual cortex at four months post‐injury, providing additional evidence of longer‐term neuronal changes in unisensory cortex following mTBI. Importantly, previous studies have observed structural and functional abnormalities in the thalamus following mTBI (Lui et al., 2014; Mayer et al., 2009b), and both incoming sensory information and top‐down modulatory signals are routed through the thalamus prior to reaching unisensory cortex. Thus, we cannot rule‐out the contribution of thalamic and other mid‐brain abnormalities to current results.
There are several limitations to the current study. First, our anatomical battery did not include more advanced structural imaging (e.g., FLAIR, T2*) that may have provided additional sensitivity for detecting lesions. Similarly, the current sample included patients both with (i.e., complicated mTBI) and without intracranial structural lesions based on CT and structural MRI scans, and evidence suggests that complicated patients may experience a more prolonged recovery (Kashluba et al., 2008). Second, abnormalities in the BOLD signal following mTBI may result from impaired neuronal function, impaired neural control of microvessels, direct damage to the vascular system, metabolic disruptions, changes in cerebral blood flow or a combination of these factors (Mayer et al., 2014; Meier et al., 2015). Standard EPI, when used alone, is not capable of disambiguating these various possible contributing sources. Similarly, it is not possible to determine whether observed differences in functional activation between patients and HC are indicative of cortical dysfunction, compensatory activation or a neuro‐protective response to brain injury. Third, patients were not assessed with a formal neurological exam following injury, which could have provided additional information regarding neurosensory dysfunction. Finally, many of the hemodynamic abnormalities in unisensory cortex were at trend levels in spite of our larger sample size, suggesting that neural evidence of sensory dysfunction is likely to be subtle even during the semi‐acute injury phase when a larger number of patients are more symptomatic.
In summary, understanding the central mechanisms underlying self‐reported neurosensory deficits following mTBI is imperative, as these sensory deficits negatively affect everyday functioning and there are currently no accepted treatments (Hoffer, 2015). Consistent with reports from military cohorts (Pogoda et al., 2012), mTBI patients self‐reported more cognitive, somatic, emotional as well as visual and vestibular symptoms during the semi‐acute stage, which only partially resolved at four months post‐injury. Evidence of multifaceted neural abnormalities in unisensory cortex was also observed, particularly within visual cortex. In contrast, current results provided no evidence of behavioral or functional deficits in cognitive control during the semi‐acute phase of mTBI. Rather, current results suggest a greater role for compensatory activation within the IPL as a function of increased cognitive load to achieve similar levels of behavioral performances. Functional abnormalities within the visual cortex and IPL persisted into the early chronic stage, suggesting that neural abnormalities may lag behind the traditional behavioral measures that are used in most clinical settings.
ACKNOWLEDGMENT
Special thanks to Diana South and Cathy Smith for assistance with data collection.
REFERENCES
- Arciniegas D, Olincy A, Topkoff J, McRae K, Cawthra E, Filley CM, Reite M, Adler LE (2000): Impaired auditory gating and P50 nonsuppression following traumatic brain injury. J Neuropsychiatry Clin Neurosci 12:77–85. [DOI] [PubMed] [Google Scholar]
- Arciniegas DB, Topkoff JL (2004): Applications of the P50 evoked response to the evaluation of cognitive impairments after traumatic brain injury. Phys Med Rehabil Clin N Am 15:177–203, viii. [DOI] [PubMed] [Google Scholar]
- Baier B, Kleinschmidt A, Muller NG (2006): Cross‐modal processing in early visual and auditory cortices depends on expected statistical relationship of multisensory information. J Neurosci 26:12260–12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryer EJ, Medaglia JD, Rostami S, Hillary FG (2013): Neural recruitment after mild traumatic brain injury is task dependent: A meta‐analysis. J Int Neuropsychol Soc 19:751–762. [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Cancelliere C, Cote P, Hincapie CA, Kristman VL, Holm LW, Borg J, Nygren‐de BC, Hartvigsen J (2014): Systematic review of the prognosis after mild traumatic brain injury in adults: Cognitive, psychiatric, and mortality outcomes: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 95:S152–S173. [DOI] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A (2004): Functional abnormalities in symptomatic concussed athletes: An fMRI study. Neuroimage 22:68–82. [DOI] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Collie A, McCrory P, Ptito A (2007): A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J Neurol Neurosurg Psychiatry 78:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E (2010): Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbin RJ, Covassin T, Hakun J, Kontos AP, Berger K, Pfeiffer K, Ravizza S (2012): Do brain activation changes persist in athletes with a history of multiple concussions who are asymptomatic? Brain Inj 26:1217–1225. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG (2010): Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention.
- Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BT, Mohlberg H, Amunts K, Zilles K (2008): Cortical folding patterns and predicting cytoarchitecture. Cereb Cortex 18:1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BL, Parsons M, Durgerian S, Reece C, Mourany L, Lowe MJ, Beall EB, Koenig KA, Jones SE, Newsome MR, Scheibel RS, Wilde EA, Troyanskaya M, Merkley TL, Walker M, Levin HS, Rao SM (2014): Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. J Neurotrauma 31:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallun FJ, Lewis MS, Folmer RL, Diedesch AC, Kubli LR, McDermott DJ, Walden TC, Fausti SA, Lew HL, Leek MR (2012): Implications of blast exposure for central auditory function: A review. J Rehabil Res Dev 49:1059–1074. [DOI] [PubMed] [Google Scholar]
- Goodrich GL, Flyg HM, Kirby JE, Chang CY, Martinsen GL (2013): Mechanisms of TBI and visual consequences in military and veteran populations. Optom Vis Sci 90:105–112. [DOI] [PubMed] [Google Scholar]
- Halterman CI, Langan J, Drew A, Rodriguez E, Osternig LR, Chou LS, van DP (2006): Tracking the recovery of visuospatial attention deficits in mild traumatic brain injury. Brain 129:747–753. [DOI] [PubMed] [Google Scholar]
- Hinds OP, Rajendran N, Polimeni JR, Augustinack JC, Wiggins G, Wald LL, Diana RH, Potthast A, Schwartz EL, Fischl B (2008): Accurate prediction of V1 location from cortical folds in a surface coordinate system. Neuroimage 39:1585–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer ME (2015): Mild traumatic brain injury: Neurosensory effects. Curr Opin Neurol 28:74–77. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Henson CO, Rupert A, Galambos R (1959): “Attention” units in the auditory cortex. Science 129:1279–1280. [DOI] [PubMed] [Google Scholar]
- Kashluba S, Hanks RA, Casey JE, Millis SR (2008): Neuropsychologic and functional outcome after complicated mild traumatic brain injury. Arch Phys Med Rehabil 89:904–911. [DOI] [PubMed] [Google Scholar]
- Kato T, Nakayama N, Yasokawa Y, Okumura A, Shinoda J, Iwama T (2007): Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J Neurotrauma 24:919–926. [DOI] [PubMed] [Google Scholar]
- Kim J, Whyte J, Patel S, Europa E, Slattery J, Coslett HB, Detre JA (2012): A perfusion fMRI study of the neural correlates of sustained‐attention and working‐memory deficits in chronic traumatic brain injury. Neurorehabil Neural Repair 26:870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Rao SL, Nair RG, Pillai S, Chandramouli BA, Subbakrishna DK (2005): Sensory gating impairment in development of post‐concussive symptoms in mild head injury. Psychiatry Clin Neurosci 59:466–472. [DOI] [PubMed] [Google Scholar]
- Lew HL, Garvert DW, Pogoda TK, Hsu PT, Devine JM, White DK, Myers PJ, Goodrich GL (2009): Auditory and visual impairments in patients with blast‐related traumatic brain injury: Effect of dual sensory impairment on Functional Independence Measure. J Rehabil Res Dev 46:819–826. [DOI] [PubMed] [Google Scholar]
- Lew HL, Weihing J, Myers PJ, Pogoda TK, Goodrich GL (2010): Dual sensory impairment (DSI) in traumatic brain injury (TBI)–An emerging interdisciplinary challenge. NeuroRehabilitation 26:213–222. [DOI] [PubMed] [Google Scholar]
- Lew HL, Pogoda TK, Baker E, Stolzmann KL, Meterko M, Cifu DX, Amara J, Hendricks AM (2011): Prevalence of dual sensory impairment and its association with traumatic brain injury and blast exposure in OEF/OIF veterans. J Head Trauma Rehabil 26:489–496. [DOI] [PubMed] [Google Scholar]
- Ling JM, Klimaj S, Toulouse T, Mayer AR (2013): A prospective study of gray matter abnormalities in mild traumatic brain injury. Neurology 81:2121–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui YW, Xue Y, Kenul D, Ge Y, Grossman RI, Wang Y (2014): Classification algorithms using multiple MRI features in mild traumatic brain injury. Neurology 83:1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso E, Driver J (2005): Multisensory spatial interactions: A window onto functional integration in the human brain. Trends Neurosci 28:264–271. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Franco AR, Canive J, Harrington DL (2009a): The effects of stimulus modality and frequency of stimulus presentation on cross‐modal distraction. Cereb Cortex 19:993–1007. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Elgie R, Gasparovic C, Phillips JP, Doezema D, Yeo RA (2009b): Auditory orienting and inhibition of return in mild traumatic brain injury: A FMRI study. Hum Brain Mapp 30:4152–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Teshiba TM, Franco AR, Ling J, Shane MS, Stephen JM, Jung RE (2011): Modeling conflict and error in the medial frontal cortex. Hum Brain Mapp 33:2843–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Yang Z, Yeo RA, Pena A, Ling JM, Mannell MV, Stippler M, Mojtahed K (2012): A functional MRI study of multimodal selective attention following mild traumatic brain injury. Brain Imaging Behav 6:343–354. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Bellgowan PS, Hanlon FM (2014): Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci Biobehav Rev 49C:8–18. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian AC, Weaver JB, Yanofsky N (1999): Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology 53:1300–1308. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ (2001): Differential working memory load effects after mild traumatic brain injury. Neuroimage 14:1004–1012. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz K, Randolph C, Barr WB, Hammeke TA, Marshall SW, Powell MR, Woo AK, Wang Y, Kelly JP (2013): Incidence, clinical course, and predictors of prolonged recovery time following sport‐related concussion in high school and college athletes. J Int Neuropsychol Soc 19:22–33. [DOI] [PubMed] [Google Scholar]
- Meier TB, Bellgowan PS, Singh R, Kuplicki R, Polansky M, Mayer AR (2015): Recovery of cerebral blood flow following sports‐related concussion. JAMA Neurol 72:530–538. [DOI] [PubMed] [Google Scholar]
- Nachev P, Husain M (2006): Disorders of visual attention and the posterior parietal cortex. Cortex 42:766–773. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Nakayama N, Miwa K, Okumura A, Soeda A, Iwama T (2007): Focal brain glucose hypometabolism in patients with neuropsychologic deficits after diffuse axonal injury. Am J Neuroradiol 28:236–242. [PMC free article] [PubMed] [Google Scholar]
- Oleksiak M, Smith BM, St Andre JR, Caughlan CM, Steiner M (2012): Audiological issues and hearing loss among Veterans with mild traumatic brain injury. J Rehabil Res Dev 49:995–1004. [DOI] [PubMed] [Google Scholar]
- Pogoda TK, Hendricks AM, Iverson KM, Stolzmann KL, Krengel MH, Baker E, Meterko M, Lew HL (2012): Multisensory impairment reported by veterans with and without mild traumatic brain injury history. J Rehabil Res Dev 49:971–984. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004): The role of the medial frontal cortex in cognitive control. Science 306:443–447. [DOI] [PubMed] [Google Scholar]
- Roberts KL, Hall DA (2008): Examining a supramodal network for conflict processing: A systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. J Cogn Neurosci 20:1063–1078. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Steinberg JL, Pearson DA, Rauch RA, Mao H, Troyanskaya M, Sharma RG, Levin HS (2007): Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. Neurorehabil Neural Repair 21:36–45. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Lin X, Steinberg JL, Radaideh M, Levin HS (2012): Altered brain activation in military personnel with one or more traumatic brain injuries following blast. J Int Neuropsychol Soc 18:89–100. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD (2013): The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 79:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M, Dippel DW, Houston GC, Wielopolski PA, Koudstaal PJ, Hunink MG, van der Lugt A (2008): Postconcussion syndrome after minor head injury: Brain activation of working memory and attention. Hum Brain Mapp 30:2789–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis EA, Wilson JT, Hadley DM, Wyper DJ (2002): SPECT imaging in head injury interpreted with statistical parametric mapping. J Nucl Med 43:476–483. [PubMed] [Google Scholar]
- Stulemeijer M, Vos PE, van der Werf S, van DG, Rijpkema M, Fernandez G (2010): How mild traumatic brain injury may affect declarative memory performance in the post‐acute stage. J Neurotrauma 27:1585–1595. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐Planar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Talsma D, Senkowski D, Soto‐Faraco S, Woldorff MG (2010): The multifaceted interplay between attention and multisensory integration. Trends Cogn Sci 14:400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG (2004): The neural mechanisms for minimizing cross‐modal distraction. J Neurosci 24:10941–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Andersen RA (2000): Models of the posterior parietal cortex which perform multimodal integration and represent space in several coordinate frames. J Cogn Neurosci 12:601–614. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang KH, King AI (2004): A proposed injury threshold for mild traumatic brain injury. J Biomech Eng 126:226–236. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Kierans A, Kenul D, Ge Y, Rath J, Reaume J, Grossman RI, Lui YW (2013): Mild traumatic brain injury: Longitudinal regional brain volume changes. Radiology 267:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmigrod S (2014): The role of the parietal cortex in multisensory and response integration: Evidence from transcranial direct current stimulation (tDCS). Multisens Res 27:161–172. [DOI] [PubMed] [Google Scholar]


