Abstract
Background and aims
Despite available effective therapies, only a minority of patients with chronic hepatitis B (CHB) receive treatment. Our goal is to study treatment rates and time to treatment initiation in patients who meet treatment criteria on long-term follow-up.
Methods
We performed a retrospective cohort study of 608 consecutive treatment-eligible patients with CHB (by 2008 US Panel or 2009 American Association for the Study of Liver Disease (AASLD) criteria) at a US community gastroenterology clinic and a university liver clinic between 2007 and 2011. Patients were observed until they started treatment or last follow-up if untreated.
Results
Mean age was 44 and most were Asian (96%) with community patients being younger and having lower alanine aminotransferase (ALT) levels. A total of 62% started treatment, and 38% remained untreated after median follow-up of 17 months (IQR=1–40 months). Overall, treatment rate was significantly higher at university liver clinic than in the community (66.7% vs 59.9%, p=0.01). In multivariate analysis, older age (HR 1.02, p=0.002), male gender (HR 1.37, p=0.02), and baseline ALT >45 U/L for males and >29 U/L for females (HR 2.24, p<0.0001) were significant predictors of treatment initiation, but not practice setting.
Conclusions
Approximately 40% of treatment-eligible patients still have not started treatment on longer follow-up. Treatment rates were higher at university clinics, but practice setting was not a predictor for treatment, but older age, male gender, and higher ALT levels were. Further studies are needed to determine the barriers for treatment initiation and to improve treatment rates in treatment-eligible patients.
Keywords: CHRONIC HEPATITIS, HEPATITIS, HEPATITIS B
Summary box.
What is already known about this subject?
-
▸
Antiviral therapy has been shown to halt the progression of liver disease and the progression of hepatocellular carcinoma in patients with chronic hepatitis B.
-
▸
Despite the availability of effective therapies, only a small proportion of chronic hepatitis B patients receive treatment.
-
▸
It is unclear how likely patients who are eligible for treatment actually receive treatment.
What are the new findings?
-
▸
In this current study of approximately 600 treatment-eligible chronic hepatitis B patients, we found that only 62% of patients received treatment.
-
▸
Patients who were seen at a university liver clinic were more likely to receive treatment than patients seen at a community gastroenterology clinic.
-
▸
Most patients who received treatment started within the first year of meeting treatment criteria.
How might it impact on clinical practice in the foreseeable future?
-
▸
The low treatment rate highlights the need for increased education of chronic hepatitis B for physicians and patients.
Introduction
Chronic hepatitis B (CHB) affects approximately 350–400 million people worldwide, and can lead to complications such as cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC).1–3 Individuals who are chronically infected with hepatitis B virus (HBV) face a 25% risk of death from cirrhosis or HCC.4–6 Each year, one million people die from HBV-related causes worldwide.3
In the USA, the overall CHB prevalence is 0.3% with an estimated 1.25 million hepatitis B carriers.1–3 However, this number is considered to be an under-estimate of the true prevalence of HBV in the USA.5–7 Some estimate that there are as many as 2 million carriers when accounting for the high prevalence of HBV in immigrant populations, which are often under-represented.3 5 Prevalence of HBV infection among Asian-Americans is reported to be between 10% and 15%,5 7 and in areas with a large proportion of immigrants, almost 85% of all HBV cases are Asian.8 HBV is responsible for approximately 5000 deaths and $1 billion in medical costs in the USA each year.3 5 7 Early initiation of anti-HBV therapy can potentially reduce the number of deaths and the financial burden caused by HBV.
Antiviral therapy has been shown to slow or halt the progression of liver disease and the development of HCC by suppressing viral replication.9–12 Treatment guidelines and algorithms have been developed by various professional societies to aid physicians in the evaluation and treatment of patients with CHB.13 14 However, even with the aid of these guidelines, only 50 000 of the estimated 2 million patients with CHB in the US have received anti-HBV therapy.15 Prior studies have reported that only some patients with CHB undergo appropriate evaluation with the appropriate testing, such as hepatitis B envelope antigen (HBeAg) and HBV DNA in addition to hepatitis B surface antigen and alanine aminotransferase (ALT) levels, and only some of the patients who meet treatment guideline criteria for antiviral therapy after the initial evaluation are treated.15–17 It is unclear how likely such patients who meet treatment criteria for antiviral therapy may actually receive treatment on longer follow-up.
The primary goal of this study was to examine actual treatment rates of patients with CHB who were treatment-eligible at a community gastroenterology (GI) clinic and a university liver clinic in the San Francisco Bay Area on long-term follow-up. The secondary aim was to identify predictors of treatment initiation in treatment-eligible patients with CHB.
Methods
Study design and data collection
We conducted a retrospective cohort study of 608 consecutive patients with CHB who were treatment-eligible at initial evaluation at a community GI clinic and a university liver clinic in the San Francisco Bay Area between 2007 and 2011. Patients were identified via computer query using International Classification of Diseases 9 codes, and treatment eligibility was based on the US Panel 2008 (US Panel) and the American Association for the Study of Liver Disease (AASLD) 2009 guidelines.18 19 Patients were seen by gastroenterologists or hepatologists, and treatment decisions were made directly by these providers. Providers used clinical and laboratory data, including Fibroscan, liver biopsy, and serum markers, to determine the need for therapy. There were no mid-level providers, such as physician assistants or nurse practitioners, involved in the care of these patients in any of these clinics.
Patients were included in the cohort if they were treatment naïve at first presentation and had at least 6 months of follow-up. Data were collected from individual patient electronic medical records using a case report form. Clinical and laboratory data available during the first 6 months of initial visit were included in evaluation. Patients who qualified for the study were observed until they started treatment or until their last follow-up if they remained untreated. Reasons for non-treatment of patients were grouped into the following categories: further observation (which included physician desire for more data and perceived normal laboratory tests); patient decision (which included patient refusal and patient loss to follow-up); no longer treatment-eligible; financial difficulties; pregnancy or future pregnancy; and unknown reasons.
This study was approved by the Institutional Review Board at Stanford University (Stanford, California, USA).
Treatment eligibility criteria
Treatment eligibility was based on the US Panel 2008 and the AASLD 2009 guidelines. Patients were considered treatment-eligible if they met the criteria for either set of guidelines. US Panel treatment eligibility criteria include ALT > upper limit of normal (ULN) (30 U/L for males, 19 U/L for females) and HBV DNA ≥20 000 IU/mL for HBeAg-positive patients and HBV DNA ≥2000 IU/mL for HBeAg-negative patients. AASLD treatment eligibility criteria include ALT >2x ULN and HBV DNA >20 000 IU/mL, regardless of HBeAg status. For patients with compensated cirrhosis, treatment eligibility is based on only HBV DNA levels. According to the US Panel and the AASLD, patients with compensated cirrhosis are treatment-eligible if HBV DNA is ≥2000 IU/mL or >2000 IU/mL, respectively. Patients with decompensated cirrhosis are eligible for treatment if they have any detectable HBV DNA level.
Statistical analysis
Descriptive statistics were reported as proportions (%) for categorical variables, and mean±SD or median (range) for continuous variables. Categorical variables were evaluated using the χ2 test; normally distributed continuous variables were evaluated using the student t test; continuous variable that were not normally distributed were evaluated using the Wilcoxon rank-sum test. Univariate and multivariate Cox proportional-hazards regression models were used to estimate HRs relating various predictors to outcomes of initiating antiviral therapy. A two-tailed p value of ≤0.05 was considered significant. All statistical analyses were performed using STATA V.11 (STATA Corporation, College Station, Texas, USA).
Results
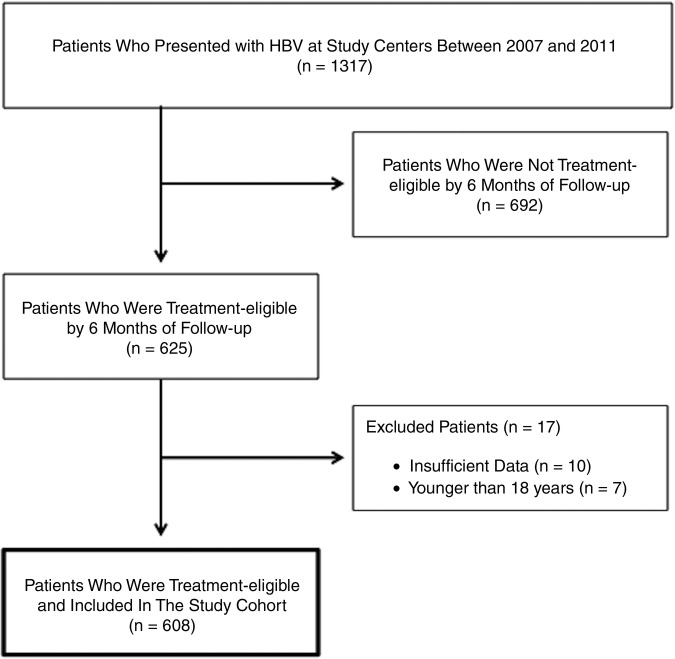
A total of 608 patients with CHB from a community GI clinic and a university liver clinic were included in this study (figure 1). Patients had a mean age of 44 years, were more commonly male (57%), and were almost all Asian (96%). The vast majority (89%) of our Asian patients were either Vietnamese or Chinese. Compared to the university clinic group, the community clinic group included significantly more Asians (99.8% vs 86.4%, p<0.0001), had more male patients (60% vs 49%, p=0.02), were more likely to be HBeAg-positive (36% vs 24%, p=0.01), and had significantly lower baseline ALT (45 vs 51 U/L, p=0.02) (table 1).
Figure 1.
Flow diagram for patients with chronic hepatitis B who were evaluated for treatment initiation. HBV, hepatitis B virus.
Table 1.
Patient baseline characteristics
| Total cohort (n=608) | Community GI clinic (n=446) | University liver clinic (n=162) | p Value | |
|---|---|---|---|---|
| Mean age (years) | 44±13 | 43±13 | 45±14 | 0.19 |
| Male | 56.9% | 59.6% | 49.4% | 0.02 |
| Asian | 96.2% | 99.8% | 86.4% | <0.0001 |
| HBeAg-positive | 32.5% | 35.9% | 24.2% | 0.01 |
| Baseline ALT, U/L | 47 (34–78) | 45 (32–77) | 51 (25–78) | 0.02 |
| Baseline HBV DNA, log10 IU/mL | 5.3 (4.2–7.3) | 5.3 (4.4–7.1) | 5.0 (4.0–7.5) | 0.07 |
| Cirrhosis | 6.9% | 5.4% | 11.1% | 0.01 |
| Months to treatment initiation | 2 (1–7) | 2 (1–8) | 1 (1–3) | 0.002 |
| Follow-up of untreated patients | 17 (1–40) | 13 (1–40) | 21 (2–40) | 0.36 |
Values are presented as mean±SD, proportions, or median (IQR).
ALT, alanine aminotransferase; GI, gastroenterology; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus DNA.
In total, 62% of patients received treatment and they did so after median follow-up of 2 months (IQR=1–7 months), but 38% remained untreated after longer follow-up (median=17 months (IQR=1–40 months)). Reasons for no treatment are shown in figure 2. The physician's desire for further observation was the most frequently cited reason for non-treatment (42.9%), followed by the patient's decision not to pursue treatment (24.5%). Patients who started treatment were more likely to be male (61% vs 50%, p=0.01), older (45 vs 41, p<0.00 001), have cirrhosis (8.8% vs 3.9%, p=0.02), and had significantly higher baseline ALT (57 vs 38 U/L, p<0.00001) and higher baseline HBV DNA (5.8 log10 vs 4.8 log10 IU/mL, p<0.00001) than patients who remained untreated (table 2).
Figure 2.
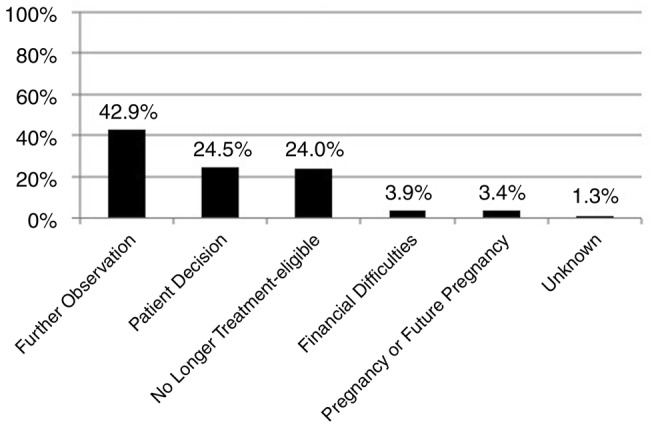
Reasons for non-treatment of treatment-eligible patients on long-term follow-up.
Table 2.
Characteristics of patients who were treated and not treated
| Total (n=608) | Treated (n=375) | Not treated (n=233) | p Value | |
|---|---|---|---|---|
| Mean age (years) | 44±13 | 45±13 | 41±13 | 0.0001 |
| Male | 57% | 61% | 50% | 0.01 |
| Asian | 96% | 97% | 96% | 0.60 |
| HBeAg-positive | 33% | 36% | 28% | 0.04 |
| Baseline ALT, U/L | 47 (34–78) | 57 (38–96) | 38 (27–50) | <0.00001 |
| Baseline HBV DNA, log10 IU/mL | 5.3 (4.2–7.3) | 5.8 (4.6–7.4) | 4.8 (4.0–6.8) | <0.00001 |
| Cirrhosis | 6.9% | 8.8% | 3.9% | 0.02 |
Values are presented as mean±SD, proportions, or median (IQR).
ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus DNA.
Of the 375 patients who received treatment, 84% were treated within their first year (median=1 month, IQR=1–3 months) of becoming treatment-eligible. Table 3 compares the characteristics of patients who received treatment within 1 year of becoming treatment-eligible to patients who received treatment after 1 year of becoming eligible. Baseline ALT was significantly higher in patients who started treatment in the first year of eligibility (61 vs 38, p<0.00001).
Table 3.
Characteristics of patients who received treatment by 12 months of eligibility and after 12 months of eligibility
| Total treated (n=375) | Treated by 12 months (n=316) | Treated after 12 months (n=59) | p Value | |
|---|---|---|---|---|
| Mean age (years) | 45±13 | 45±13 | 45±12 | 0.85 |
| Male | 61.1% | 61.7% | 57.6% | 0.56 |
| Asian | 96.5% | 96.2% | 98.3% | 0.42 |
| HBeAg-positive | 35.9% | 37.5% | 28.8% | 0.21 |
| Baseline ALT, U/L | 57 (38–96) | 61 (41–104) | 38 (27–52) | <0.00001 |
| Baseline HBV DNA, log10 IU/mL | 5.8 (4.6–7.4) | 5.9 (4.6–7.5) | 5.2 (4.7–7.1) | 0.45 |
| Cirrhosis | 8.8% | 9.8% | 3.4% | 0.11 |
Values are presented as mean±SD, proportions, or median (IQR).
ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus DNA.
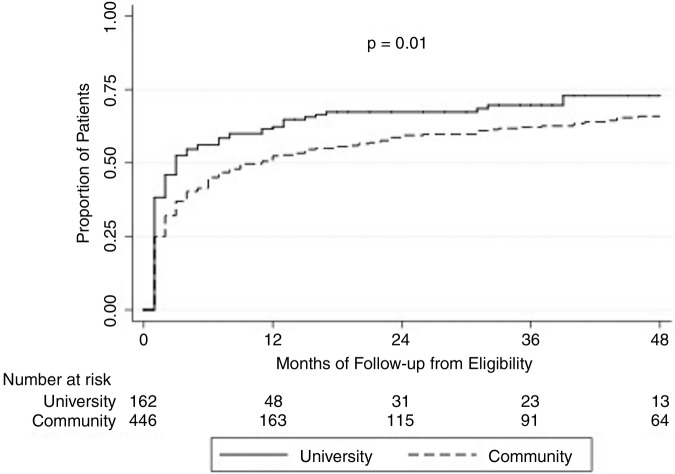
Patients seen at the university clinic were more likely to be treated than patients at the community GI clinic (66.7% vs 59.9%, p=0.01). Figure 3 compares the rates of treatment at the two sites over time. Treatment rate within the first year of treatment eligibility was significantly higher at the university clinic than at the community clinic (60% vs 49%, p=0.02). Community and university patients who were treated within 1 year of becoming treatment eligible had similar baseline ALT, but community patients were more likely to be male (65% vs 54%, p=0.05), Asian (99.5% vs 88.7%, p<0.0001), HBeAg-positive (46% vs 22%, p<0.0001), and had significantly higher baseline HBV DNA (6.1 log10 vs 5.5 log10 IU/mL, p=0.03) (table 4). Table 5 compares patients who received treatment after 1 year of becoming treatment eligible at the two sites. Baseline ALT was significantly higher in patients at the university clinic than at the community clinic (52 vs 34 U/L, p=0.01).
Figure 3.
Treatment rates in patients who were treatment-eligible at a community GI clinic and a university liver clinic through 4 years.
Table 4.
Characteristics of patients who received treatment by 12 months of eligibility at a community GI clinic and a university liver clinic
| Total treated by 12 months (n=316) | Community GI clinic (n=219) | University liver clinic (n=97) | p Value | |
|---|---|---|---|---|
| Mean age (years) | 45±13 | 44±13 | 47±13 | 0.06 |
| Male | 61.7% | 65.3% | 53.6% | 0.05 |
| Asian | 96.2% | 99.5% | 88.7% | <0.0001 |
| HBeAg-Positive | 37.5% | 45.7% | 22.3% | <0.0001 |
| Baseline ALT, U/L | 61 (41–104) | 61 (41–106) | 60 (42–102) | 0.95 |
| Baseline HBV DNA, log10 IU/mL | 5.9 (4.6–7.5) | 6.1 (4.8–7.4) | 5.5 (4.0–7.6) | 0.03 |
| Cirrhosis | 9.8% | 9.1% | 11.3% | 0.54 |
Values are presented as mean±SD, proportions, or median (IQR).
ALT, alanine aminotransferase; GI, gastroenterology; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus DNA.
Table 5.
Characteristics of patients who received treatment after 12 months of eligibility at a community GI clinic and a university liver clinic
| Total treated after 12 months (n=59) | Community GI clinic (n=48) | University liver clinic (n=11) | p Value | |
|---|---|---|---|---|
| Mean age (years) | 45±12 | 45±12 | 46±15 | 0.84 |
| Male | 57.6% | 64.6% | 27.3% | 0.02 |
| Asian | 98.3% | 100.0% | 90.9% | 0.04 |
| HBeAg-Positive | 28.8% | 31.3% | 18.2% | 0.39 |
| Baseline ALT, U/L | 38 (27–52) | 34 (25–46) | 52 (44–54) | 0.01 |
| Baseline HBV DNA, log10 IU/mL | 5.2 (4.7–7.1) | 5.3 (4.8–7.4) | 4.8 (4.3–5.8) | 0.14 |
| Cirrhosis | 3.4% | 2.1% | 9.1% | 0.25 |
Values are presented as mean±SD, proportions, or median (IQR).
ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus DNA.
On multivariate analysis, older age (HR 1.02, p=0.002), male gender (HR 1.37, p=0.02), baseline ALT >45 for males and >29 for females (HR 2.24, p<0.0001), higher HBV DNA (HR 1.11, p=0.05), and cirrhosis (HR 1.65, p=0.04) were significant independent predictors of treatment initiation overall. Practice setting was not a predictor (table 6). Significant independent predictors for treatment initiation within the first year of treatment eligibility were older age (HR 1.02, p=0.003), male gender (HR 1.42, p=0.02), and baseline ALT >45 U/L for males and >29 U/L for females (HR 2.77, p<0.0001), but practice setting was not (table 7).
Table 6.
Predictors of treatment in patients who were treatment-eligible
| Unadjusted HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value | |
|---|---|---|---|---|
| Age | 1.01 (1.00 to 1.02) | 0.002 | 1.02 (1.01 to 1.03) | 0.002 |
| Male | 1.33 (1.07 to 1.64) | 0.01 | 1.37 (1.05 to 1.78) | 0.02 |
| Non-Asian | 0.88 (0.48 to 1.61) | 0.69 | 0.61 (0.27 to 1.35) | 0.22 |
| Community Site | 0.82 (0.66 to 1.03) | 0.09 | 0.82 (0.62 to 1.10) | 0.19 |
| HBeAg-Positive | 1.37 (1.09 to 1.73) | 0.01 | 1.19 (0.82 to 1.73) | 0.35 |
| Baseline ALT | 2.15 (1.68 to 2.75) | <0.0001 | 2.24 (1.66 to 3.04) | <0.0001 |
| Baseline HBV DNA | 1.14 (1.06 to 1.21) | <0.0001 | 1.11 (1.00 to 1.24) | 0.05 |
| Cirrhosis | 1.74 (1.21 to 2.51) | 0.003 | 1.65 (1.03 to 2.64) | 0.04 |
Values are presented as median (range).
ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus DNA.
Table 7.
Predictors of treatment by 12 months of eligibility in patients who were treatment-eligible
| Unadjusted HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value | |
|---|---|---|---|---|
| Age | 1.01 (1.00 to 1.02) | 0.003 | 1.02 (1.01 to 1.03) | 0.003 |
| Male | 1.31 (1.04 to 1.66) | 0.02 | 1.42 (1.06 to 1.90) | 0.02 |
| Non-Asian | 0.88 (0.47 to 1.66) | 0.70 | 0.54 (0.23 to 1.29) | 0.17 |
| Community Site | 0.77 (0.61 to 0.98) | 0.04 | 0.76 (0.55 to 1.03) | 0.08 |
| HBeAg-Positive | 1.36 (1.06 to 1.76) | 0.02 | 1.28 (0.86 to 1.91) | 0.23 |
| Baseline ALT | 2.51 (1.89 to 3.33) | <0.0001 | 2.77 (1.93 to 3.99) | <0.0001 |
| Baseline HBV DNA | 1.13 (1.05 to 1.21) | 0.001 | 1.09 (0.97 to 1.22) | 0.15 |
| Cirrhosis | 1.83 (1.26 to 2.68) | 0.002 | 1.45 (0.89 to 2.38) | 0.14 |
Values are presented as median (range).
ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus DNA.
Discussion
While treatment of CHB can decrease the risk of disease progression and HCC development, the treatment rate of patients who meet treatment eligibility criteria is quite poor with only one-half to two-thirds actually receiving treatment.20–23 Results from prior studies are limited by small sample size, lack of follow-up, and/or lack of practice setting diversity. In addition, according to a recent systematic review of barriers to care and treatment for patients with CHB in Europe, most studies do not provide detailed treatment data or treatment eligibility data; and when available, the data suggest that some patients may have been initiated on therapy inappropriately, thus true treatment rate in treatment-eligible patients may even be lower than perceived.24
In our current study of 608 patients with median follow-up of approximately 1½ years from community and university practices, a total of 62% of patients received therapy after becoming treatment-eligible. The most frequently cited reason for non-treatment was the treating physician's desire for further observation (43%). Another common reason for non-treatment in this study was the patient's decision not to pursue treatment (25%). It appears that the factors for non-treatment are both at the provider and patient levels. Other studies have identified potential physician-factors and patient-factors that range from inadequate screening practice for HBV infection to suboptimal management of patients with known CHB.15–17 20–22 In a review by Cohen et al,15 the authors postulate that suboptimal screening of at-risk patients is an important barrier to receiving appropriate medical care. Supporting this theory is a survey-based study by Kallman et al25 that showed only 58% of primary care physicians and 81% of gastroenterologists were aware of professional guidelines for the management of patients with CHB. In another survey-based study, Chao et al26 found that physicians at multiple levels of training, from recent medical graduates to attending physicians, did not have adequate knowledge of screening and managing patients with CHB—only 24% of the physicians were able to identify the correct serological tests to screen for CHB, and only 13% knew the appropriate next steps to refer patients who tested positive for HBV. Meanwhile, studies have also shown that patients have very limited knowledge of their disease and the treatment options available.27–30 What these findings highlight is the need for increased education of CHB for both physicians and patients on a greater scale, since several studies have demonstrated the effectiveness of this strategy in targeted populations.25 29–33
In regards to appropriate linkage to care of patients who have been screened and diagnosed with HBV infection, prior studies have also shown that there are barriers at various stages, from the suboptimal evaluation of newly diagnosed patients to the under-treatment of patients who have undergone treatment evaluation and have met criteria for anti-HBV therapy.8 21 34 35 For example, in a study conducted by Juday et al,36 the authors found that only 35% of untreated patients with CHB received laboratory monitoring of HBV DNA and ALT levels at least once every year. This could likely lead to an underestimate of untreated patients who are eligible for antiviral therapy. When comparing treatment rates between clinical settings, patients seen in the university were more likely to be treated than patients at the community GI clinic (67% vs 60%, p=0.12). This difference in treatment rates could be due to the larger number of patients with advanced liver disease seen in the university versus the community GI clinic, as indicated by the significantly higher baseline ALT levels (51 vs 45, p=0.02) and a higher proportion of patients with cirrhosis seen at university clinics (11.1% vs 5.4%, p=0.01).
In the current study, for patients who received treatment, 84% started within the first year of becoming treatment-eligible. Compared to patients who received treatment after 1 year, these patients had significantly higher ALT at baseline (61 vs 38 U/L, p<0.00001). On multivariate analysis, elevated baseline ALT was also a significant independent predictor of treatment within the first year of eligibility and for treatment overall. Both physicians and patients could have perceived the higher ALT to be a more urgent indication of significant hepatic damage and more serious illness, leading patients to start treatment on shorter follow-up. Since ALT fluctuates over time, patients who meet treatment criteria but are reluctant to start anti-HBV therapy should be monitored more closely. The appearance of high ALT elevation may encourage both physicians and patients to recognise the disease severity and become more receptive or proactive with treatment initiation. Even patients who do not meet treatment criteria within the first 6 months of presentation can become treatment-eligible on longer follow-up (20%).37
While this study is the first of its kind to examine actual treatment rates in patients who are eligible for treatment with longer follow-up and in diverse practice settings, it has some limitations. First, the majority of patients included in this study are Asians, so our findings may not be generalisable to other ethnic groups. While our study included university and community clinics, both subspecialty clinics, we did not include clinics in a primary care setting, where a large proportion of chronically infected patients are seen and where patients are even more likely to be under-treated. However, in most urban areas, the vast majority of patients with CHB probably would at least be referred to gastroenterologists in their community, and the results of our study are more likely to reflect the true treatment rates seen in real-life setting.21 22
In conclusion, in this study of approximately 600 consecutive treatment-eligible patients with CHB followed at two specialty clinics in the San Francisco Bay area, approximately 4 of 10 patients did not receive anti-HBV therapy even on longer follow-up. Eligible patients are more likely to initiate anti-HBV therapy in university liver clinics than in community GI clinics during the first year of their follow-up but treatment rates over long-term follow-up did not differ significantly between the two practice settings. In both practice settings, higher ALT level was an independent predictor of higher treatment rates among these treatment-eligible patients, but it is not clear how much of this influence by ALT levels is physician driven versus patient driven. However, it appears that both provider and patient factors contribute to under-treatment, and educational efforts should be targeted at both providers and patients. Additional studies are also needed to better understand the potential socioeconomic, cultural and medical barriers that prevent patients who should receive anti-HBV therapy from actually getting initiated on therapies that may benefit them, as many of these factors are likely modifiable.
Footnotes
Contributors: VDV was involved in the study design, data collection, data analysis and interpretation, and drafting of the manuscript. AD was involved in the data collection and critical review of the manuscript. NHN was involved in the data analysis and interpretation and critical review of the manuscript. LHK was involved in the data analysis and interpretation and critical review of the manuscript. HNT was involved in the data collection and critical review of the manuscript. HAN was involved in the data collection and critical review of the manuscript. KKN was involved in the data collection and critical review of the manuscript. MN was involved in the data collection and critical review of the manuscript. AH was involved in the data collection and critical review of the manuscript. MHN was involved in the study concept and design, data analysis and interpretation, and critical revision of the manuscript.
Competing interests: HNT has served as a speaker for Vertex and Bristol-Myers Squibb, consultant for Bristol-Myers Squibb, advisory board member for Gilead Sciences Inc. and Bristol-Myers Squibb, has received funding from Gilead Sciences Inc. and Roche, and owns stocks and shares in Gilead Sciences Inc. HAN has served as a speaker and advisory board member for Gilead Sciences Inc. and Bristol-Myers Squibb. MHN has served as a consultant for Bristol-Myers Squibb, Gilead Sciences Inc., and Roche Laboratories, and has received research funding from Bristol-Myers Squibb, Gilead Sciences, Novartis Pharmaceuticals, and Roche Laboratories.
Ethics approval: Stanford University Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis 2005;25(Suppl 1):3–8. doi:10.1055/s-2005-915644 [DOI] [PubMed] [Google Scholar]
- 2.Custer B, Sullivan SD, Hazlet TK, et al. . Global epidemiology of hepatitis B virus. J Clin Gastroenterol 2004;38(10 Suppl 3):S158–68. doi:10.1097/00004836-200411003-00008 [DOI] [PubMed] [Google Scholar]
- 3.Elgouhari HM, Abu-Rajab Tamimi TI, Carey WD. Hepatitis B virus infection: understanding its epidemiology, course, and diagnosis. Cleve Clin J Med 2008;75:881–9. doi:10.3949/ccjm.75a.07019 [DOI] [PubMed] [Google Scholar]
- 4.Wasley A, Kruszon-Moran D, Kuhnert W, et al. . The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis 2010;202:192–201. doi:10.1086/653622 [DOI] [PubMed] [Google Scholar]
- 5.Cohen C, Evans AA, London WT, et al. . Underestimation of chronic hepatitis B virus infection in the United States of America. J Viral Hepat 2008;15:12–13. doi:10.1111/j.1365-2893.2007.00888.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology 2007;46:1034–40. doi:10.1002/hep.21784 [DOI] [PubMed] [Google Scholar]
- 7.Kim WR. Epidemiology of hepatitis B in the United States. Hepatology 2009;49(5 Suppl):S28–34. doi:10.1002/hep.22975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ku KC, Li J, Ha NB, et al. . Chronic hepatitis B management based on standard guidelines in community primary care and specialty clinics. Dig Dis Sci 2013;58:3626–33. doi:10.1007/s10620-013-2889-1 [DOI] [PubMed] [Google Scholar]
- 9.Gordon SC, Lamerato LE, Rupp LB, et al. . Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol 2014;12:885–93. doi:10.1016/j.cgh.2013.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dienstag JL. Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology 2009;49(5 Suppl):S112–21. doi:10.1002/hep.22920 [DOI] [PubMed] [Google Scholar]
- 11.Liaw YF, Sung JJ, Chow WC, et al. . Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521–31. doi:10.1056/NEJMoa033364 [DOI] [PubMed] [Google Scholar]
- 12.Marcellin P, Chang TT, Lim SG, et al. . Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med 2003;348:808–16. doi:10.1056/NEJMoa020681 [DOI] [PubMed] [Google Scholar]
- 13.Lok AS, McMahon BJ, Practice guidelines committee AAftSoLD. Chronic hepatitis B. Hepatology 2001;34:1225–41. doi:10.1053/jhep.2001.29401 [DOI] [PubMed] [Google Scholar]
- 14.Keeffe EB, Dieterich DT, Han SH, et al. . A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin Gastroenterol Hepatol 2004;2:87–106. doi:10.1016/S1542-3565(03)00312-4 [DOI] [PubMed] [Google Scholar]
- 15.Cohen C, Holmberg SD, McMahon BJ, et al. . Is chronic hepatitis B being undertreated in the United States? J Viral Hepat 2011;18:377–83. doi:10.1111/j.1365-2893.2010.01401.x [DOI] [PubMed] [Google Scholar]
- 16.Jung CW, Tan J, Tan N, et al. . Evidence for the insufficient evaluation and undertreatment of chronic hepatitis B infection in a predominantly low-income and immigrant population. J Gastroenterol Hepatol 2010;25:369–75. doi:10.1111/j.1440-1746.2009.06023.x [DOI] [PubMed] [Google Scholar]
- 17.Giannini EG, Torre F, Basso M, et al. . A significant proportion of patients with chronic hepatitis B who are candidates for antiviral treatment are untreated: a region-wide survey in Italy. J Clin Gastroenterol 2009;43:1001–7. doi:10.1097/MCG.0b013e31818e876f [DOI] [PubMed] [Google Scholar]
- 18.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661–2. doi:10.1002/hep.23190 [DOI] [PubMed] [Google Scholar]
- 19.Keeffe EB, Dieterich DT, Han SH, et al. . A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008;6:1315–41; quiz 286 doi:10.1016/j.cgh.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 20.Nguyen VG, Wan K, Trinh HN, et al. . Chronic hepatitis B treatment eligibility and actual treatment rates in patients in community gastroenterology and primary care settings. J Clin Gastroenterol 2015;49:145–9. doi:10.1097/MCG.0000000000000132 [DOI] [PubMed] [Google Scholar]
- 21.Kim LH, Nguyen VG, Trinh HN, et al. . Low treatment rates in patients meeting guideline criteria in diverse practice settings. Dig Dis Sci 2014;59:2091–9. doi:10.1007/s10620-014-3283-3 [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Ristau JT, Trinh HN, et al. . Undertreatment of Asian chronic hepatitis B patients on the basis of standard guidelines: a community-based study. Dig Dis Sci 2012;57:1373–83. doi:10.1007/s10620-012-2137-0 [DOI] [PubMed] [Google Scholar]
- 23.Sun YT, Zhang YX, Tang H, et al. . Clinical characteristics and current management of hepatitis B and C in China. World J Gastroenterol 2014;20:13582–90. doi:10.3748/wjg.v20.i37.13582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papatheodoridis GV, Tsochatzis E, Hardtke S, et al. . Barriers to care and treatment for patients with chronic viral hepatitis in Europe: a systematic review. Liver Int 2014;34:1452–63. doi:10.1111/liv.12565 [DOI] [PubMed] [Google Scholar]
- 25.Kallman JB, Arsalla A, Park V, et al. . Screening for hepatitis B, C and non-alcoholic fatty liver disease: a survey of community-based physicians. Aliment Pharmacol Ther 2009;29:1019–24. doi:10.1111/j.1365-2036.2009.03961.x [DOI] [PubMed] [Google Scholar]
- 26.Chao SD, Wang BM, Chang ET, et al. . Medical training fails to prepare providers to care for patients with chronic hepatitis B infection. World J Gastroenterol 2015;21:6914–23. doi:10.3748/wjg.v21.i22.6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu KQ. Hepatitis B virus (HBV) infection in Asian and Pacific Islander Americans (APIAs): how can we do better for this special population? Am J Gastroenterol 2008;103:1824–33. doi:10.1111/j.1572-0241.2008.01878.x [DOI] [PubMed] [Google Scholar]
- 28.Ha NB, Trinh HN, Nguyen TT, et al. . Prevalence, risk factors, and disease knowledge of chronic hepatitis B infection in Vietnamese Americans in California. J Cancer Educ 2013;28:319–24. doi:10.1007/s13187-013-0466-0 [DOI] [PubMed] [Google Scholar]
- 29.Wu CA, Lin SY, So SK, et al. . Hepatitis B and liver cancer knowledge and preventive practices among Asian Americans in the San Francisco Bay Area, California. Asian Pac J Cancer Prev 2007;8:127–34. [PubMed] [Google Scholar]
- 30.Nguyen TT, Taylor V, Chen MS Jr, et al. . Hepatitis B awareness, knowledge, and screening among Asian Americans. J Cancer Educ 2007;22:266–72. doi:10.1080/08858190701645751 [DOI] [PubMed] [Google Scholar]
- 31.Coronado GD, Taylor VM, Tu SP, et al. . Correlates of hepatitis B testing among Chinese Americans. J Community Health 2007;32:379–90. doi:10.1007/s10900-007-9060-x [DOI] [PubMed] [Google Scholar]
- 32.Khalili M, Guy J, Yu A, et al. . Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci 2011;56:1516–23. doi:10.1007/s10620-010-1439-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu D, Yang JD, Lok AS, et al. . Hepatitis B screening and vaccination practices in Asian American primary care. Gut Liver 2013;7:450–7. doi:10.5009/gnl.2013.7.4.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarkar M, Shvachko VA, Ready JB, et al. . Characteristics and management of patients with chronic hepatitis B in an integrated care setting. Dig Dis Sci 2014;59:2100–8. doi:10.1007/s10620-014-3142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Johnson KB, Roccaro G, et al. . Poor adherence to AASLD guidelines for chronic hepatitis B Management and treatment in a large academic medical center. Am J Gastroenterol 2014;109:867–75. doi:10.1038/ajg.2014.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juday T, Tang H, Harris M, et al. . Adherence to chronic hepatitis B treatment guideline recommendations for laboratory monitoring of patients who are not receiving antiviral treatment. J Gen Intern Med 2011;26:239–44. doi:10.1007/s11606-010-1549-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen NH, Nguyen V, Trinh HN, et al. . Treatment eligibility of patients with chronic hepatitis B initially ineligible for therapy. Clin Gastroenterol Hepatol 2013;11:565–71. doi:10.1016/j.cgh.2012.12.028 [DOI] [PubMed] [Google Scholar]