Abstract
Background:
This study was performed to evaluate the learning curve and related complications of ultrasound (US) guided central venous catheter (CVC) insertion in infants.
Materials and Methods:
This study was performed in Imam Hosein Hospital of Isfahan from September 2014 to March 2015. Participants were infants consecutively candidate for CVC insertion. Three steps were designed to complement the learning. For each step of learning, 20 patients were considered and for every patient one CVC was inserted: (1) In the first step, venous puncture and guide wire passage was performed by an experienced radiologist and the surgeon was taught how to do it, then CVC was placed by the surgeon. (2) In the second step, venous puncture and guide-wire passage was performed by the surgeon under the supervision of the same radiologist, and then CVC was placed by the surgeon. (3) In the third step, US-guided CVC insertion was performed by the surgeon completely, and the radiologist came to the operating room only if it was necessary. In each of these steps, the time spent of the US probe on the skin until the guide wire passage into the vein was recorded for every patient. All perioperative complications were recorded.
Results:
The mean point for the time spent of the US probe on the skin until the guide wire passage into the vein was 84.9 ± 13.6, 119.1 ± 15.2, and 90.3 ± 11.2 s in the step 1, 2 and 3, respectively (P = 0.04). There was no significant difference between the frequencies of complications among tree steps.
Conclusion:
US-guided percutaneous CVC insertion is a safe and reliable method which can be easily and rapidly learned.
Keywords: Learning curve, perioperative complications, ultrasound-guided central venous catheter insertion
INTRODUCTION
Central venous catheter (CVC) placement is a reliable vascular access and is necessary for anticancer chemotherapy or bone marrow transplantation.[1] Also, these catheters are indicated for the administration of intravenous medication treatments, fluids, or total parenteral nutrition and repeated blood sampling.[2] More than 5 million CVCs are placed each year in the United States.[3] CVC placement in the young children is more difficult than the adult patient because the vessel dimensions are smaller, and routes of the subclavian and internal jugular veins (IJVs) are sharper and more angulated in infants.[4] Common methods for CVC placement include the traditional open surgical venous cut-down (OSC) procedures and percutaneous procedures. OSC can be either onto a peripheral tributary such as the cephalic, facial, or external jugular vein, or directly into the IJV.[5,6,7] In OSC approach, the vein is dissected and controlled via small skin incision and an appropriate size catheter is tunneled from a separate stab wound into the previous incision and inserted under direct vision via a venotomy that is, then repaired with a fine nonabsorbable suture or ligated.[6] The risk of thrombosis and the difficulty of reoperation will be increased after OSC because of the traumatization of the vein due to dissection and repair of the venotomy.[8,9]
In the percutaneous procedures, Seldinger wire technique is used. The desired vessel is punctured with a sharp hollow needle. A round-tipped guide wire is then advanced through it, and the needle is withdrawn. The track is then dilated, and a cannula is passed over the guide wire into the vessel and the guide-wire is withdrawn.[10] Traditionally anatomical landmarks on the skin surface were used for localization of the vein but it was a blind procedure and several complications included hemothorax (accumulation of blood in the space between the lung and the chest wall), pneumothorax (the presence of air in the pleural space), failure to cannulate the vein, pericardial tamponade (collection of blood in the pericardial sac), arterial puncture (insertion of the needle into the artery instead of the vein), and even death have been described.[9,11,12] Alderson et al. reported that among children undergoing cardiac surgery 18% had abnormal jugular venous anatomy.[13] The use of ultrasound (US) for percutaneous central venous cannulation results in fewer needles passes to cannulate the vein and decreased complication rate and increased success rate compared with the landmark technique.[14,15,16,17,18,19] The UK's National Institute of Clinical Excellence has recommended that the preferred method for CVC insertion into the IJV is two-dimensional imaging US guidance in elective situations and also should be considered in most clinical circumstances either electively or in an emergency situation.[20] Shifting to US-guided percutaneous CVC insertion from OSC involves a learning curve, which is probably associated with higher complication rates.
This study focused on learning curve and related complications experimenting US-guided percutaneous technique in infants as a novel technique at our hospital (Imam Hosein Children Hospital located in Isfahan, Iran).
MATERIALS AND METHODS
This descriptive study was performed in the pediatric surgery ward of Imam Hosein Hospital in Isfahan from September 2014 to March 2015 to evaluate the learning curve and related complications of US-guided CVC. Approval for the study was obtained from the university and hospital Ethics Committees. Written consent was obtained from the parents after discussion of study propose for them.
Participants were infants consecutively candidate for CVC insertion. Patients with established coagulopathy (international normalized ratio > 1.5) or platelet count below 100,000/mm3 and patients with body weight <2 kg were excluded.
Three steps were designed to complement the learning. According to previous studies, after a theoretical course, placement of eight CVCs under the supervision of a person conversant with US-guided CVC insertion is sufficient for training.[21,22] Therefore, in this study to ensure adequate training for each step of learning, 20 patients were considered.
In the first step, venous puncture and guide wire passage was performed by an experienced radiologist and the surgeon was taught how to do it, and then CVC was placed by the surgeon.
In the second step, venous puncture and guide wire passage was performed by the surgeon under the supervision of the same radiologist, and then CVC was placed by the surgeon.
In the third step, US-guided CVC insertion was performed by the surgeon completely, and the radiologist came to the operating room only if it was necessary.
Demographic data, type of disease (infectious, cardiologic, hematological, metabolic, gastroenterological, neurological and others), and the site of insertion of the catheter was recorded for every patient. In each of the steps, the time spent of the US probe on the skin, until the guide wire passage into the vein was recorded. All intraoperative and postoperative complications such as failure to cannulate the vein, arterial puncture, hemothorax, pneumothorax, hematoma, and death were recorded up to 48 h after operation.
To analyze the data, we use SPSS 20 software (SPSS Inc, Chicago, IL). Required tests were Chi-square, one-way ANOVA and post-hoc. Quantitative variables were shown as a mean ± standard deviation and quantitative ones as percent. P < 0.05 was considered as significant.
Intervention
All patients underwent IJV cannulation. For this purpose under the sterile field, a shoulder roll was placed to hyper extent the neck and the patient was placed in trendelenburg positione with the head turned slightly away from the insertion site. The vein was located under the guide of US. The 2100 Honda US machine made in Japan and a linear 5–7.5 mHz probe was used [Figure 1]. By keeping the probe gently on the IJV the needle was advanced into the vein. After aspiration of venous blood, the guide wire was introduced, and the catheter was placed using standard Seldinger technique by a 16 G. Arrow catheter, and its proper position was established by C-arm. The catheter was anchored using nylon 3-0 suture placed on the skin at the exit site. Chest X-ray was done to rule out intraoperative complications.
Figure 1.
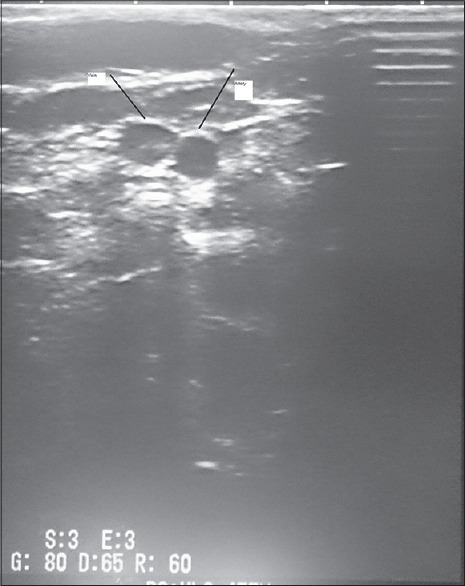
Ultrasonic view of neck vessels
RESULTS
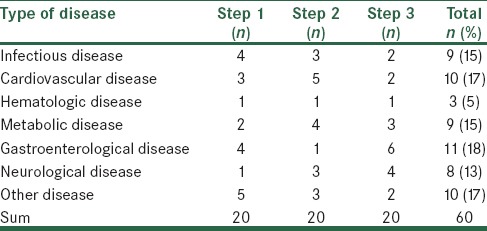
In each step, 20 CVCs were inserted for 20 patients. The mean age of the patients was 50.7 ± 9.1, 53.6 ± 11 and 39.5 ± 8.2 days in step 1, 2 and 3 respectively (P = not significant [NS]). The mean weight of the patients was 4225 ± 251, 4250 ± 288 and 4260 ± 262 g in step 1, 2, and 3, respectively (P = NS). Male to female ratio was 1.2, 1.1, and 0.8 in step 1, 2, and 3, respectively. The causes of hospitalization among patients were shown in Table 1.
Table 1.
Classification of patients based on the cause of hospitalization
The mean point of time spent of the US probe on the skin until the guide-wire passage into the vein was 84.9 ± 13.6, 119.1 ± 15.2, 90.3 ± 11.2 s in the step 1, 2, and 3, respectively [Figure 2].
Figure 2.
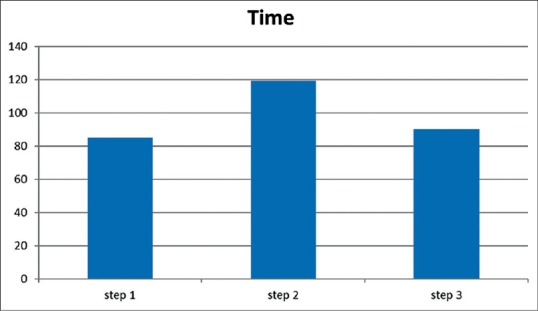
The mean of time (s) spent of the ultrasound probe on the skin until the guide wire passage into the vein
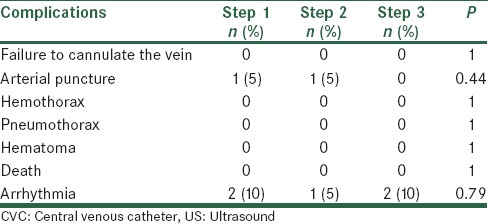
The mean of time spent of the US probe on the skin until the guide wire passage into the vein was not similar in three steps (P = 0.04). Post-hoc LSD showed the mean time was similar between step 1, step 3 (P = 0.39), but was significantly more in step 2 than step 1 (P = 0.03) and step 3 (P = 0.045). Complications were listed in Table 2. The Chi-square test showed that there was no significant difference between the frequencies of complications among tree steps.
Table 2.
Perioperative complications related to US guided CVC insertion
DISCUSSION
Central venous lines insertion is one of the most common procedures in a major tertiary-level pediatric hospital, often involving the dedicated surgical team. As far as our surgical group is involved, the OSC has been the technique of choice, especially in very young babies requiring central venous cannulation. We have recently introduced and experienced the use of US guidance and progressively shifted the preferred approach to the percutaneous technique.
In our patients, by using The US, the perioperative complication rate of 3% was similar to that of 3% reported by Basford et al.[23] in their small series of 18 pediatric patients undergoing Hickman lines insertion using a similar method. The overall complication rate in US-guided CVC insertion in pediatric age ranges between 2.4% and 4.6% according to the international literature.[17,18] This suggests that visualization of the central vein at the time of insertion of the venous catheter using the US is important in reducing the rate of failure and complications relating to damage to adjacent structures.
Failure to cannulate the vein is a known complication of CVC insertion without US guidance.[24] No failure occurred in our series. This finding is similar to Arul et al. study[9] in which only one failure of vein cannulation was reported in early stages but is in contrast of Grebenik et al.[25] study in which they found that their success rates were significantly greater in the landmark group (89.3% vs. 78%).
An arterial puncture rate in our series is higher in first two steps than a third step but is similar to literature values. However, Grebenik et al.[25] found that their arterial puncture rates were significantly lower (6.2% vs. 11.9%) using landmark technique. It seems that the learning curve of each surgeon involved may have deeply influenced the arterial puncture complication rate. As the study period proceeded, there have been an increasing number of percutaneous procedures along with a decrease in arterial puncture rate, in the last study period. We may hypothesize that the learning curve of the involved surgeons has been progressively improving during the study, leading to a more reduction of this complication as compared to international standards.
In our experience, the time added to the CVC insertion process by US use was fairly short, as also reported by Froehlich et al.[26] Although in step 2, the senior resident had no experience of US-guided CVC placement, but total time decreased rapidly after a few procedures, to finally reach a mean of 90 s in step 3, similar to that reported in a study by Nguyen et al. study.[21] The main area in which time was saved with experience was between the first skin puncture and aspiration of venous blood into the syringe. Slama et al.[22] considered the technique as being acquired when this time was <3 min. In our experience, this time can be reduced to <2 min after repeated experiences.
Our study revealed that the transient arrhythmia (nonsustained ventricular tachycardia due to the guide-wire being advanced into the right ventricle) happened in all steps of study at the rate of 5–10%. These findings are similar to results of Dodge et al.'s study.[15] It seems this complication is due to failure of correct percutaneous, or ultrasonic land marking of the tip of the catheter and this experience should be developed in future experiences of ultrasonic guided catheter placement.
One of the limitations of this method is that the US machine is not always in the operating room and sometimes takes a long time to get it out of the radiology department.
CONCLUSION
Standard Seldinger approach combined with US-guided needle insertion have made CVC insertion in children of all ages a safe and reliable method in our hands. Our results demonstrate that CVC placement under US guidance is a simple and safe procedure that can be easily and rapidly learned.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fratino G, Molinari AC, Parodi S, Longo S, Saracco P, Castagnola E, et al. Central venous catheter-related complications in children with oncological/hematological diseases: An observational study of 418 devices. Ann Oncol. 2005;16:648–54. doi: 10.1093/annonc/mdi111. [DOI] [PubMed] [Google Scholar]
- 2.Napalkov P, Felici DM, Chu LK, Jacobs JR, Begelman SM. Incidence of catheter-related complications in patients with central venous or hemodialysis catheters: A health care claims database analysis. BMC Cardiovasc Disord. 2013;16(13):86. doi: 10.1186/1471-2261-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feller-Kopman D. Ultrasound-guided internal jugular access: A proposed standardized approach and implications for training and practice. Chest. 2007;132:302–9. doi: 10.1378/chest.06-2711. [DOI] [PubMed] [Google Scholar]
- 4.Janik JE, Conlon SJ, Janik JS. Percutaneous central access in patients younger than 5 years: Size does matter. J Pediatr Surg. 2004;39:1252–6. doi: 10.1016/j.jpedsurg.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Broviac JW, Cole JJ, Scribner BH. A silicone rubber atrial catheter for prolonged parenteral alimentation. Surg Gynecol Obstet. 1973;136:602–6. [PubMed] [Google Scholar]
- 6.Heimbach DM, Ivey TD. Technique for placement of a permanent home hyperalimentation catheter. Surg Gynecol Obstet. 1976;143:634–6. [PubMed] [Google Scholar]
- 7.Ogata ES, Schulman S, Raffensperger J, Luck S, Rusnak M. Caval catheterization in the intensive care nursery: A useful means for providing parenteral nutrition to the extremely low birth-weight infant. J Pediatr Surg. 1984;19:258–62. doi: 10.1016/s0022-3468(84)80181-5. [DOI] [PubMed] [Google Scholar]
- 8.Male C, Julian JA, Massicotte P, Gent M, Mitchell L, PROTEKT Study Group. Significant association with location of central venous line placement and risk of venous thrombosis in children. Thromb Haemost. 2005;94:516–21. doi: 10.1160/TH03-02-0091. [DOI] [PubMed] [Google Scholar]
- 9.Arul GS, Lewis N, Bromley P, Bennett J. Ultrasound-guided percutaneous insertion of Hickman lines in children. Prospective study of 500 consecutive procedures. J Pediatr Surg. 2009;44:1371–6. doi: 10.1016/j.jpedsurg.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953;39:368–76. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 11.Vegunta RK. Vascular access. In: Holcomb GW III, Murphy JP, Ostlie DJ, editors. Ashcraft's Pediatric Surgery. 6th ed. London: Elsevier Health Sciences; 2014. pp. 118–9. [Google Scholar]
- 12.Zeller KA, Petty JK. Vascular access procedures. In: Ziegler MM, Azizkhan RG, von Allmen D, Weber TR, editors. Operative Pediatric Surgery. 2nd ed. New York: McGraw Hill Education; 2014. pp. 140–5. [Google Scholar]
- 13.Alderson PJ, Burrows FA, Stemp LI, Holtby HM. Use of ultrasound to evaluate internal jugular vein anatomy and to facilitate central venous cannulation in paediatric patients. Br J Anaesth. 1993;70:145–8. doi: 10.1093/bja/70.2.145. [DOI] [PubMed] [Google Scholar]
- 14.McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;20(348):1123–33. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- 15.Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31:1519–26. doi: 10.7863/jum.2012.31.10.1519. [DOI] [PubMed] [Google Scholar]
- 16.Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, Augoustides JG, Elbarbary M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38:1105–17. doi: 10.1007/s00134-012-2597-x. [DOI] [PubMed] [Google Scholar]
- 17.Leyvi G, Taylor DG, Reith E, Wasnick JD. Utility of ultrasound-guided central venous cannulation in pediatric surgical patients: A clinical series. Paediatr Anaesth. 2005;15:953–8. doi: 10.1111/j.1460-9592.2005.01609.x. [DOI] [PubMed] [Google Scholar]
- 18.Gebauer B, Teichgräber UM, Werk M, Beck A, Wagner HJ. Sonographically guided venous puncture and fluoroscopically guided placement of tunneled, large-bore central venous catheters for bone marrow transplantation-high success rates and low complication rates. Support Care Cancer. 2008;16:897–904. doi: 10.1007/s00520-007-0378-9. [DOI] [PubMed] [Google Scholar]
- 19.Koroglu M, Demir M, Koroglu BK, Sezer MT, Akhan O, Yildiz H, et al. Percutaneous placement of central venous catheters: Comparing the anatomical landmark method with the radiologically guided technique for central venous catheterization through the internal jugular vein in emergent hemodialysis patients. Acta Radiol. 2006;47:43–7. doi: 10.1080/02841850500406845. [DOI] [PubMed] [Google Scholar]
- 20.Technology Appraisal Guidance No. 49. c2002. [cited 2002 september]. National Institute for Clinical Excellence [Internet]. Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters. Available from: https://www.nice.org.uk/guidance/ta49 . [Google Scholar]
- 21.Nguyen BV, Prat G, Vincent JL, Nowak E, Bizien N, Tonnelier JM, et al. Determination of the learning curve for ultrasound-guided jugular central venous catheter placement. Intensive Care Med. 2014;40:66–73. doi: 10.1007/s00134-013-3069-7. [DOI] [PubMed] [Google Scholar]
- 22.Slama M, Novara A, Safavian A, Ossart M, Safar M, Fagon JY. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23:916–9. doi: 10.1007/s001340050432. [DOI] [PubMed] [Google Scholar]
- 23.Basford TJ, Poenaru D, Silva M. Comparison of delayed complications of central venous catheters placed surgically or radiologically in pediatric oncology patients. J Pediatr Surg. 2003;38:788–92. doi: 10.1016/jpsu.2003.50168. [DOI] [PubMed] [Google Scholar]
- 24.Skladal D, Horak E, Maurer K, Simma B. Complications of percutaneous insertion of Hickman catheters in children. J Pediatr Surg. 1999;34:1510–3. doi: 10.1016/s0022-3468(99)90114-8. [DOI] [PubMed] [Google Scholar]
- 25.Grebenik CR, Boyce A, Sinclair ME, Evans RD, Mason DG, Martin B. NICE guidelines for central venous catheterization in children. Is the evidence base sufficient? Br J Anaesth. 2004;92:827–30. doi: 10.1093/bja/aeh134. [DOI] [PubMed] [Google Scholar]
- 26.Froehlich CD, Rigby MR, Rosenberg ES, Li R, Roerig PL, Easley KA, et al. Ultrasound-guided central venous catheter placement decreases complications and decreases placement attempts compared with the landmark technique in patients in a pediatric intensive care unit. Crit Care Med. 2009;37:1090–6. doi: 10.1097/CCM.0b013e31819b570e. [DOI] [PubMed] [Google Scholar]


