Abstract
Background:
An important role of nitric oxide (NO) in neuroinflammation has been suggested. It is also suggested that NO has a critical role in learning and memory. Neuro-inflammation induced by lipopolysaccharide (LPS) has been reported that deteriorates learning and memory. The effect of L-arginine (LA) as a precursor of NO on LPS-induced spatial learning and memory and neuronal plasticity impairment was evaluated.
Materials and Methods:
The animals were grouped into: (1) Control, (2) LPS, (3) LA-LPS, and (4) LA. The rats received intraperitoneally LPS (1 mg/kg) 2 h before experiments and LA (200 mg/kg) 30 min before LPS. The animals were examined in Morris water maze (MWM). Long-term potentiation (LTP) from CA1 area of the hippocampus was also assessed by 100 Hz stimulation in the ipsilateral Schaffer collateral pathway.
Results:
In MWM, time latency and traveled path were higher in LPS group than the control group (P < 0.001) whereas in LA-LPS group they were shorter than LPS group (P < 0.001). The amplitude and slope of field excitatory postsynaptic potential (fEPSP) decreased in LPS group compared to control group (P < 0.05 and P < 0.01) whereas, there was not any significant difference in these parameters between LPS and LA-LPS groups.
Conclusion:
Administration of LPS impaired spatial memory and synaptic plasticity. Although LA ameliorated deleterious effects of LPS on learning of spatial tasks, it could not restore LPS-induced LTP impairment.
Keywords: L-arginine, lipopolysaccharide, long-term potentiation, memory
INTRODUCTION
A large number of people in the world suffer from different degrees of learning and memory impairments. Learning and memory impairment not only lessen the quality of life for the individuals but also burden the cost of healthcare for society.[1] The underlying mechanisms of learning and memory deficits have been not well-understood. It is suggested that overproduction of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6) followed by neuroinflammation have a role in cognitive impairments.[2,3] Lipopolysaccharide (LPS), as a potent bacterial endotoxin, triggers immune responses and promotes the generation of inflammatory cytokines such as TNF-α, IL-1β, and IL-6.[4] LPS has frequently used to induce a neuroinflammation model in rodents.[5] This model has been repeatedly used to investigate the role of neuroinflammation on learning and memory and to evaluate the responsible mechanisms for cognitive impairments[6] and finally to investigate the benefits of the drugs on learning and memory impairments due to inflammation.[7] It has been well-documented that activation of immune cells, including macrophages and neutrophils by LPS, is associated with memory and synaptic plasticity impairment.[8] In addition, researchers have reported that administration of LPS leads to spatial memory impairment and cognition, reduction of antioxidant defense, and an increased level of inflammatory cytokines such as TNF-α and IL-1β in the hippocampus.[9] It has also been reported that intraperitoneally administration of LPS inhibits hippocampal-dependent learning and long-term potentiation (LTP) induction in perforant path granule cell synapses.[10]
L-arginine (LA) is the physiological substrate of nitric oxide synthase (NOS) which regulates inflammatory responses, redox stress, glucose metabolism, and neurogenesis.[11] On the other hand, LA affects learning and memory, synaptic plasticity, and has a neuroprotective effect in the brain.[12] It has been reported that passive avoidance memory retention was disturbed in rats when LA is administrated in high dose.[13] The results of many studies have also indicated that LA restores learning and memory impairment caused by L-nitro-LA (L-NA) and 7-nitroindazol (7-NI).[14]
In spite of all these findings, the effects of LA as a precursor of nitric oxide (NO) on LPS-induced memory and synaptic plasticity impairments have not been studied, therefore; the current research was aimed to understand more details about this subject.
MATERIALS AND METHODS
Animals and drugs
In current research, 56 male Wistar rats (8 weeks old and weighing 200–250 g) were prepared from the animal house of Mashhad University of Medical Sciences, Mashhad, Iran. The rats were kept in standard conditions (temperature (22 ± 2°C) and 12 h light/dark cycle) with free access to food and water. Working with animals was carried out in accordance with approved procedures by the Committee on Animal Research of Mashhad University of Medical Sciences. For Morris water maze (MWM), 32 of the animals were divided into four groups: (1) Control (2) LPS, (3) LA-LPS, and (4) LA (n = 8 in each group). After dissolving in saline, drugs were administrated intraperitoneally (LPS; 1 mg/kg[15] and LA; 200 mg/kg[16]). LPS was injected 2 h before training trails in MWM test and recording fast excitatory postsynaptic potential (fEPSP) in electrophysiological experiments, and LA was used 30 min before LPS or saline in LA-LPS and LA groups, respectively.[17] The animals of LPS group were treated with saline (2 ml/kg) instead of LA. In the animals of control and LA groups, 2 ml/kg of saline was injected instead of LPS. In this study, 32 animals were treated with drugs or vehicles for 6 constitutive days. The rest of the animals (24) were used for electrophysiological experiments after receiving a single dose of drugs or vehicle. All drugs were provided freshly. LPS and LA were purchased from Sigma (Sigma-Aldrich Chemical Co.).
Behavioral analysis
Spatial learning and memory was evaluated using MWM test. Before each experiment, for familiarizing with the apparatuses, the animals were placed in filled maze with water without a platform for 30 s. In the hidden platform acquisition test, the animals were released in the tank and allowed to freely swim to find the hidden platform within 60 s. If the rat got rich to find the platform within 60 s, it was allowed to remain on the platform for 20 s before the next trail otherwise; it was guided to the platform by the experimenter and permitted to stay on it for 20 s. The animals performed four trials on each of the five consecutive days, and each trial began with the rat being placed inside the pool and released facing the side wall at one of four positions. The time spent and traveled distance were measured using a software (Radiab, made by Mr. Nomiri) to evaluate the spatial learning ability. Twenty-four hours after acquisition, the platform was removed, and probe test was performed. The time spent, and the traveled path in the target quadrant (Q1) was compared between groups. The MWM test was done between 9 and 12 a.m.
Electrophysiological study
The results of MWM showed that there was a significant difference between LPS-LA and LPS groups, but no significant difference was observed between LA and control groups. For electrophysiological experiments, 24 of the animals were divided into three groups: (1) Control (2) LPS and (3) LA-LPS (n = 8 in each group).
The animals were deeply anesthetized with urethane (1.6 g/kg). The head was fixed in a stereotaxic apparatus. After removing the skin and exposing the skull, the proper location of CA1 area of hippocampus and Schaffer collateral pathway were drilled according to the atlas of Paxinos and Watson. For recording of fEPSP, a bipolar stimulating stainless steel electrode with 0.125 mm in diameter (A-M system, England) was lowered in Schaffer collateral pathway of right hippocampus[18] (AP = 3 mm; ML = 3.5 mm; DV = 2.8–3 mm). A unipolar recording electrode with the same characteristics of stimulating electrode was fixed to the CA1 area of the ipsilateral (AP = 4.1 mm; ML = 3 mm; DV = 2.5 mm). Physiological and stereotaxic indicators were used for determining the proper location of the electrodes. The stimulating electrode and recording electrode were connected to a stimulator and an amplifier, respectively. After stimulating of the Schaffer collateral pathway and recording from CA1 area of the hippocampus, fEPSP was amplified (100×) and filtered (1 Hz to 3 kHz band pass) using a differential amplifier.
After a 30 min resting period, the input-output (I/O) protocol was carried out for determination of synaptic potency. For this purpose, the intensity of stimuli (as input) was enhanced gradually with a constant current and fEPSP (as output) was detected. A stimulus intensity, which produced 50% of a maximum response was used for recording baseline before and after high-frequency stimuli (HFS).[18] A baseline recording was performed at 30 min before LTP induction. Then LTP induction was exerted by the HFS protocol of 100 Hz.[19] Finally, fEPSP was recorded for 90 min. Computer-based stimulating and recording was carried out using neurotrace software version 9 and electro module 12 (Science Beam Institute, Tehran, Iran). The analysis of responses was achieved using custom software from the same institute. The values of the slope and amplitude of the fEPSP in each graph revealed the average of the 30 consecutive traces.
Statistical analysis
All data were expressed as means ± standard error of the mean. The data of the time and distance during 5 days of MWM and the data of LTP criteria were compared using repeated measures analysis of variance (ANOVA) followed by Tukey's post hoc comparisons test. The data of probe trail in MWM were compared by one-way ANOVA followed by Tukey's post hoc comparisons test. Differences were considered statistically significant when P < 0.05.
RESULTS
Morris water maze test
The animals of LPS group had higher latency and longer traveled path in comparison with control group (P < 0.01 and P < 0.001). The animals in LA-LPS and LA groups had significantly lower latency and traveled length to reach platform compared those of LPS group (P < 0.01 and P < 0.001). There was no significant difference in the time and traveled distance to reach the platform between control, LA-LPS, and LA groups [Figures 1 and 2].
Figure 1.
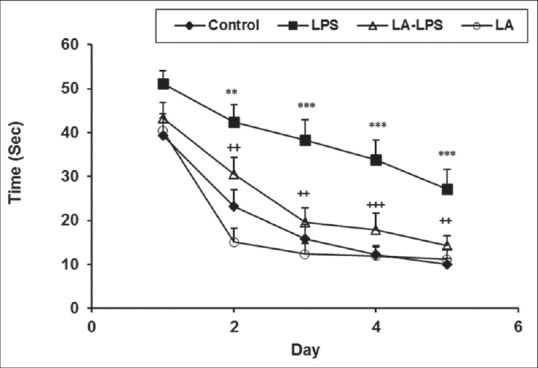
Comparison of time latency to reach the platform in Morris water maze test between four groups. Data are presented as mean ± standard error of the mean (n = 8 in each group). The time latency of the lipopolysaccharide group was significantly higher than those of the control group whereas the animals of L-arginine-lipopolysaccharide group spent a less time to reach the platform than lipopolysaccharide ones. There was no significant difference between control, L-arginine-lipopolysaccharide, and L-arginine groups. **P < 0.01 and ***P < 0.001 compared with control group, ++P < 0.01 and +++P < 0.001 compared with lipopolysaccharide group
Figure 2.
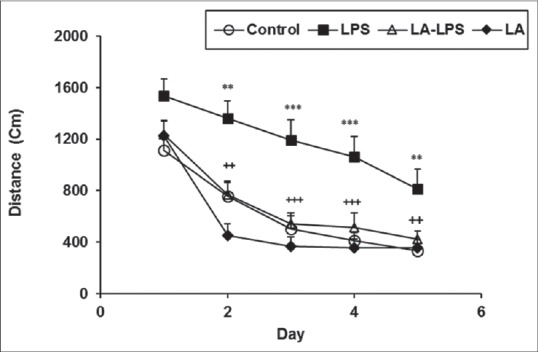
Comparison of traveled distance to reach the platform in Morris water maze test between four groups. Data are presented as mean ± standard error of the mean (n = 8 in each group). The traveled distance of the lipopolysaccharide group was significantly higher than those of the control group whereas the animals of L-arginine-lipopolysaccharide group traveled a shorter distance to reach the platform than lipopolysaccharide ones. There was no significant difference between control, L-arginine-lipopolysaccharide, and L-arginine groups. **P < 0.01 and ***P < 0.001 compared with control group, ++P < 0.01 and +++P < 0.001 compared with lipopolysaccharide group
In the probe trail, the animals of LPS group spent lower time and traveled less distance in target Q1 compared to control group (P < 0.001). The animals in LA-LPS and LA groups spent more time and traveled more distance in Q1 compared to LPS group (P < 0.01). We did not observe any significant difference in the time spent and traveled distance in the Q1 among control, LA-LPS, and LA groups. The results also showed that there was no significant difference in the time spent and traveled distance in the nontarget quadrants (Q2, Q3, and Q4) between four groups [Figures 3 and 4].
Figure 3.
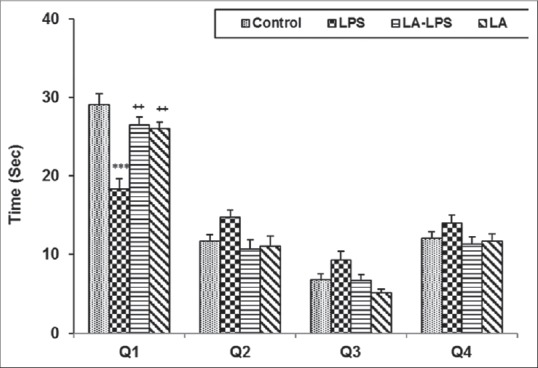
The results of the time spent in target quadrant in probe day, 24 h after the last learning secession. The platform was removed and the time spent in target quadrant was compared between groups. Data are shown as mean ± standard error of the mean (n = 8 in each group). ***P < 0.001 compared with control group, +P < 0.05 compared with lipopolysaccharide group
Figure 4.
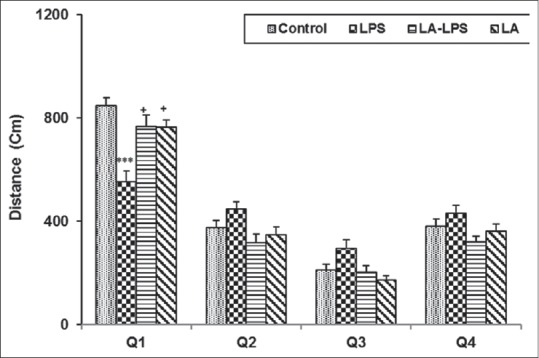
The results of the traveled distance in target quadrant in probe day, 24 h after the last learning secession. The platform was removed, and the traveled distance in target quadrant was compared between groups. Data are shown as mean ± standard error of the mean (n = 8 in each group). ***P < 0.001 compared with control group, +P < 0.05 compared with lipopolysaccharide group
Electrophysiological results
Figure 5c shows single traces recorded before and after induction of long-term potentiation in CA1 area of the hippocampus. After applying HFS, the fEPSP amplitude significantly decreased in the LPS group with respect to control group (P < 0.05 and P < 0.01). There was no significant difference in the fEPSP amplitude between the LA-LPS and LPS groups [Figure 5a]. The fEPSP amplitude in LA-LPS was also lower than that of the control group (P < 0.05 and P < 0.01).
Figure 5.
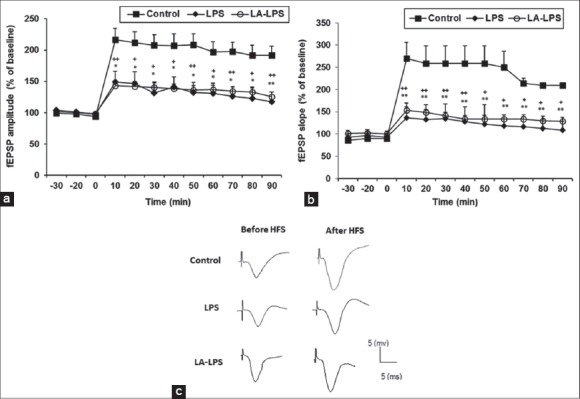
The results of long-term potentiation induction in CA1 area of the hippocampus using 100 Hz tetanic stimulation at: (a) The field excitatory postsynaptic potential slope and (b) the field excitatory postsynaptic potential amplitude. Data are presented as the average percentage change from baseline responses. Each point shows mean ± standard error of the mean (n = 8 in each group). Slope and amplitude of field excitatory postsynaptic potential in lipopolysaccharide group (*P < 0.05 and **P < 0.01) and L-arginine group (+P < 0.05 and ++P < 0.01) were lower than control group. There is not any significant difference in slope and amplitude of field excitatory postsynaptic potential between lipopolysaccharide and L-arginine groups, (c) Single traces recorded before and after induction of long-term potentiation in CA1 area of the hippocampus
After inducing HFS, the fEPSP slope was significantly lower in the LPS and LA-LPS groups compared to control group (P < 0.05 and P < 0.01). The results did not show any significant difference in the fEPSP amplitude between the LA-LPS and LPS groups [Figure 5b].
DISCUSSION
The results of the present research indicated that the animals of LPS group spent more time and traveled a longer distance to find the hidden escape platform in comparison with those of control group [Figures 1 and 2]. The results of probe day also revealed that the rats of LPS group did not remember the location of the platform and spent lower time and traveled shorter distance in the target Q1 where the platform had been previously located [Figures 3 and 4]. In parallel with behavioral results, LPS also attenuated synaptic plasticity in the present study. In supporting this claim, the amplitude and slope of fEPSP in LPS-treated animals was lower with respect to the vehicle-injected ones [Figures 5a–5c].
Similar to our results, it has been reported that administration of 1 mg/kg LPS leads to cognitive deficits in rats.[15] It has also been previously shown that intraperitoneal administration of LPS impaired contextual fear conditioning in rats.[20] A direct injection of LPS into the hippocampus of rats also deteriorates spatial memory.[21] The exact mechanism(s) responsible for deleterious effects of LPS on learning and memory has not been well-known. It is suggested that detrimental effects of LPS on synaptic function, learning and memory is probably mediated by excessive production of inflammatory cytokines, particularly IL-1β in the brain.[22,23] It has also been documented that an increased level of IL-1β followed by injection of LPS is accompanied with a reduction of glutamate release and LTP impairment.[24] It has also been shown that IL-1 receptor antagonists reverse LPS-induced deficits when were administrated before LPS in contextual fear conditioning.[25] Considering this evidence, it seems LPS-induced inflammation and overproduction of inflammatory cytokines and also decreased level of glutamate release have a role in learning, memory, and LTP impairment caused by LPS which was seen in the present study.
LA as a precursor of NO affects learning and memory. It has been reported that memory consolidation was ameliorated in rats when LA was administrated intracerebroventricularly or subcutaneously.[26] Furthermore, injection LA (200 mg/kg) restored learning and memory caused by 7-NI in passive avoidance and elevated plus maze tests in rats.[16] In line with these findings, in our study administration of 200 mg/kg LA 30 min before LPS improved the spatial learning and memory impairments in rats. In the present experiments, the rats of LA-LPS group not only spent shorter time and traveled less distance to find the hidden escape platform but also looked for the location of platform better in probe day than the animals of LPS group.
LA is considered to be as a potent regulatory of the immune system. LA not only postpones the onset disorders associated with inflammation such as allergic encephalomyelitis disease in rats but also prevents the development of neurological symptoms and inflammatory responses.[27] It has been propounded that in low concentrations, LA increases activation-related functions of macrophages such as cytotoxicity against tumor cells, generation of superoxide, and phagocytosis in culture media while, in high concentration it inhibits production of superoxide, cytotoxicity, phagocytosis, and protein synthesis.[28] Regarding this fact, the preventive effects of LA in a high dose, which was used in the current study on learning and memory impairments is conceivable. On the other hand, agmatine important metabolites of LA considered to have neuroprotective and anti-apoptotic effects in the brain areas such as the hippocampus.[29,30,31] It also exerts antidepressant, anxiolytic, anti-tumor, and anticonvulsive effects.[32] Therefore, a mediatory role for agmatine in improving effects of LA on learning and memory deficits caused by LPS which observed in the present study might be suggested.
It is suggested that peripheral administration of LPS increases the level of β-amyloid (Aβ) in the hippocampus, which has an important role in Alzheimer's disease and neuronal death.[6,33] It has been reported that Aβ increases calcium influx through voltage-gated calcium channels (VGCC)[34] and finally is followed by neuronal death and apoptosis.[29] It has also been indicated that agmatine strongly suppresses activation of VGCC in cultured hippocampal neurons in rats[35] which might be considered as a possible mechanism for improving effects of LA which was seen in the present study, however, it needs to be more investigated.
Additionally, agmatine has been proposed to act as a competitive inhibitor for both of neuronal NOS and inducible NOS.[36] Prevention of LPS-induced NO overproduction and the brain tissues oxidative damage by agmatine may also have a role in the results of the present study.[37,38]
In the present study, we did not observe any significant difference in behavioral parameters in LA-treated rats with respect to vehicle-treated ones in MWM test. Therefore, it thought that LA by itself did not affect spatial learning and memory. Previously in our laboratory, it has been shown that chronic administration of LA does not affect learning of spatial task in ovariectomized rats.[39] It has also been revealed that in spite of reversing of L-nitro-L-arginine-methyl ester (L-NAME)-induced learning and memory impairment, LA by itself did not affect memory retention when it was administered into the hippocampus.[40]
In contrast to the behavioral results, injection of LA before LPS could not restore LPS-caused synaptic plasticity impairment in the current study. There was no any significant difference in slope and amplitude of fEPSP in the animals of LA-LPS group with respect to the rats of LPS group. There is a little information about the effect of LA on LTP impairment. In a study, intracerebroventricular injection of LA revered the effect of L-NAME on LTP inhibition.[40] It has also been proposed that agmatine-derived LA, which is co-stored with L-glutamate in the hippocampal pyramidal neurons inhibits N-methyl-D-aspartate (NMDA) receptors.[41] Considering this fact that LTP induction in perforant pathway dependents on the activation of hippocampal NMDA receptors,[42] it is possible that blockage of these receptors by LA-obtained agmatine take parts in the results of present study. Meanwhile, in behavioral experiments LA was injected during six consecutive days while, in electrophysiological experiments it was administrated as a single dose before LPS in current research. Therefore, it seems that injection of LA as a single dose may not be able to prevent malefic effects of LPS on LTP induction in the hippocampus of rats. However, further research is needed to elucidate the exact mechanism(s).
Briefly, in current study, LPS disturbed spatial learning and memory and synaptic plasticity. The result of the present study also indicated that pretreatment by LA was able to restore spatial learning and memory deficit without affecting synaptic plasticity impairment caused by LPS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The results described in this paper were from a PhD student's thesis. The authors would like to thank the Vice Presidency of Research of Mashhad University of Medical Sciences for their financial support.
REFERENCES
- 1.Paulsen JS, Heaton RK, Sadek JR, Perry W, Delis DC, Braff D, et al. The nature of learning and memory impairments in schizophrenia. J Int Neuropsychol Soc. 1995;1:88–99. doi: 10.1017/s135561770000014x. [DOI] [PubMed] [Google Scholar]
- 2.Donzis EJ, Tronson NC. Modulation of learning and memory by cytokines: Signaling mechanisms and long term consequences. Neurobiol Learn Mem. 2014;115:68–77. doi: 10.1016/j.nlm.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palin K, Bluthé RM, Verrier D, Tridon V, Dantzer R, Lestage J. Interleukin-1beta mediates the memory impairment associated with a delayed type hypersensitivity response to bacillus Calmette-Guérin in the rat hippocampus. Brain Behav Immun. 2004;18:223–30. doi: 10.1016/j.bbi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Hou CC, Lin H, Chang CP, Huang WT, Lin MT. Oxidative stress and pyrogenic fever pathogenesis. Eur J Pharmacol. 2011;667:6–12. doi: 10.1016/j.ejphar.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Zhao L, Fu H, Wu Y, Wang T. Ulinastatin suppresses lipopolysaccharide induced neuro-inflammation through the downregulation of nuclear factor-κB in SD rat hippocampal astrocyte. Biochem Biophys Res Commun. 2015;458:763–70. doi: 10.1016/j.bbrc.2015.01.155. [DOI] [PubMed] [Google Scholar]
- 6.Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyns H, Plessers E, De Backer P, Meyer E, Croubels S. In vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs. Vet Immunol Immunopathol. 2015 doi: 10.1016/j.vetimm.2015.06.001. pii: S0165-242700127-0. [DOI] [PubMed] [Google Scholar]
- 8.Frühauf PK, Ineu RP, Tomazi L, Duarte T, Mello CF, Rubin M, et al. Spermine reverses lipopolysaccharide-induced memory deficit in mice. J Neuroinflammation. 2015;12:3. doi: 10.1186/s12974-014-0220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sell KM, Crowe SF, Kent S. Lipopolysaccharide induces memory-processing deficits in day-old chicks. Pharmacol Biochem Behav. 2001;68:497–502. doi: 10.1016/s0091-3057(01)00463-4. [DOI] [PubMed] [Google Scholar]
- 10.Hauss-Wegrzyniak B, Lynch MA, Vraniak PD, Wenk GL. Chronic brain inflammation results in cell loss in the entorhinal cortex and impaired LTP in perforant path-granule cell synapses. Exp Neurol. 2002;176:336–41. doi: 10.1006/exnr.2002.7966. [DOI] [PubMed] [Google Scholar]
- 11.Yi J, Horky LL, Friedlich AL, Shi Y, Rogers JT, Huang X. L-arginine and Alzheimer's disease. Int J Clin Exp Pathol. 2009;2:211–38. [PMC free article] [PubMed] [Google Scholar]
- 12.Böhme GA, Bon C, Lemaire M, Reibaud M, Piot O, Stutzmann JM, et al. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci U S A. 1993;90:9191–4. doi: 10.1073/pnas.90.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.dos Reis EA, de Oliveira LS, Lamers ML, Netto CA, Wyse AT. Arginine administration inhibits hippocampal Na(+), K(+)-ATPase activity and impairs retention of an inhibitory avoidance task in rats. Brain Res. 2002;951:151–7. doi: 10.1016/s0006-8993(02)03077-9. [DOI] [PubMed] [Google Scholar]
- 14.Paul V, Ekambaram P. Involvement of nitric oxide in learning and memory processes. Indian J Med Res. 2011;133:471–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Terrando N, Rei Fidalgo A, Vizcaychipi M, Cibelli M, Ma D, Monaco C, et al. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit Care. 2010;14:R88. doi: 10.1186/cc9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yildiz Akar F, Ulak G, Tanyeri P, Erden F, Utkan T, Gacar N. 7-Nitroindazole, a neuronal nitric oxide synthase inhibitor, impairs passive-avoidance and elevated plus-maze memory performance in rats. Pharmacol Biochem Behav. 2007;87:434–43. doi: 10.1016/j.pbb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Orzelska J, Talarek S, Listos J, Fidecka S. Divergent effects of L-arginine-NO pathway modulators on diazepam and flunitrazepam responses in NOR task performance. Behav Brain Res. 2015;284:179–86. doi: 10.1016/j.bbr.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Saadati H, Sheibani V, Esmaeili-Mahani S, Hajali V, Mazhari S. Prior regular exercise prevents synaptic plasticity impairment in sleep deprived female rats. Brain Res Bull. 2014;108:100–5. doi: 10.1016/j.brainresbull.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Piao MH, Liu Y, Wang YS, Qiu JP, Feng CS. Volatile anesthetic isoflurane inhibits LTP induction of hippocampal CA1 neurons through a4ß2 nAChR subtype-mediated mechanisms. Ann Fr Anesth Reanim. 2013;32:e135–41. doi: 10.1016/j.annfar.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–29. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- 21.Deng XH, Ai WM, Lei DL, Luo XG, Yan XX, Li Z. Lipopolysaccharide induces paired immunoglobulin-like receptor B (PirB) expression, synaptic alteration, and learning-memory deficit in rats. Neuroscience. 2012;209:161–70. doi: 10.1016/j.neuroscience.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues FS, Souza MA, Magni DV, Ferreira AP, Mota BC, Cardoso AM, et al. N-acetylcysteine prevents spatial memory impairment induced by chronic early postnatal glutaric acid and lipopolysaccharide in rat pups. PLoS One. 2013;8:e78332. doi: 10.1371/journal.pone.0078332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hennigan A, Trotter C, Kelly AM. Lipopolysaccharide impairs long-term potentiation and recognition memory and increases p75NTR expression in the rat dentate gyrus. Brain Res. 2007;1130:158–66. doi: 10.1016/j.brainres.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 24.Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000;275:26252–8. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- 25.Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32:14641–8. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plech A, Klimkiewicz T, Maksym B. Effect of L-arginine on memory in rats. Pol J Pharmacol. 2003;55:987–92. [PubMed] [Google Scholar]
- 27.Scott GS, Bolton C. L-arginine modifies free radical production and the development of experimental allergic encephalomyelitis. Inflamm Res. 2000;49:720–6. doi: 10.1007/s000110050652. [DOI] [PubMed] [Google Scholar]
- 28.Albina JE, Caldwell MD, Henry WL, Jr, Mills CD. Regulation of macrophage functions by L-arginine. J Exp Med. 1989;169:1021–9. doi: 10.1084/jem.169.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarifkar A, Choopani S, Ghasemi R, Naghdi N, Maghsoudi AH, Maghsoudi N, et al. Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. Eur J Pharmacol. 2010;634:84–8. doi: 10.1016/j.ejphar.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Gilad GM, Gilad VH, Finberg JP, Rabey JM. Neurochemical evidence for agmatine modulation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity. Neurochem Res. 2005;30:713–9. doi: 10.1007/s11064-005-6865-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhu MY, Piletz JE, Halaris A, Regunathan S. Effect of agmatine against cell death induced by NMDA and glutamate in neurons and PC12 cells. Cell Mol Neurobiol. 2003;23:865–72. doi: 10.1023/A:1025069407173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halaris A, Plietz J. Agmatine: Metabolic pathway and spectrum of activity in brain. CNS Drugs. 2007;21:885–900. doi: 10.2165/00023210-200721110-00002. [DOI] [PubMed] [Google Scholar]
- 33.Butterfield DA. Amyloid beta-peptide [1-42]-associated free radical-induced oxidative stress and neurodegeneration in Alzheimer's disease brain: Mechanisms and consequences. Curr Med Chem. 2003;10:2651–9. doi: 10.2174/0929867033456422. [DOI] [PubMed] [Google Scholar]
- 34.Zhong SZ, Ge QH, Li Q, Qu R, Ma SP. Peoniflorin attentuates Abeta((1-42))-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J Neurol Sci. 2009;280:71–8. doi: 10.1016/j.jns.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Zheng JQ, Weng XC, Gai XD, Li J, Xiao WB. Mechanism underlying blockade of voltage-gated calcium channels by agmatine in cultured rat hippocampal neurons. Acta Pharmacol Sin. 2004;25:281–5. [PubMed] [Google Scholar]
- 36.Auguet M, Viossat I, Marin JG, Chabrier PE. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn J Pharmacol. 1995;69:285–7. doi: 10.1254/jjp.69.285. [DOI] [PubMed] [Google Scholar]
- 37.Yamada K, Komori Y, Tanaka T, Senzaki K, Nikai T, Sugihara H, et al. Brain dysfunction associated with an induction of nitric oxide synthase following an intracerebral injection of lipopolysaccharide in rats. Neuroscience. 1999;88:281–94. doi: 10.1016/s0306-4522(98)00237-1. [DOI] [PubMed] [Google Scholar]
- 38.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosseini M, Headari R, Oryan S, Hadjzadeh MA, Saffarzadeh F, Khazaei M. The effect of chronic administration of L-arginine on the learning and memory of estradiol-treated ovariectomized rats tested in the morris water maze. Clinics (Sao Paulo) 2010;65:803–7. doi: 10.1590/S1807-59322020000800012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harooni HE, Naghdi N, Sepehri H, Rohani AH. The role of hippocampal nitric oxide (NO) on learning and immediate, short-and long-term memory retrieval in inhibitory avoidance task in male adult rats. Behav Brain Res. 2009;201:166–72. doi: 10.1016/j.bbr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Wang WP, Iyo AH, Miguel-Hidalgo J, Regunathan S, Zhu MY. Agmatine protects against cell damage induced by NMDA and glutamate in cultured hippocampal neurons. Brain Res. 2006;1084:210–6. doi: 10.1016/j.brainres.2006.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori K, Togashi H, Ueno KI, Matsumoto M, Yoshioka M. Aminoguanidine prevented the impairment of learning behavior and hippocampal long-term potentiation following transient cerebral ischemia. Behav Brain Res. 2001;120:159–68. doi: 10.1016/s0166-4328(00)00371-5. [DOI] [PubMed] [Google Scholar]


