Summary
Background
Many unicellular organisms age: as time passes, they divide more slowly and ultimately die. In budding yeast, asymmetric segregation of cellular damage results in aging mother cells and rejuvenated daughters. We hypothesize that the organisms in which this asymmetry is lacking, or can be modulated, may not undergo aging.
Results
We performed a complete pedigree analysis of microcolonies of the fission yeast Schizosaccharomyces pombe growing from a single cell. When cells were grown under favorable conditions, none of the lineages exhibited aging, which is defined as a consecutive increase in division time and increased death probability. Under favorable conditions, few cells died, and their death was random and sudden rather than following a gradual increase in division time. Cell death correlated with the inheritance of Hsp104-associated protein aggregates. After stress, the cells that inherited large aggregates aged, showing a consecutive increase in division time and an increased death probability. Their sisters, who inherited little or no aggregates, did not age.
Conclusions
We conclude that S. pombe does not age under favorable growth conditions, but does so under stress. This transition appears to be passive rather than active and results from the formation of a single large aggregate, which segregates asymmetrically at the subsequent cell division. We argue that this damage-induced asymmetric segregation has evolved to sacrifice some cells so that others may survive unscathed after severe environmental stresses.
Introduction
Aging and eventual death has fascinated humans since ancient times, yet a central question remains unanswered: do all living organisms age [1, 2]? Aging is defined as slower reproduction and increased probability of death with time. In unicellular organisms, replicative aging is defined by an increase in division time and increased probability of cell death with an increasing number of divisions. It was hypothesized that an asymmetry in the distribution of aging factors, which are cell components which contribute to aging, at cell division is required to define the identity of the aged mother cell and the young daughter [3]. This hypothesis is in agreement with the observed aging in asymmetrically dividing prokaryotes and eukaryotes [4–6] and in symmetrically dividing prokaryotic cells that segregate damage asymmetrically [7, 8]. These findings were interpreted as evidence that aging is a conserved feature of all living organisms [9]. Mechanistically, the asymmetric segregation of damaged proteins, such as protein aggregates or carbonylated proteins, at division was proposed to underlie replicative aging [10–13]. The role of asymmetric segregation raises the possibility that equal partition of ‘‘aging factors’’ might prevent aging.
Does the symmetrically dividing fission yeast, Schizosaccharomyces pombe, age? Evidence for aging includes the observations that selected individual cells asymmetrically inherit fission scars [13, 14] and damaged proteins [13], exhibit an increase in volume and altered cell morphology [14], and die after a limited number of divisions [13, 14]. Evidence against includes the physical restriction in the accumulation of a large number of fission scars due to the bipolar growth and symmetrical division character of S. pombe [15], the random segregation of damaged proteins between the two daughter cells [16], and the absence of telomere shortening, a common marker of cellular aging [17, 18].
To resolve this controversy, it is essential to look for the defining criteria for replicative aging in unicellular organisms [4, 7, 19]: an increase in the time between consecutive divisions (division time) and an increased probability of cell death with the number of times the cell has previously divided (replicative age). The existence of an aging lineage can be further supported by the identification of an aging factor that is inherited by the aging cell. Cell components that segregate asymmetrically to aging cells in other organisms, such as the old cell pole [7], protein aggregates [10], ribosomal DNA circles [20], the recently replicated spindle-pole body (new SPB) [21] or centrosome [22], the vacuole, which acidifies with age [23], or even a larger cell volume [24], could be related to aging in S. pombe.
By performing pedigree analysis of microcolonies growing from single S. pombe cells, we analyzed division times, inheritance of cell components, and cell death across many lineages. Here we show that S. pombe is able to avoid aging under favorable conditions, but ages in response to stressful environments. Under stressful conditions, the asymmetric segregation of protein aggregates correlates with and likely causes slower division and eventual cell death.
Results
Asymmetric Segregation of Cell Components Does Not Correlate with an Increase in Division Time in S. pombe
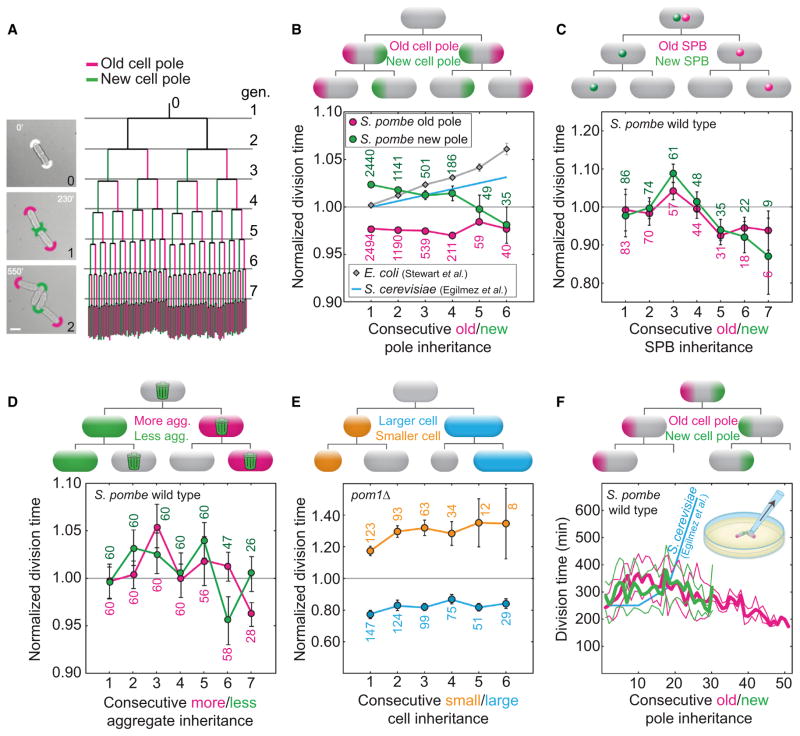
Consecutive inheritance of specific cell components over many divisions may correlate with a consecutive increase in division time and in the probability of cell death, which would define an aging lineage in S. pombe. To test this hypothesis, we performed a complete pedigree analysis of individual fission yeast cells in three distinct wild-type strains (NCYC132, L972, and RumNRRL), randomly selected from exponentially growing cultures. The rod-shaped cells of S. pombe grew and divided by medial fission continuously for up to eight generations, forming a monolayer microcolony (Movie S1 available online).We generated a complete pedigree tree for the founder cell of each microcolony and all its descendants (n = 20–52 microcolonies; Figure 1A), and we tested whether the inheritance of cell components correlated with an increase in division time.
Figure 1. Asymmetric Inheritance of Aging Factors in Pedigree Lineages Does Not Correlate with Aging.
(A) Left: the pole identity in the founder cell is not known (white arcs at 0′). After the first division (generation 1), the old (magenta arc) and new (green arc) pole segregate asymmetrically (generation 2). Right: pedigree tree of 52 microcolonies (NCYC132) representing average division times (length of vertical lines) of new pole (left branch, green) and old pole (right branch, magenta) cells. The bifurcations represent cell divisions. Horizontal lines (gray) mark the first division in each generation (gen). The scale bar represents 5 μm.
(B) Cells that consecutively inherit the old pole (magenta) or the new pole (green) do not exhibit an increase in division time (strain NCYC132; n = 52 cell lineages; Movie S1). For comparison, we show the division times for E. coli (estimated for old-pole cells from Figure 3A in [7]) and S. cerevisiae (estimated from a linear fit for mother cells of age two to ten generations from Figure 2 in [19], normalized by the division time of the cells of the second generation).
(C–E) Cells that consecutively inherit the old spindle pole body (magenta) or the new spindle pole body (green, labeled with Cdc7-GFP, strain IH1106; n = 13 cell lineages; Movie S2) (C) or inherit a higher amount of protein aggregates (magenta) or a lower amount of protein aggregates (green, strain MC19; n = 30 cell lineages; Movie S2) (D) or are born smaller (orange) and larger (blue) in asymmetrically dividing cells (pom1Δ strain JB107; n = 32 cell lineages; Movie S2)
(E) do not show an increase in division time with an increasing division number. Data are mean ± SEM; the number of cells is given in the graphs.
(F) Average division time of cells that consecutively inherit the old pole (thick magenta line, n = 10 spores) or the new pole (thick green line, n = 32 spores, T = 23°C ± 2°C; thin lines represent the SEM) from micromanipulation experiments (inset). Death events related to the old/new pole inheritance were not observed. For comparison, we show division times for S. cerevisiae (estimated for mothers cells from Figure 1 in [19], multiplied by 3.6 to match the scale).
See also Figure S1 and Movies S1 and S2.
The first cell component that we tested was the old cell pole, a pre-existing structure that is inherited from the mother cell. In different experiments on E. coli, continued inheritance of the old pole has been correlated with an increase in division time [7] or filament formation [8]. The division time of the cells that consecutively inherited the old pole for up to six divisions (Figures 1B and S1A) decreased, on average, by 0.1% per division. However, fission yeast cells typically grow to a larger extent at the old than at the new pole [25, 26], and the cell that inherits the new pole typically inherits a larger part of the old cell wall and the scar from the previous division [15]. Therefore, we tested whether the new-pole cell inheritance [13, 14] was correlated with an increase in division time. The division time decreased, on average, by 0.5% per division (Figures 1B, S1A, and S1B). We conclude that there was no correlation between cell pole inheritance and an increase in division time, in three wild-type strain isolates, which indicates that this feature is conserved in the species.
We decided to repeat the analysis we performed for the cell pole to study whether other cell components in S. pombe would correlate with aging. The newly synthesized SPB, which is segregated asymmetrically to the slowly dividing mother cell in S. cerevisiae [21], can be distinguished from the old SPB in S. pombe by the specific localization of Cdc7 to the new SPB during anaphase [27, 28]. Using a strain where Cdc7 was labeled with GFP (Figure S1C), we tested whether the different SPBs correlated with an increase in division time. Neither the cells consecutively inheriting the new SPB nor the ones inheriting the old SPB exhibited an increase in division time (Figure 1C and Movie S2). Another component that segregates asymmetrically to aging E. coli [10] and S. cerevisiae [12, 29, 30] cells that exhibit an increase in division time are protein aggregates. By following the inheritance of GFP-labeled Hsp104, a molecular chaperone that associates with aggregates [12] (Figure S1D and Movie S2), we observed no significant increase in division time in cells inheriting a large or small number of aggregates, respectively (Figure 1D). As a consequence of their morphologically symmetric division, S. pombe cells might avoid an asymmetry in the segregation of a diffusible aging factor beyond that associated with the binomial partitioning of a finite number of aggregates.
Thus, we hypothesized that genetically modified S. pombe cells that divide into a larger and smaller daughter cell might age because the larger daughter cell may inherit a larger amount of an aging factor, and dilute it less in the next division. In this way, the smaller daughter would inherit a smaller amount of an aging factor and dilute it more in the next division, avoiding aging. We tested this by using a pom1Δ mutant [31], which divides off-center (Figures S1E and S1F) and where larger and smaller cells grew on average 7 μm in length to a constant division size over consecutive divisions (Figure S1F). We did not observe a significant increase in division time, when the larger or the smaller sibling was followed for consecutive divisions (asymmetry, measured by the ratio between the length of the smaller and larger cells and their respective sisters, was 30%–70%; Figure 1E and Movie S2). A summary of these results is found in Table 1 (see Figure S1G for absolute division times).
Table 1.
Summary of Pedigree Analysis for All of the Strains and Conditions Tested
| Strain and Condition | Followed Feature | Change in Division Time, per Division (%) |
|---|---|---|
| NCYC132 WT (30°C) | Old pole | −0.09 ± 0.14 (4,533) |
| NCYC132 WT (30°C) | New pole | −0.54 ± 0.16 (4,352)a |
| L972 WT (30°C) | Old pole | −0.06 ± 0.29 (618) |
| L972 WT (30°C) | New pole | 0.06 ± 0.29 (555) |
| RumNRRL WT (30°C) | Old pole | −0.56 ± 0.40 (538) |
| RumNRRL WT (30°C) | New pole | −1.35 ± 0.55 (461) |
| Cdc7-GFP WT (30°C) | Old SPB | −1.58 ± 0.88 (243) |
| Cdc7-GFP WT (30°C) | New SPB | −2.50 ± 0.93 (226)b |
| Hsp104-GFP WT (30°C) | High aggregate amount | 0.18 ± 1.90 (60) |
| Hsp104-GFP WT (30°C) | Low aggregate amount | −0.57 ± 2.06 (60) |
| pom1Δ (30°C), asymmetric cell division | Old pole | −1.42 ± 0.74 (318) |
| pom1Δ (30°C), asymmetric cell division | New pole | 0.90 ± 0.78 (319) |
| pom1Δ (30°C), asymmetric cell division | Larger sibling | −0.01 ± 0.86 (463) |
| pom1Δ (30°C), asymmetric cell division | Smaller sibling | 1.01 ± 1.61 (308) |
| Hsp104-GFP WT (40°C, 1 hr) | High aggregate amount | 19.7 ± 10.8 (49)a |
| Hsp104-GFP WT (40°C, 1 hr) | Low aggregate amount | −1.62 ± 3.29 (30) |
| Hsp104-GFP WT (30°C, H2O2 1 mM) | High aggregate amount | 100 ± 18.6 (72)a |
| Hsp104-GFP WT (30°C, H2O2 1 mM) | Low aggregate amount | −1.29 ± 2.64 (30) |
Change in division time is shown in percent per division, with the number of cells in parentheses. Division times were normalized by the average for the corresponding generation of each colony. WT, wild-type.
p < 0.005.
0.005 < p < 0.05.
To test whether signs of aging appear after a larger number of divisions, we used micromanipulation to follow cells consecutively inheriting the old or the new pole (Figure S1H). Starting with a spore, individual cells inheriting the old or the new pole were kept on an agar plate while all other cells were removed with a microneedle after each one to three cell divisions (Figure S1H). During over 50 divisions for cells inheriting the old pole and 30 divisions for cells inheriting the new poles, the cells divided continuously without a significant increase in the division time (Figure 1F). For comparison, E. coli cells that inherited the old cell pole grown in a microfluidic device did not show an increase in division time for more than 200 divisions, but were more likely to die than younger cells [8]. Other reports detected slower cell division after as few as three to five divisions in E. coli [7] and 20 divisions in S. cerevisiae and human fibroblasts [19, 32, 33]. Unlike these experiments, we were unable to detect an increase in division time related to the age of the cell poles in S. pombe.
We conclude that an increase in division time in S. pombe is not associated with the consecutive inheritance of known aging factors for other organisms and that the absence of aging is independent from the morphological symmetry of division, at least when the imposed asymmetry is up to 70%. We are not asserting that the individual components, such as cell poles, of S. pombe cells are immortal. We are confident that if any indivisible component is followed for enough cell divisions, the cell that harbors it will eventually die, but our evidence suggests that the probability of this death will be constant rather than increasing over time.
S. pombe Cell Division Time Does Not Increase over Consecutive Divisions
It is possible that aging, if present, is correlated with the inheritance of a cell component other than the ones we tested. Therefore it is necessary to test for an increase in cell division time over consecutive divisions. We consider three general scenarios for replicative aging (Figure 2A): (1) the increase in division time occurs only in one sister cell (the aging cell), as in S. cerevisiae and E. coli [7, 19]; (2) the increase in division time occurs in both sister cells, such as in clonal aging of human somatic cells [34]; or (3) there is no increase in division time in sister cells, and aging does not occur. We used cells from the NCYC132 strain to test whether cells that carry the feature of slow division (identified by a longer division time and/or slower growth than the average of the colony) would transmit this feature to their daughters, which should exhibit an increase in their division time. Mother cells with a division time exceeding the mean by at least 1 SD gave rise to two daughters, both of which divided faster than their mother. The slowest dividing of the two daughters, which should represent the aging lineage, had a division time 12% shorter than that of their mother (mothers, 155.9 ± 7.0 min; slower daughters, 136.9 ± 17.3 min; mean ± SD; n = 107; p = 10−18; Figures 2B and 2C). We got similar results for two other wild-type strains (L972 and RumNRRL; Figure S2, left panels). The simplest explanation of these results is that S. pombe divides at a roughly constant cell size [35, 36]: slower-dividing cells are larger at division, which implies that their daughters will need to grow for a shorter period of time before they can divide. Since we did not detect the presence of aging in the most slowly dividing cells, we decided to analyze the division times of all mother and daughter cells in the population in the three wild-type strains (Figure S2). We observed that there was no correlation between the division time of the mother and each of its two daughter cells (Figure S2, middle panels). The difference in division time of daughter cells, which is known to increase during aging of asymmetrically dividing cells as the mother cell divides slower [7], was not correlated with the division time of the mother (Figure S2, right panels).
Figure 2. Daughter Cells of Slowly Dividing Mothers Divide Faster Than Their Mothers.
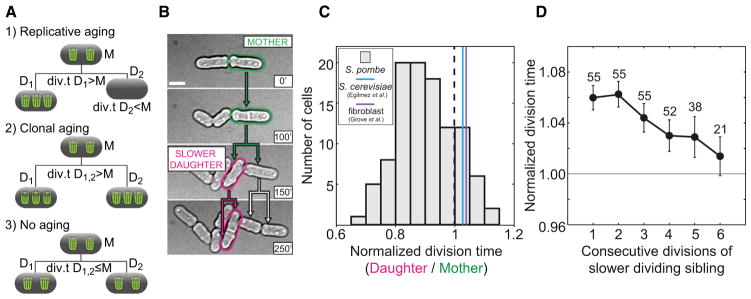
(A) Aging scenarios: (1) one daughter cell (D1) inherits more damage and divides slower than its mother (M), (2) both daughter cells (D1, D2) divide slower than their mother (M), and (3) both daughter cells (D1, D2) divide equally fast or faster than their mother (M), hence there is no aging. Green trash bins represent aging factors.
(B) Identification of an S. pombe lineage of putatively aging cells: the slower-dividing mothers (green) and the slower-dividing daughters (magenta) that divide later than their siblings. The scale bar represents 5 μm. The time is given in minutes.
(C) S. pombe mother cells with a long division time (1 SD above the average, n = 107) generated daughters with a shorter division time. A histogram of division times normalized by the mother’s division time is shown. The mean value of the daughter division time was significantly smaller than 1 (p = 10−21). In cells that exhibit aging, the average normalized daughter division time was greater than 1 (S. cerevisiae, [19]; human fibroblasts, [33]).
(D) The division time of the cells with a higher division time than their sibling (slower-dividing sibling) decreased by 0.0099 per division (r = −0.96, 95% confidence interval for r = [−1.00, −0.71], p = 0.002, the number of cells is shown).
See also Figure S2.
A similar analysis was also performed for slowly growing cells. When we analyzed mother cells with a growth rate below the mean, we found that the daughter cells were, on average, growing at a higher rate than their mothers (mothers, 46.4 ± 2.69 nm/min; slower daughters, 49.8 ± 3.84 nm/min; mean ± SD; n = 34; p = 10−4). Finally, we expect that the progeny of the daughter that has the longer cell division time will show an increase in their cell division times over several consecutive divisions. We followed the slower-dividing sister cell over six consecutive divisions and observed that the mean division time did not increase (>50 individual lineages; Figure 2D). We conclude that the feature of slow division, a conserved feature of aging, was not transmitted from mothers to daughters, contrary to what occurs in early divisions in E. coli [7] and S. cerevisiae [37], further supporting the absence of aging depicted in scenario 3, where aging factors segregate binomially at division (Figure 2A).
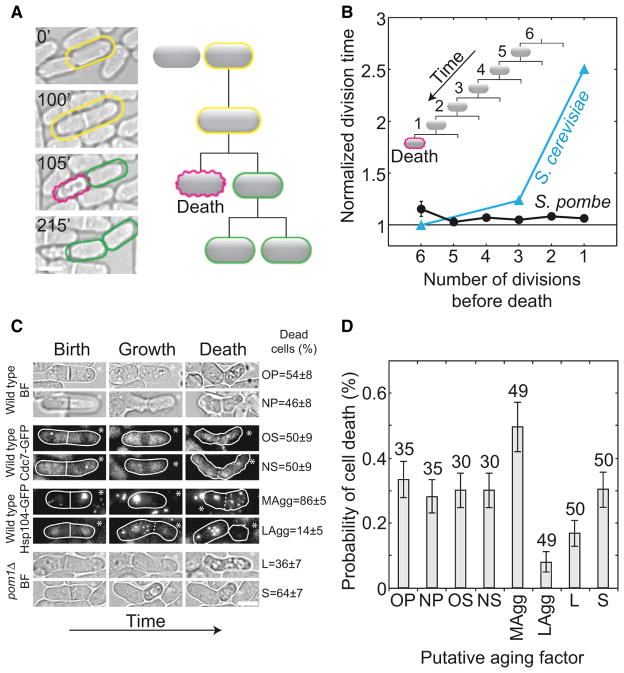
Cell Death Is Not Preceded by Aging
Aging in other organisms is more pronounced in the last few divisions prior to cell death [37]. Indeed, cells of S. cerevisiae and Candida albicans die on average after 20 divisions [5, 6], and in S. cerevisiae cell death is preceded by a 2-fold increase in division time over a period of one to two divisions [19]. If S. pombe exhibits aging, we expect a similar increase in division time before death. We screened 10,000 S. pombe cells and detected 36 individual cell death events (Figure 3A). The total number of death events registered was low compared to the number of total cell divisions observed (0.1%–0.3% in NCYC132, L972, RumNRRL wild-type strains, n > 5,000 cell divisions). The death frequency in S. pombe (0.3%) was higher than the aging-related death frequency in S. cerevisiae and C. albicans (0.0001% ≈ 1 death event/220 cell divisions [5, 6]). If in S. pombe death was only a consequence of aging, the expected lifespan of an individual cell would be around eight divisions (0.3% ≈ 1 death event/28 cell divisions). Therefore, aging would have been detected in a fraction of our microcolony experiments (Figure 1A) and in the long-term experiments (Figure 1F).
Figure 3. Cell Death Is Not Preceded by an Increase in Division Time and Correlates with the Inheritance of Protein Aggregates.
(A) A cell (yellow) divided (100 min) and one of the daughter cells died (magenta at 105 min). Cell death was recognized by a distinct cell morphology [38] (shrinkage of cell volume and surface irregularities), as well as by the absence of growth. The morphology, growth, and division of the cell before death (yellow), as well as of the surviving sister cell (green), were normal (Movie S1).
(B) Normalized division time as a function of the number of divisions before death decreased on average by 0.7% ± 0.6% per division; p = 0.2, n = 36 cells (34 dead cells with surviving sisters and two dead sister cells; 174 cell divisions in total). For comparison, division time for S. cerevisiae is shown (taken from Figure 2 and the text in [19]).
(C) Time lapse of the last division before cell death after inheritance of a putative aging factor (OP, old cell pole; NP, new cell pole; OS, old SPB; NS, new SPB; MAgg, more aggregates; LAgg, less aggregates; L, larger cell; S, smaller cell). The percentage of cell deaths associated with the inheritance of a factor is shown on the right; strains are shown on the left; BF, bright field. White lines encircle cells.
(D) The probability of death in the next cell cycle after inheritance of a putative aging factor is shown (n > 5,000 cell divisions, the number of cells is given in the graph). Data are means ± SEM; scale bars represent 5 μm.
See also Figure S3.
We identified the ancestors of the dead cells and measured their division times for six divisions preceding death. The division time did not increase before death (Figure 3B and Movie S2). In S. cerevisiae and E. coli, the difference in the division time of aging cells and their siblings increases with the number of divisions before cell death [7, 19]. We found no increase in the difference in division time between S. pombe siblings in the cell lineage preceding death (Figure S3A). The morphology of the dying cells and their divisional symmetry before death were unaffected, and their siblings continued to divide (Figure 3A and Movie S1). Cell death typically occurred in one of the siblings within ~3 min after their separation, suggesting that death is due to a catastrophic failure in some process rather than the gradual decline of aging.
It is possible that unstressed S. pombe cells undergo a slower type of aging that, running in the background, results in a less frequent death. In this situation, aging may occur due to the asymmetric segregation of an unidentified aging factor. However, this would represent a much less significant percentage of the cell death in the population and would most likely negligibly contribute to the population fitness.
The Inheritance of Protein Aggregates Correlates with Death
We next tested whether there was a correlation between the inheritance of cell components with cell death (Figure 3C). Cells that inherited a higher amount of aggregated proteins, measured by the total intensity of aggregate-associated Hsp104-GFP (arbitrary units [a.u.]) in the puncta, exhibited a higher probability of death than cells that inherited a lower amount (Figures 3C and 3D). We observed that at the moment of death both the amount and the number of aggregates correlate with cell death (Figures 4A and 4B and Movie S3). To determine whether inheriting aggregate amount or number at birth above a threshold d (d = 5 a.u. for amount ord=2 for aggregate number; Figure 4C) is linked with death, we observed that cells inheriting a high aggregate amount died with a high frequency, while cells born with a number of aggregates above d exhibited only a small increase in death frequency. Therefore, the amount rather than the number of aggregates inherited by a cell at birth is associated with survival in the next cell cycle. Thus, aggregates are able to accumulate in symmetrically dividing S. pombe cells and correlate with cell death. In support of this observation, protein aggregates grew 10-fold faster (Figures S3B and S3C) or overlapped with the division plane (Figure S3D) before death. The probability of cell death after an overlap event was higher for a larger overlap region between the aggregate and the cell division plane (Figures S3E and S3F), suggesting that the aggregates may disrupt cytokinesis or the integrity of the daughter cell wall [39].
Figure 4. Cell Death Correlates with the Amount of Protein Aggregates.
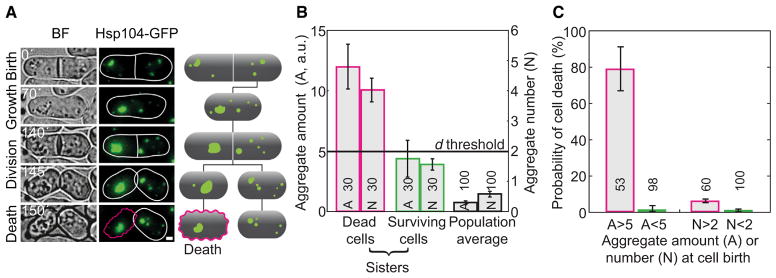
(A) Bright-field (BF) and fluorescence images of a strain expressing Hsp104-GFP, and the corresponding schemes. The cell with a large amount of protein aggregates died (magenta edge), while its sister survived (white edge). The scale bar represents 1 μm. The time is given in minutes.
(B) Aggregate amount (A, Hsp104-GFP intensity in arbitrary units, a.u.) and puncta number (N) for dead cells (magenta), their sisters (green), and the population (black).
(C) Death frequency in cells born with Hsp104-GFP intensity or aggregate number above (magenta) and below (green) the death threshold, d (d=5 a.u. for A, defined as three times the average of the population; or 2 aggregates for N, see B).
The data are means ± SEM. The number of cells from more than three independent experiments is given in the graphs. See also Figures S3 and S4 and Movie S3.
We performed a variety of tests to demonstrate that the puncta labeled with Hsp104-GFP represent endogenous aggregates. First, we compared the following properties in strains where a fluorescent protein label was either present or absent in Hsp104: (1) the molecular weight of aggregates and the distribution of Hsp104 in different molecular weight fractions (Figures S4A–S4C), (2) the Hsp104 in vitro and Hsp104-GFP in vivo disaggregase activity (Figures S4D– S4F), and (3) the cell death and thermotolerance response (Figures S4G and S4H). We also compared the Hsp104 puncta number and cell-cycle properties using different fluorescent labels (Figures S5A–S5C). We found that the tested properties were similar in the presence and in the absence of the GFP label in Hsp104 (see also the Supplemental Experimental Procedures). We conclude that Hsp104 labeled with GFP is a reliable in vivo marker for protein aggregation.
In summary, the analysis of the final cell divisions when aging in other organisms is most pronounced [40] showed that there was no increase in division time or growth arrest before death and that death occurs catastrophically, most likely as a consequence of the accumulation of protein aggregates. Thus, our results show that aging does not occur in S. pombe, at least under favorable growth conditions.
Cells that Inherit Protein Aggregates Undergo Aging after Stress
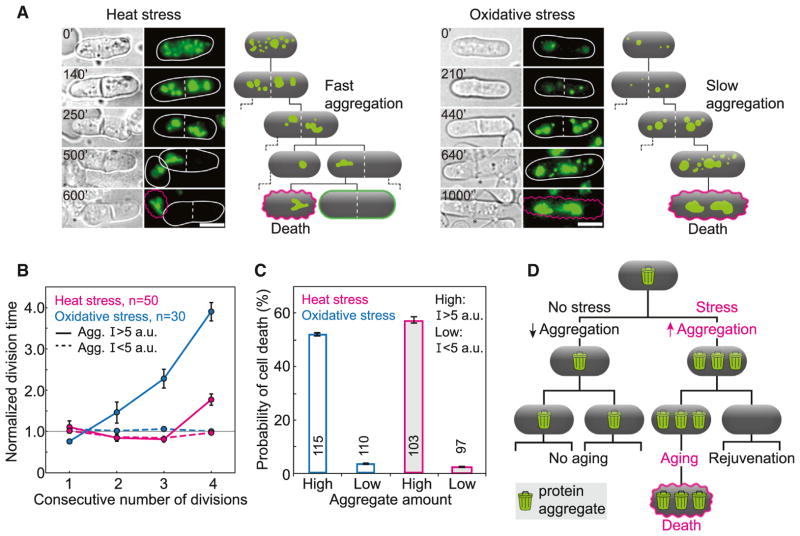
Under favorable conditions, which produce low levels of protein aggregates, random segregation of aggregates in symmetrically dividing cells distributes the aggregates across the population. In this way, aggregates are prevented from accumulating faster than they are diluted, which is likely to be the ultimate cause of aging. In contrast, environmental stress may produce enough aggregated proteins to kill both daughter cells. Under these conditions, strongly asymmetric segregation of the aggregates would ensure that the cell born with fewer aggregates survives and its sister ages and dies. To test this hypothesis, we subjected exponentially growing cells to two independent types of stress: heat (40°C) or oxidizing agents (1 mM H2O2) for 1 hr. Cells were allowed to recover from stress in rich media at 30°C and were then monitored for five divisions. During growth arrest after stress, there was an increase in the total amount of aggregates (Figures S5D and S5E), and when cells resumed division, a single large aggregate was formed and inherited by one of the sister cells, while its sister was born clean (Figure 5A).
Figure 5. After Stress, Cells that Inherit Large Protein Aggregates Show Aging.
(A) Images of cells that inherit large aggregates (Hsp104-GFP, green) after heat (40°C, 1 hr; left) and oxidative (1 mM H2O2; right) stress. Schemes depict aggregate formation and cell death (magenta), which occurred two to five cell divisions after stress. Scale bars represent 5 μm. Time is given in minutes.
(B) Normalized division time before death increased for cells inheriting large aggregates (solid lines, Hsp104-GFP intensity I > 5 a.u.), but not for cells clean of aggregates (dashed lines, Hsp104-GFP intensity I < 5 a.u.).
(C) Cells inheriting a larger amount of aggregates had a higher probability of death than cells inheriting a smaller amount, indicating that after stress protein aggregates behave as an aging factor. Data are means ± SEM. The number of cells is shown in the graphs.
(D) Scheme representing the transition between nonaging and aging in S. pombe. Under favorable growth conditions, aging factors (protein aggregates, depicted as trash bins) distribute equally between both siblings and aging is not present. After stress, a high amount of aging factors is asymmetrically segregated to one cell, giving rise to a clean sibling. The cell that inherits a large amount of aging factors undergoes aging and death.
We investigated whether aging was linked to this newly established asymmetry in aggregate segregation by monitoring the division time and the probability of death in cells that consecutively inherited the large aggregate and their sisters (Figure 5A and Movie S4). We identified a consecutive increase in division time for cells that inherit large aggregates, but not for cells that inherited a small amount of aggregates (Figure 5B and Table 1). Moreover, there was a higher probability, relative to unstressed control cells, of cell death associated with the inheritance of large protein aggregates, but not for cells that were born clean of aggregates (Figure 5C). We observed that after four divisions, there was a higher probability to segregate damage to the cell that inherited the old cell pole at division (Figures S5F and S5G), which may be a consequence of the nuclear movement during anaphase, displacing large aggregates toward the old cell pole. The formation of an aging lineage, defined by the inheritance of a large protein aggregate, was verified for both types of stress, indicating that aging is independent of the origin of stress. This is similar to the scenario where aging factors are retained by one cell, the cell that inherits the old cell pole at division (Figure 2A).
We next compared how the two types of stress trigger aging. In the case of heat stress, the increase in division time occurred only in the cell cycle immediately before death, whereas for oxidative stress, this increase occurred consecutively over three divisions before death (Figure 5B). This difference might be explained by the rate of aggregate formation: after heat stress the majority of the total aggregate amount was generated prior to the first division, whereas after oxidative stress there was a gradual accumulation of aggregated proteins over three divisions after stress (Figure 5A). The percentage of cell death after stress was similar for heat and oxidative stresses: after heat stress most cells died suddenly after division, whereas after oxidative stress there was a long arrest in cell growth before death (Figure 5A). Therefore, for different stresses, the increase in division time and the phenotype of cell death manifest differently, suggesting that the aging phenotype reflects the amount and type of damage. We conclude that after stress, aggregate segregation causes aging in the lineage that retains the large aggregate, enabling the generation of clean daughters, as depicted in the scheme of Figure 5D.
Discussion
A Transition from Nonaging to Aging that Requires Asymmetric Segregation of Damage
A major limitation in studying aging in morphologically symmetrically dividing unicellular organisms is the identification of a biochemical marker whose inheritance correlates with aging. In S. pombe, we identified protein aggregates as a marker for aging under stress conditions. After stress, cells that retained large aggregates exhibited features of aging: a consecutive increase in division time and probability of death. However, under favorable growth conditions in S. pombe, protein aggregates segregate randomly at division and cells do not undergo aging. The comparison of our results with previous studies of aging in S. pombe can be found in Table S1. Upon inheritance of a large aggregate amount, the inability to assemble a protective stress response during favorable growth conditions is likely to culminate in aggregate growth and toxicity that lead to death. The differences in growth conditions might also explain the observed discrepancy between the aging phenotype of E. coli cells that grow in solid agarose pads [7] or liquid media [8].
A number of different explanations could account for the slower cell cycles of the cells that retain large aggregates after stress. The rapid formation of protein aggregates after heat stress to an amount above the death threshold is initially tolerated, most likely due to the protective stress response [41]; however, as cells divide this ability may be lost [42] due to a decreased expression or buffering ability of molecular chaperones. As essential proteins are titrated and sequestered by aggregates [43], cell-cycle checkpoint activation may result in the observed cell-cycle delays followed by cell death [44]. Alternatively, the composition of the protein aggregates after stress might differ from the nonstressed situation, and essential proteins might be specifically sequestered or enriched in the stress-related aggregates [45], leading to the aging phenotype observed.
How is aging reset in cells born with small aggregate amounts? The sisters of cells containing large aggregates, which are born with an aggregate amount below the death threshold, divide without exhibiting an asymmetry in damage segregation or an increase in division time or probability of death. This occurs both under favorable and stress conditions and suggests that the inheritance of aggregates per se, and not the stress treatment, makes cells age and die. Therefore, cells can avoid aging by lowering or maintaining the total levels of damage below the death threshold. Aging in S. pombe seems to be modulated by fluctuations in the total levels of damage: accumulation of damage under unfavorable growth conditions triggers aging, and aggregate clearance due to asymmetric segregation keeps the cleared cells from aging and allows survival after substantial damage. Mechanisms that prevent and repair damage are also likely to play an important role in the survival of cells that do not exhibit aging.
Aging and Random Segregation—A Different Way to Handle Damage?
Our data suggest the existence of a nonaging unicellular eukaryotic organism, the fission yeast S. pombe. In other organisms, aging is thought to be beneficial because damage is segregated only to some cells in the population, while others are born damage free [46]. Nonaging organisms may use a different life strategy that does not depend on the segregation of damaged material to a few cells in the population, but rather on the maintenance of the fitness of each cell. The maintenance of individual fitness can be achieved actively by a directed segregation mechanism, in which both cells inherit nearly identical numbers of aging factors. Alternatively, random segregation of damage at division may effectively distribute low levels of spontaneous damage, without the need of dedicated cellular machinery, while allowing a higher variability of damage levels in individuals. If the gap between the mean number of new aging factors produced per generation and the number required to trigger aging is large enough, repeated rounds of random segregation followed by dilution will produce only a tiny fraction of cells that age and die. In evolutionary terms, sacrificing a few individuals that randomly inherit high damage amounts may have a lower cost than an active damage segregation mechanism, at least in certain symmetrically dividing cells. Organisms that exhibit aging, such as S. cerevisiae, C. elegans, and D. melanogaster, can respond to stress either by accelerating the rate of aging and death, or by exhibiting a lifespan extension due to hormesis in response to mild stress [47]. Lifespan extension also occurs in mutants that have increased capacity to handle stress-related damage and in species that acquired more efficient stress resistance mechanisms [48, 49]. In organisms in which aging is not present, stress may trigger aging either due to an increase in the damage production rate or by changing the way damage is segregated.
Conclusions
The current paradigm in aging research argues that all organisms age. We have challenged this view by failing to detect aging in S. pombe cells grown in favorable conditions. We have shown that S. pombe undergoes a transition between nonaging and aging, due to asymmetric segregation of a high amount of damage. Further studies will elucidate the mechanisms underlying the transition to aging and its dependence on environmental components.
Human somatic cells show aging, dividing for a limited number of times in vitro [34], whereas cancer cells, germ cells, and self-renewing stem cells are thought to exhibit replicative immortality. While S. cerevisiae is a widely used model for cell aging [46, 50], S. pombe may be a model system for immortal cells, such as the germline. In addition, S. pombe represents an attractive tool for studying aging as a gain of function: manipulation of growth conditions rapidly generates high numbers of fluorescently labeled aging cells, amenable to sorting and genetic and biochemical manipulation. Comparative studies of aging and nonaging life strategies across single-cell species will help to clarify what determines the replicative potential and aging of cells in higher eukaryotic organisms [51].
Supplementary Material
Acknowledgments
We thank J. Bähler, I. Hagan, M.G. Ferreira, and G. Rödel for strains; J. Peychl, B. Schroth-Diez, T. Franzmann, and C. Iserman for help with experiments; I. Šarić for the drawings; and J. Howard, T. Kurzchalia, E. Paluch, M.G. Ferreira, J. Matos, J.H. Koschwanez, and the members of the Tolić-Nørrelykke group for discussions and comments on the manuscript. This work was supported by the Max Planck Society. M.C. received a fellowship (SFRH/BD/37056/2007) from the Portuguese Foundation for Science and Technology (FCT).
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, five figures, two tables, and four movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.07.084.
References
- 1.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jazwinski SM. The genetics of aging in the yeast Saccharomyces cerevisiae. Genetica. 1993;91:35–51. doi: 10.1007/BF01435986. [DOI] [PubMed] [Google Scholar]
- 4.Ackermann M, Stearns SC, Jenal U. Senescence in a bacterium with asymmetric division. Science. 2003;300:1920. doi: 10.1126/science.1083532. [DOI] [PubMed] [Google Scholar]
- 5.Fu XH, Meng FL, Hu Y, Zhou JQ. Candida albicans, a distinctive fungal model for cellular aging study. Aging Cell. 2008;7:746–757. doi: 10.1111/j.1474-9726.2008.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortimer RK, Johnston JR. Life span of individual yeast cells. Nature. 1959;183:1751–1752. doi: 10.1038/1831751a0. [DOI] [PubMed] [Google Scholar]
- 7.Stewart EJ, Madden R, Paul G, Taddei F. Aging and death in an organism that reproduces by morphologically symmetric division. PLoS Biol. 2005;3:e45. doi: 10.1371/journal.pbio.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P, Robert L, Pelletier J, Dang WL, Taddei F, Wright A, Jun S. Robust growth of Escherichia coli. Curr Biol. 2010;20:1099–1103. doi: 10.1016/j.cub.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyström T. A bacterial kind of aging. PLoS Genet. 2007;3:e224. doi: 10.1371/journal.pgen.0030224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindner AB, Madden R, Demarez A, Stewart EJ, Taddei F. Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc Natl Acad Sci USA. 2008;105:3076–3081. doi: 10.1073/pnas.0708931105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilaniu H, Gustafsson L, Rigoulet M, Nyström T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Larsson L, Caballero A, Hao X, Oling D, Grantham J, Nyström T. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Erjavec N, Cvijovic M, Klipp E, Nyström T. Selective benefits of damage partitioning in unicellular systems and its effects on aging. Proc Natl Acad Sci USA. 2008;105:18764–18769. doi: 10.1073/pnas.0804550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker MG, Walmsley RM. Replicative ageing in the fission yeast Schizosaccharomyces pombe. Yeast. 1999;15:1511–1518. doi: 10.1002/(sici)1097-0061(199910)15:14<1511::aid-yea482>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Calleja GB, Zuker M, Johnson BF, Yoo BY. Analyses of fission scars as permanent records of cell division in Schizosaccharomyces pombe. J Theor Biol. 1980;84:523–544. doi: 10.1016/s0022-5193(80)80018-x. [DOI] [PubMed] [Google Scholar]
- 16.Minois N, Frajnt M, Dölling M, Lagona F, Schmid M, Küchenhoff H, Gampe J, Vaupel JW. Symmetrically dividing cells of the fission yeast schizosaccharomyces pombe do age. Biogerontology. 2006;7:261–267. doi: 10.1007/s10522-006-9025-y. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura TM, Cooper JP, Cech TR. Two modes of survival of fission yeast without telomerase. Science. 1998;282:493–496. doi: 10.1126/science.282.5388.493. [DOI] [PubMed] [Google Scholar]
- 19.Egilmez NK, Jazwinski SM. Evidence for the involvement of a cytoplasmic factor in the aging of the yeast Saccharomyces cerevisiae. J Bacteriol. 1989;171:37–42. doi: 10.1128/jb.171.1.37-42.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinclair DA, Guarente L. Extrachromosomal rDNA circles— a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 21.Pereira G, Tanaka TU, Nasmyth K, Schiebel E. Modes of spindle pole body inheritance and segregation of the Bfa1p-Bub2p checkpoint protein complex. EMBO J. 2001;20:6359–6370. doi: 10.1093/emboj/20.22.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tkemaladze JV, Chichinadze KN. Centriolar mechanisms of differentiation and replicative aging of higher animal cells. Biochemistry (Mosc) 2005;70:1288–1303. doi: 10.1007/s10541-005-0261-6. [DOI] [PubMed] [Google Scholar]
- 23.Hughes AL, Gottschling DE. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature. 2012;492:261–265. doi: 10.1038/nature11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zadrag R, Kwolek-Mirek M, Bartosz G, Bilinski T. Relationship between the replicative age and cell volume in Saccharomyces cerevisiae. Acta Biochim Pol. 2006;53:747–751. [PubMed] [Google Scholar]
- 25.Mitchison JM, Nurse P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1985;75:357–376. doi: 10.1242/jcs.75.1.357. [DOI] [PubMed] [Google Scholar]
- 26.Baumgärtner S, Tolić-Nu˛rrelykke IM. Growth pattern of single fission yeast cells is bilinear and depends on temperature and DNA synthesis. Biophys J. 2009;96:4336–4347. doi: 10.1016/j.bpj.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grallert A, Krapp A, Bagley S, Simanis V, Hagan IM. Recruitment of NIMA kinase shows that maturation of the S. pombe spindle-pole body occurs over consecutive cell cycles and reveals a role for NIMA in modulating SIN activity. Genes Dev. 2004;18:1007–1021. doi: 10.1101/gad.296204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohrmann M, Schmidt S, Hagan I, Simanis V. Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase Cdc7p. Genes Dev. 1998;12:84–94. doi: 10.1101/gad.12.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spokoini R, Moldavski O, Nahmias Y, England JL, Schuldiner M, Kaganovich D. Confinement to organelle-associated inclusion structures mediates asymmetric inheritance of aggregated protein in budding yeast. Cell Rep. 2012;2:738–747. doi: 10.1016/j.celrep.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 30.Zhou C, Slaughter BD, Unruh JR, Eldakak A, Rubinstein B, Li R. Motility and segregation of Hsp104-associated protein aggregates in budding yeast. Cell. 2011;147:1186–1196. doi: 10.1016/j.cell.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bähler J, Nurse P. Fission yeast Pom1p kinase activity is cell cycle regulated and essential for cellular symmetry during growth and division. EMBO J. 2001;20:1064–1073. doi: 10.1093/emboj/20.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macieira-Coelho A, Pontén J, Philipson L. The division cycle and RNA-synthesis in diploid human cells at different passage levels in vitro. Exp Cell Res. 1966;42:673–684. doi: 10.1016/0014-4827(66)90280-1. [DOI] [PubMed] [Google Scholar]
- 33.Grove GL, Cristofalo VJ. Characterization of the cell cycle of cultured human diploid cells: effects of aging and hydrocortisone. J Cell Physiol. 1977;90:415–422. doi: 10.1002/jcp.1040900305. [DOI] [PubMed] [Google Scholar]
- 34.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 35.Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- 36.Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy BK, Austriaco NR, Jr, Guarente L. Daughter cells of Saccharomyces cerevisiae from old mothers display a reduced life span. J Cell Biol. 1994;127:1985–1993. doi: 10.1083/jcb.127.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyata M, Miyata H, Johnson BF. Sibling differences in cell death of the fission yeast, Schizosaccharomyces pombe, exposed to stress conditions. Antonie van Leeuwenhoek. 2000;78:203–207. doi: 10.1023/a:1026556111051. [DOI] [PubMed] [Google Scholar]
- 39.Sipiczki M. Splitting of the fission yeast septum. FEMS Yeast Res. 2007;7:761–770. doi: 10.1111/j.1567-1364.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Luo C, Zou K, Xie Z, Brandman O, Ouyang Q, Li H. Single cell analysis of yeast replicative aging using a new generation of microfluidic device. PLoS ONE. 2012;7:e48275. doi: 10.1371/journal.pone.0048275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lackner DH, Schmidt MW, Wu S, Wolf DA, Bähler J. Regulation of transcriptome, translation, and proteome in response to environmental stress in fission yeast. Genome Biol. 2012;13:R25. doi: 10.1186/gb-2012-13-4-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sørensen JG, Nielsen MM, Kruhøffer M, Justesen J, Loeschcke V. Full genome gene expression analysis of the heat stress response in Drosophila melanogaster. Cell Stress Chaperones. 2005;10:312–328. doi: 10.1379/CSC-128R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, Vabulas RM. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144:67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 44.Lu C, Brauer MJ, Botstein D. Slow growth induces heat-shock resistance in normal and respiratory-deficient yeast. Mol Biol Cell. 2009;20:891–903. doi: 10.1091/mbc.E08-08-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinkraus KA, Kaeberlein M, Kennedy BK. Replicative aging in yeast: the means to the end. Annu Rev Cell Dev Biol. 2008;24:29–54. doi: 10.1146/annurev.cellbio.23.090506.123509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rattan SI. Aging, anti-aging, and hormesis. Mech Ageing Dev. 2004;125:285–289. doi: 10.1016/j.mad.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Pérez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci USA. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ungvari Z, Ridgway I, Philipp EE, Campbell CM, McQuary P, Chow T, Coelho M, Didier ES, Gelino S, Holmbeck MA, et al. Extreme longevity is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal. J Gerontol A Biol Sci Med Sci. 2011;66:741–750. doi: 10.1093/gerona/glr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- 51.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.