Abstract
OBJECTIVE
One-dose varicella vaccination for children was introduced in the United States in 1995. In 2006, a second dose was recommended to further decrease varicella disease and outbreaks. We describe the impact of the 2-dose vaccination program on varicella incidence, severity, and outbreaks in 2 varicella active surveillance areas.
METHODS
We examined varicella incidence rates and disease characteristics in Antelope Valley (AV), CA, and West Philadelphia, PA, and varicella outbreak characteristics in AV during 1995–2010.
RESULTS
In 2010, varicella incidence was 0.3 cases per 1000 population in AV and 0.1 cases per 1000 population in West Philadelphia: 76% and 67% declines, respectively, since 2006 and 98% declines in both sites since 1995; incidence declined in all age groups during 2006–2010. From 2006–2010, 61.7% of case patients in both surveillance areas had been vaccinated with 1 dose of varicella vaccine and 7.5% with 2 doses. Most vaccinated case patients had <50 lesions with no statistically significant differences among 1- and 2-dose cases (62.8% and 70.3%, respectively). Varicella-related hospitalizations during 2006–2010 declined >40% compared with 2002–2005 and >85% compared with 1995–1998. Twelve varicella outbreaks occurred in AV during 2007–2010, compared with 47 during 2003–2006 and 236 during 1995–1998 (P < .01).
CONCLUSIONS
Varicella incidence, hospitalizations, and outbreaks in 2 active surveillance areas declined substantially during the first 5 years of the 2-dose varicella vaccination program. Declines in incidence across all ages, including infants who are not eligible for varicella vaccination, and adults, in whom vaccination levels are low, provide evidence of the benefit of high levels of immunity in the population.
Keywords: varicella/epidemiology, varicella vaccination, chickenpox/epidemiology, chickenpox vaccine, population surveillance, United States/epidemiology
Before the introduction of varicella vaccine in the United States in 1995, there were an estimated 4 million cases of varicella, 10 000 varicella-related hospitalizations, and 100 deaths annually.1–4 During the 1-dose varicella vaccination era (1995–2005), varicella incidence, deaths, and hospitalizations declined by 90%, 88%, and >65% respectively.5–7
Despite dramatic declines in varicella incidence and severe disease, varicella outbreaks continued to occur in highly vaccinated school populations. In 2006, based on disease epidemiology during the 1-dose varicella vaccination program, including outbreaks among highly vaccinated school populations, immune response to vaccination, and vaccine effectiveness, the Advisory Committee on Immunization Practices recommended a routine second dose of varicella vaccine for all children.8 We used data from 2 varicella active surveillance areas from 1995 to 2010 to describe the impact of the 2-dose varicella vaccination program on varicella incidence, disease characteristics, and outbreaks.
METHODS
Active surveillance for varicella was conducted in Antelope Valley (AV), CA, and West Philadelphia (WP), PA, from 1995 to 2010 with funding from the Centers for Disease Control and Prevention (CDC) by County of Los Angeles Department of Public Health and Philadelphia Department of Public Health, as described previously.5,9–12 AV, a geographically defined health services district ~70 miles outside the city of Los Angeles, had a population of 373 098 in 2010, of which 42% were white non-Hispanic and 40% were white Hispanic. WP, an inner-city area of Philadelphia, had a population of 272 260 in 2010, of whom 74% were African American. The populations of the 2 surveillance areas were disproportionately African American and Hispanic compared with the overall US population in 2010 (13.6% African American, 16.3% Hispanic).13
A varicella case was defined as an illness characterized by acute onset of a generalized maculopapulovesicular rash without other known cause.14 One-dose breakthrough varicella was defined as a varicella case in a person >1 year of age vaccinated with 1 dose of varicella vaccine >42 days before onset of rash. Two-dose breakthrough varicella was defined as a varicella case in a person >1 year of age vaccinated with 2 doses of varicella vaccine, with the second dose received >42 days before rash onset. A varicella outbreak was defined as ≥5 cases of varicella from a common setting, epidemiologically linked, and occurring within ≥1 incubation periods (ie, ≥21 days) of another case. In 2010, there were 263 reporting sites in AV and 330 in WP. Reporting sites included schools, day cares, health care providers, hospitals, public health clinics, correctional facilities, and homeless shelters. Reporting sites reported biweekly, even when no cases were identified. Sites that failed to report biweekly were contacted to verify the absence of cases. Surveillance staff conducted case investigations with each case patient or parent/guardian to collect demographic, clinical, and epidemiologic data through a structured telephone interview. Medical records were reviewed for all serious varicella-related complications or hospitalizations. Immunocompromising conditions were defined as “chronic medical conditions that depress the immune system (eg, cancer, leukemia, HIV/AIDS, organ transplant).” Vaccination status was verified by using vaccination registries and school or provider records.
National Immunization Survey results from Los Angeles County and Philadelphia County were used to estimate 1-dose varicella vaccination coverage among children 19 to 35 months of age in AV and WP during 2006–2010.15,16 Because the National Immunization Survey does not capture 2-dose varicella vaccination coverage, a variety of data sources were used to derive estimates of 2-dose varicella vaccination coverage. In AV, 2-dose varicella vaccination data were obtained from health forms completed by parents or guardians of kindergarten students who entered an AV public school district during the 2009–2010 school year, similar to methods used by CDC to annually analyze school vaccination coverage data from federally funded immunization programs.17 Two-dose varicella vaccination coverage among Kaiser Permanente Southern California enrollees aged 4 to 6 years who represented ~20% of the insured population residing in AV was determined using the Kaiser Immunization Tracking System using methods described previously.18 Two-dose varicella vaccination data were extracted from the Philadelphia Department of Public Health’s Kids Immunization Database/Tracking System for children aged 4 to 12 years living in WP who had ≥1 vaccinations of any type recorded.
SAS (version 9.2; SAS Institute, Cary, NC) was used for data analysis. We calculated incidence rates of reported varicella per 1000 population using population estimates from the US Census Bureau. We used Poisson regression to estimate the annual trend in age-specific incidence rates over the periods from 1995, the year varicella vaccine was introduced, and 2006, the last full year of the 1-dose vaccination program, to 2010, the fourth full year of the routine 2-dose varicella vaccination program for children in the United States.
RESULTS
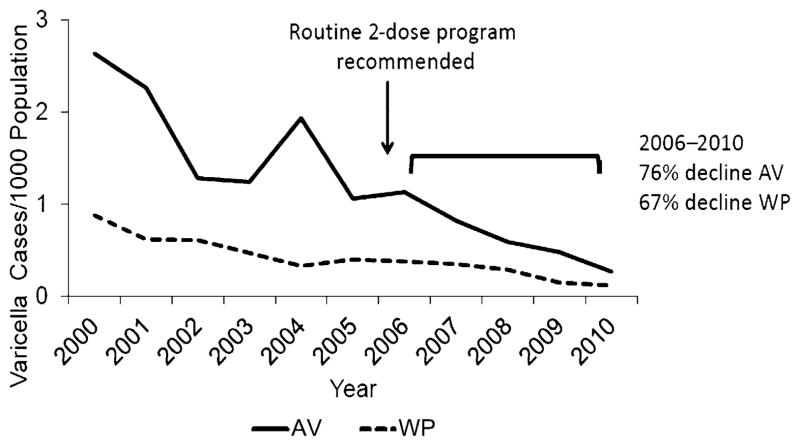
There were 1528 cases reported from 2006 to 2010, 1183 from AV and 345 from WP; 145 (9.5%) were laboratory confirmed. From 2006 to 2010, varicella incidence declined by 76.3% in WP (P < .001) and 67.1% in AV (P < .001; Fig 1). Age-specific varicella incidence rates declined 81.3% to 98.7% during 1995–2010 (P < .001) and were lower for all age groups in 2010 compared with 2006 (Table 1).
FIGURE 1.
Varicella incidence: AV (Los Angeles County, CA) and WP (PA), 2000–2010.
TABLE 1.
Reported Numbers of Varicella Cases and Incidence Rates (Per 1000 Population) by Age Group, AV (CA) and WP (PA), 1995 and 2006–2010
| Age, y | 1995
|
2006
|
2007
|
2008
|
2009
|
2010
|
Percentage Change
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | 2010 vs 1995 | 2010 vs 2006 | |
| AV | ||||||||||||||
| <1 | 134 | 19.7 | 20 | 3.4 | 12 | 2.0 | 11 | 1.7 | 12 | 1.9 | 4 | 0.6 | −96.7*** | −81.3** |
| 1–4 | 1127 | 48.8 | 42 | 1.9 | 40 | 1.7 | 32 | 1.3 | 35 | 1.4 | 23 | 0.9 | −98.2*** | −54.0** |
| 5–9 | 1228 | 54.9 | 164 | 6.3 | 109 | 4.3 | 70 | 2.8 | 42 | 1.6 | 20 | 0.7 | −98.7*** | −88.3*** |
| 10–14 | 235 | 10.8 | 121 | 3.7 | 95 | 3.1 | 73 | 2.4 | 54 | 1.9 | 24 | 0.9 | −91.5*** | −75.3*** |
| 15–19 | 65 | 3.1 | 22 | 0.7 | 19 | 0.5 | 18 | 0.5 | 16 | 0.5 | 15 | 0.5 | −84.9*** | −29.3a |
| ≥20 | 145 | 0.8 | 25 | 0.1 | 20 | 0.1 | 13 | 0.1 | 18 | 0.1 | 14 | 0.1 | −92.9*** | −50.0* |
| Overall | 2934 | 10.3 | 394 | 1.1 | 295 | 0.8 | 217 | 0.6 | 177 | 0.5 | 100 | 0.3 | −97.4*** | −76.3*** |
| WP | ||||||||||||||
| <1 | 38 | 8.8 | 2 | 0.5 | 4 | 1.1 | 5 | 1.3 | 4 | 1.0 | 3 | 0.7 | −91.6*** | 40.8a |
| 1–4 | 358 | 20.8 | 31 | 2.0 | 23 | 1.5 | 22 | 1.4 | 13 | 0.8 | 9 | 0.6 | −97.3*** | −72.7** |
| 5–9 | 534 | 27.3 | 27 | 1.5 | 28 | 1.6 | 16 | 0.9 | 7 | 0.4 | 6 | 0.3 | −98.8*** | −78.7** |
| 10–14 | 162 | 8.9 | 25 | 1.3 | 11 | 0.6 | 8 | 0.4 | 8 | 0.4 | 2 | 0.1 | −98.8*** | −91.4** |
| 15–19 | 39 | 1.9 | 5 | 0.2 | 10 | 0.4 | 3 | 0.1 | 6 | 0.2 | 4 | 0.1 | −92.4*** | −25.1a |
| ≥20 | 60 | 0.3 | 12 | 0.1 | 17 | 0.1 | 21 | 0.1 | 3 | 0.0 | 10 | 0.1 | −81.3*** | −17.3a |
| Overall | 1197 | 4.1 | 102 | 0.4 | 93 | 0.4 | 75 | 0.3 | 41 | 0.2 | 34 | 0.1 | −97.0*** | −67.1*** |
Nonsignificant (P ≥.05).
P < .05.
P < .01.
P < .001.
Of the 1302 (89.7%) case patients ≥1 year of age from AV and WP during 2006–2010 with information on vaccination status, 61.7% had received 1 dose of varicella vaccine and 7.5% 2 doses. Although the overall proportion of case patients with ≥1 doses of varicella vaccine did not vary significantly during 2006–2010, the proportion among case patients 10–14 and 15–19 years of age increased from 56.2% to 92.3% and 11.1% to 31.6%, respectively. Among vaccinated case patients 4 to 14 years of age, the proportion that had received 2 doses of varicella vaccine increased from 0.9% in 2006 to 43.4% in 2010. The median age among vaccinated case patients in AV increased from 8 years in 2006 to 9 years in 2010 and from 6 years in 2006 to 7 years in 2010 in WP. Among unvaccinated case patients, the median age in AV increased from 12 years in 2006 to 16 years in 2010 and from 15 years in 2006 to 20 years in 2010 in WP.
Information on vaccination status and number of lesions was available for 1216 (83.8%) case patients ≥1 year of age during 2006–2010 (Table 2). Among those ≥4 years of age, there were no statistically significant differences in the proportions by number of lesions comparing 1- to 2-dose breakthrough case patients within age groups or comparing age groups within 1- and 2-dose vaccination-status categories.
TABLE 2.
Number of Lesions by Age Group and Vaccination Status of Case Patients by Age Group, AV (Los Angeles County, CA) and WP (PA), 2006–2010, n = 1216
| No. of Lesions | Vaccinated With 2 Doses, n (%) | Vaccinated With 1 Dose, n (%) | Unvaccinated, n (%) |
|---|---|---|---|
| 1–3 y | n = 1 | n = 111 | n = 43 |
| <50 lesions | 1 (100) | 81 (73.0) | 21 (48.8) |
| 50–500 lesions | 0 | 29 (26.1) | 22 (51.2) |
| >500 lesions | 0 | 1 (0.9) | 0 |
| 4–7 y | n = 52 | n = 196 | n = 23 |
| <50 lesions | 38 (73.1) | 134 (68.4) | 16 (69.6) |
| 50–500 lesions | 14a (26.9) | 62 (31.6) | 6 (26.1) |
| >500 lesions | 0 | 0 | 1 (4.4) |
| 8–14 y | n = 36 | n = 435 | n = 136 |
| <50 lesions | 23 (63.9) | 299 (68.7) | 47 (34.6) |
| 50–500 lesions | 13a (36.1) | 133 (30.6) | 84 (61.8) |
| >500 lesions | 0 | 3 (0.7) | 5 (3.7) |
| 15–19 y | n = 2 | n = 18 | n = 65 |
| <50 lesions | 2 (100) | 11 (61.1) | 19 (29.2) |
| 50–500 lesions | 0 | 7 (38.9) | 40 (61.5) |
| >500 lesions | 0 | 0 | 6 (9.2) |
| ≥20 y | n = 0 | n = 3 | n = 95 |
| <50 lesions | — | 3 (100) | 31 (32.6) |
| 50–500 lesions | — | 0 | 61 (64.2) |
| >500 lesions | — | 0 | 3 (3.2) |
None of the 2-dose breakthrough case patients had >250 lesions.
A smaller proportion of the case patients reported during 2006–2010 had immunocompromising conditions compared with 1995–2005 (0.4% vs 11.7% for 0- to 14-year-olds, 0% vs 13.7% for 15- to 19-year-olds, and 3.6% vs 13.6% for ≥20-year-olds, respectively, P < .01 for all 3 age groups). A larger proportion of the adolescent and adult case patients reported during 2006–2010 received antiviral treatment of varicella compared with during 1995–2005 (26.8% vs 16.7% for 15- to 19-year-olds and 59.6% vs 26.1% for ≥20- year-olds, respectively, P < .01 for both age groups).
During 2006–2010, there were 3 varicella-related hospitalizations in AV and 7 in WP for varicella-related hospitalization rates of 0.17 per 100 000 population (95% confidence interval 0.05–0.51) in AV and 0.52 per 100 000 population (95% confidence interval 0.25–1.10) in WP. This represents declines in varicella-related hospitalizations of 43.3% and 92.2% in AV compared with 2002–2005 and 1995–1998, respectively, and 47.4% and 86.8% in WP. Among the 10 hospitalized case patients from AV and WP during 2006–2010, 1 was <1 year old, 1 was 14 years old, and 8 were ≥20 years of age. None of the hospitalized case patients had been vaccinated. Only 1 of the hospitalized case patients had an immunocompromising condition. Five of the 10 hospitalized case patients had varicella-related complications; 2 had pneumonia, and 3 had skin/soft tissue infections.
Between 2003 and 2006, 3 varicella outbreaks were reported from WP (2 in schools and 1 in a day care), and no outbreaks occurred after implementation of the 2-dose recommendation. There were 12 varicella outbreaks in AV during 2007–2010, compared with 47 during 2003–2006 and 236 during 1995–1998 (P < .01; Table 3), representing 74.5% and 94.9% declines during 2007–2010 compared with 2003–2006 and 1995–1998, respectively. There were no outbreaks in AV reported in 2011. Overall, 66 (65%) outbreak-related case patients identified during 2007–2010 in AV had been vaccinated, all with 1 dose. A larger proportion of outbreak-related case patients during 2007–2010 had mild disease (<50 lesions) compared with earlier time periods.
TABLE 3.
Characteristics of Varicella Outbreaks and Outbreak-Related Cases in an Active Surveillance Site by 4-Year Time Periods, AV (CA), 1995–2010
| Characteristic | 1995–1998 | 1999–2002 | 2003–2006 | 2007–2010 | Pa |
|---|---|---|---|---|---|
| Total number of outbreaks | 236 | 52 | 47 | 12 | <.01b |
| Duration of outbreak, median (range), d | 45 (7–198) | 41 (1–149) | 30 (3–72) | 38 (5–61) | <.01 |
| Outbreak-related cases | |||||
| Total number | 5409 | 703 | 499 | 102 | <.01c |
| Proportion of outbreak-related cases among total number of reported cases | 57.8 | 27.6 | 27.6 | 12.9 | <.01 |
| Number of cases per outbreak, median (range) | 15 (5–124) | 11 (5–56) | 9 (5–45) | 9 (5–11) | <.01c |
| Outbreak size, n (%) of outbreaks | |||||
| <10 cases | 65 (27.5) | 24 (46.2) | 30 (63.8) | 7 (58.3) | <.01 |
| 10–24 cases | 106 (44.9) | 22 (42.3) | 15 (31.9) | 5 (41.7) | <.01 |
| 25–49 cases | 39 (16.5) | 5 (9.6) | 2 (4.3) | 0 | <.01 |
| ≥50 cases | 26 (11.0) | 1 (1.9) | 0 | 0 | <.01 |
| Age of case patients, median (range), y | 6 (0–59) | 7 (0–49) | 9 (0–43) | 11 (0–41) | <.01c |
| Total outbreak case patients, by age, n (%), y | |||||
| <1 | 150 (2.8) | 20 (2.8) | 5 (1.0) | 1 (1.0) | <.01 |
| 1–4 | 1596 (29.5) | 115 (16.4) | 25 (5.0) | 3 (2.9) | <.01 |
| 5–9 | 2987 (55.2) | 442 (62.9) | 254 (50.9) | 32 (31.4) | <.01 |
| 10–14 | 402 (7.4) | 107 (15.2) | 194 (38.9) | 61 (59.8) | <.01 |
| 15–19 | 73 (1.4) | 3 (0.4) | 10 (2.0) | 3 (2.9) | <.01 |
| ≥20 | 201 (3.7) | 16 (2.3) | 11 (2.2) | 2 (2.0) | <.01 |
| Vaccinated outbreak case patients, by age, n (%), y | |||||
| 1–4 | 32 (2.0) | 38 (33.0) | 16 (64.0) | 1 (33.3) | <.01 |
| 5–9 | 40 (1.0) | 95 (21.5) | 201 (79.1) | 29 (90.6) | <.01 |
| 10–14 y | 1 (0.2) | 17 (15.9) | 73 (37.6) | 36 (59.0) | <.01 |
| 15–19 y | 0 (0) | 1 (33.3) | 2 (20.0) | 0 | NS |
| ≥20 y | 6 (3.0) | 1 (6.3) | 2 (18.2) | 0 | NS |
| Total | 79 (1.5) | 152 (21.6) | 294 (58.9) | 66 (64.7) | <.01 |
| Lesion count, n (%) of cases | |||||
| <50 | 1894 (35.0) | 287 (40.9) | 252 (51.0) | 59 (58.4) | <.01 |
| ≥50 | 3515 (65.0) | 414 (59.1) | 242 (49.0) | 42 (41.6) | <.01 |
| Complications, n (%) of cases | 502 (9.3) | 39 (5.6) | 14 (2.8) | 1 (1.0) | <.01 |
NS, not significant.
Determined by χ2 test for trend, unless otherwise specified.
Linear regression used to determine P value.
Kruskal-Wallis test.
In AV, starting in 2001, children entering kindergarten or entering California schools from out of state were required to have 1 dose of varicella vaccine or evidence of immunity. By school year 2010–2011, students in kindergarten through 10th grade were covered by the 1-dose requirement. In Los Angeles County, estimated 1-dose varicella vaccination coverage among 19- to 35-month-olds increased from 90.5% in 2006 to 95.1% in 2010. Two-dose varicella vaccination coverage among kindergarten students in an AV public school district (n = 2026) at the start of the 2009–2010 school year was 84%. Two-dose varicella vaccination coverage among Kaiser Permanente Southern California enrollees residing in AV was 76% among 4-year-olds, 98% among 5-year-olds, and 99% among 6-year-olds in 2010.
In Philadelphia, school entry requirements for 1 dose of varicella vaccine were implemented in 2000 for kindergarten and 2001 for sixth grade with grades K through 12 covered by the 2007–2008 school year. Two doses of varicella vaccine were required for students entering kindergarten and first grade in 2007–2008 and sixth grade in 2009–2010. One-dose varicella vaccination coverage among children 19 to 35 months of age was 92.7% to 94.6% during 2006–2010. During 2007–2010, 2-dose varicella vaccine coverage among children aged 4 to 6 years increased from 39% to 59%. By 2010, 2-dose varicella vaccine coverage rates among children aged 7 to 9 years and 10 to 12 years were 61% and 45%.
DISCUSSION
The United States transitioned from a 1-dose to a 2-dose varicella vaccination program for children in 2006 to further decrease varicella and its complications.8 The second dose of varicella vaccine was expected to provide higher vaccine efficacy and offer improved protection to the 15% to 20% of children who do not respond adequately to the first dose.19 Declines in varicella incidence and outbreaks reported since 2006 through vaccine effectiveness studies and passive surveillance have provided early indications of the impact of the 2-dose varicella vaccination program.20–22 The substantial declines in varicella incidence and outbreaks we report on from these 2 active surveillance areas during the first 5 years of the 2-dose varicella vaccination program provide additional evidence of the program’s sustained impact. Supporting the attribution of these declines in varicella incidence to the 2-dose vaccination program is the fact that the greatest declines in varicella incidence have been noted among children for whom the second dose was recommended. Declines in varicella incidence were seen across all age groups, including infants, who are not eligible for varicella vaccination, and adults, in whom vaccination levels are considered to be low, with a smaller proportion of cases among persons with immunocompromising conditions, providing evidence of the benefit of high levels of immunity in the population. Varicella-related hospitalizations in the active surveillance areas declined nearly 50% during the first 5 years of the 2-dose varicella vaccination program. With full implementation of the 2-dose varicella vaccination program, it may be possible to eliminate the most severe outcomes of varicella.
Opportunities for further declines in varicella incidence remain; in 2010, 57% of cases among 4- to 14-year-olds occurred in children who had received 1 or no doses of varicella vaccine. Although data are limited, there does appear to have been rapid uptake of the second dose of varicella vaccine among elementary school–age children in the years immediately after it was recommended.18,20 However, challenges to ensuring protection of all children before they enter adolescence and adulthood remain. In 2011, an estimated 20% of adolescents had never had varicella and had not received the second dose of varicella vaccine.23 Catch-up vaccination of children and adolescents who previously received 1 dose of varicella vaccine is recommended; providers should use all routine health care visits to ensure that all children without evidence of varicella immunity have received 2 doses of varicella vaccine.8
An important consideration in the adoption of a 2-dose varicella vaccination program in the United States was the burden posed by varicella outbreaks, which, although greatly reduced in number and size, continued to occur even in settings with high 1-dose varicella vaccination coverage.11,24,25 During 2007–2010, there were no outbreaks in WP. In addition to the school entry regulations for vaccination, Philadelphia Department of Public Health was extremely proactive in implementing prevention and control measures when notified of a case in a school or day care setting. In AV, the number of outbreaks during 2007–2010 declined nearly fourfold compared with the last 4 years of the 1-dose varicella vaccination program. Although none of the outbreak-related cases identified in AV had received 2 doses of varicella vaccine, large outbreaks involving 2-dose vaccinees have been reported.26 Investigating cases and outbreaks among 1- and 2-dose vaccinees who present with modified disease is challenging in that it can be difficult to distinguish mild breakthrough varicella from other causes of rash and collect adequate specimens from maculopapular lesions for laboratory testing.26 As the positive predictive value of clinical diagnosis declines, better laboratory tools, including alternative specimen types suitable for collection in outbreak investigations that commence after rash resolution, are needed.27 Going forward, clinician education on the growing importance of laboratory confirmation of varicella will be important to ensure appropriate implementation of varicella control strategies.
Although the proportion of varicella cases that occurred among vaccinees remained stable since implementation of the 2-dose varicella vaccination program, nearly half of them were among 2-dose vaccinees in 2010. Data on clinical characteristics of breakthrough varicella in 2-dose vaccinees are limited, although cases with >300 lesions have not been reported to date.20,28,29 From our data as well as from a prelicensure varicella vaccine efficacy study, no statistically significant differences in the clinical characteristics of breakthrough varicella between 1- and 2-dose vaccinees have been reported.28 It is unclear if breakthrough varicella severity is truly similar across 1- and 2-dose vaccinees or if only the more severe end of the clinical spectrum of 2-dose varicella is coming to medical attention. The extent to which 2-dose breakthrough disease is contagious is not known, although it is likely to be mediated at least in part by the number of lesions as was demonstrated for 1-dose breakthrough varicella.30
A number of limitations to these data should be considered. Laboratory confirmation was not performed for all cases, and some cases clinically diagnosed as having varicella, especially breakthrough varicella, may not have had varicella. This would lead to an underestimation of declines in varicella. It is also possible that some varicella cases in the active surveillance areas did not come to medical attention, leading to an overestimation of declines in varicella incidence. Data sources for estimating 2-dose varicella vaccination coverage levels are limited; reliance on registry data may result in imprecise coverage estimates.
CONCLUSIONS
The varicella vaccination program in the United States has resulted in dramatic declines in varicella incidence, hospitalizations, and deaths. Ongoing surveillance will be needed to describe more fully the impact of the routine 2-dose varicella vaccination program in the United States. Full implementation of varicella vaccination recommendations across all age groups, including adolescents, adults, and women postpartum, remains critically important for ensuring the greatest possible protection of susceptible individuals at risk for severe varicella disease.
WHAT’S KNOWN ON THIS SUBJECT
The 1-dose childhood varicella vaccination program in the United States resulted in dramatic declines in varicella incidence, hospitalizations, and deaths. There is little information on the impact of the 2006 recommendation for 2-dose varicella vaccination of children on varicella epidemiology.
WHAT THIS STUDY ADDS
In the first 5 years of the 2-dose varicella vaccination program, declines in varicella incidence were seen in all age groups, including infants who are not eligible for varicella vaccination, providing evidence of the benefit of high population immunity.
Acknowledgments
FUNDING: Funded through a cooperative agreement with the Centers for Disease Control and Prevention.
We thank Niya Spells, BA, Rodrerica Pierre, BA, Salini Mohanty, MPH, Jimmy John, BA, E. Claire Newbern, PhD, MPH, Irini Daskalaki, MD, Mia Renwick, MPH, and Priya Abraham, BS, from Philadelphia Department of Public Health; Karen Kuguru, MPA, from County of Los Angeles Department of Public Health; and Jane Seward, MBBS, MPH, Scott Schmid, MS, PhD, and Kay Radford, BS, from the Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC.
ABBREVIATIONS
- AV
Antelope Valley
- CDC
Centers for Disease Control and Prevention
- WP
West Philadelphia
Footnotes
Dr Bialek conceptualized and designed the study; acquired, analyzed, and interpreted the data; and drafted and revised the manuscript; Ms Perella, Dr Viner, Ms Jackson, and Dr Civen each coordinated and supervised data collection at 1 of the 2 sites, analyzed and interpreted the data, and critically revised the manuscript; Dr Zhang analyzed and interpreted the data and critically revised the manuscript; Drs Mascola and Watson conceptualized and designed the study, acquired and interpreted the data, and critically revised the manuscript; Ms Lopez coordinated data collection, analyzed and interpreted the data, and critically revised the manuscript; and all authors approved the final manuscript as submitted.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, US Department of Health and Human Services, County of Los Angeles Department of Public Health, or the Philadelphia Department of Public Health.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Wharton M. The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am. 1996;10(3):571–581. doi: 10.1016/s0891-5520(05)70313-5. [DOI] [PubMed] [Google Scholar]
- 2.Galil K, Brown C, Lin F, Seward J. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr Infect Dis J. 2002;21(10):931–935. doi: 10.1097/00006454-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med. 2005;352(5):450–458. doi: 10.1056/NEJMoa042271. [DOI] [PubMed] [Google Scholar]
- 4.Meyer PA, Seward JF, Jumaan AO, Wharton M. Varicella mortality: trends before vaccine licensure in the United States, 1970–1994. J Infect Dis. 2000;182(2):383–390. doi: 10.1086/315714. [DOI] [PubMed] [Google Scholar]
- 5.Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites—United States, 1995–2005. J Infect Dis. 2008;197(suppl 2):S71–S75. doi: 10.1086/522156. [DOI] [PubMed] [Google Scholar]
- 6.Marin M, Zhang JX, Seward JF. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics. 2011;128(2):214–220. doi: 10.1542/peds.2010-3385. [DOI] [PubMed] [Google Scholar]
- 7.Lopez AS, Zhang J, Brown C, Bialek S. Varicella-related hospitalizations in the United States, 2000–2006: the 1-dose varicella vaccination era. Pediatrics. 2011;127(2):238–245. doi: 10.1542/peds.2010-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marin M, Güris D, Chaves SS, Schmid S, Seward JF Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC) Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-4):1–40. [PubMed] [Google Scholar]
- 9.Seward JF, Watson BM, Peterson CL, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA. 2002;287(5):606–611. doi: 10.1001/jama.287.5.606. [DOI] [PubMed] [Google Scholar]
- 10.Marin M, Watson TL, Chaves SS, et al. Varicella among adults: data from an active surveillance project, 1995–2005. J Infect Dis. 2008;197(suppl 2):S94–S100. doi: 10.1086/522155. [DOI] [PubMed] [Google Scholar]
- 11.Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis. 2008;197(suppl 2):S114–S119. doi: 10.1086/522144. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds MA, Watson BM, Plott-Adams KK, et al. Epidemiology of varicella hospitalizations in the United States, 1995–2005. J Infect Dis. 2008;197(suppl 2):S120–S126. doi: 10.1086/522146. [DOI] [PubMed] [Google Scholar]
- 13.United States Census Bureau. [Accessed May 30, 2013];American fact finder. Available at: http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_DP_DPDP1&-prodType=table.
- 14.Council of State and Territorial Epidemiologists. [Accessed February 19, 2013];Public health reporting and national notification for varicella. Available at: http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/09-ID-68.pdf.
- 15.Centers for Disease Control and Prevention (CDC) National, state, and local area vaccination coverage among children aged 19–35 months—United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56(34):880–885. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among children aged 19–35 months—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(34):1157–1163. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Vaccination coverage among children in kindergarten—United States, 2012–13 school year. MMWR Morb Mortal Wkly Rep. 2013;62(30):607–612. [PMC free article] [PubMed] [Google Scholar]
- 18.Hechter RC, Chao C, Li Q, Jacobsen SJ, Tseng HF. Second-dose varicella vaccination coverage in children and adolescents in a managed care organization in California, 2006–2009. Pediatr Infect Dis J. 2011;30(8):705–707. doi: 10.1097/INF.0b013e31820eae6a. [DOI] [PubMed] [Google Scholar]
- 19.Marin M, Meissner HC, Seward JF. Varicella prevention in the United States: a review of successes and challenges. Pediatrics. 2008;122(3) doi: 10.1542/peds.2008-0567. Available at: www.pediatrics.org/cgi/content/full/122/3/e744. [DOI] [PubMed] [Google Scholar]
- 20.Baxter R, Ray P, Tran TN, et al. Long-term effectiveness of varicella vaccine: a 14-year, prospective cohort study. Pediatrics. 2013;131(5) doi: 10.1542/peds.2012-3303. Available at: www.pediatrics.org/cgi/content/full/131/5/e1389. [DOI] [PubMed] [Google Scholar]
- 21.Kattan JA, Sosa LE, Bohnwagner HD, Hadler JL. Impact of 2-dose vaccination on varicella epidemiology: Connecticut—2005–2008. J Infect Dis. 2011;203(4):509–512. doi: 10.1093/infdis/jiq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) Evolution of varicella surveillance—selected states, 2000–2010. MMWR Morb Mortal Wkly Rep. 2012;61(32):609–612. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13–17 years—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(34):671–677. [PubMed] [Google Scholar]
- 24.Lopez AS, Guris D, Zimmerman L, et al. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics. 2006;117(6) doi: 10.1542/peds.2005-2085. Available at: www.pediatrics.org/cgi/content/full/117/6/e1070. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Outbreak of varicella among vaccinated children—Michigan, 2003. MMWR Morb Mortal Wkly Rep. 2004;53(18):389–392. [PubMed] [Google Scholar]
- 26.Mahamud A, Wiseman R, Grytdal S, et al. Challenges in confirming a varicella outbreak in the two-dose vaccine era. Vaccine. 2012;30(48):6935–6939. doi: 10.1016/j.vaccine.2012.07.076. [DOI] [PubMed] [Google Scholar]
- 27.Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51(1):23–32. doi: 10.1086/653113. [DOI] [PubMed] [Google Scholar]
- 28.Kuter B, Matthews H, Shinefield H, et al. Study Group for Varivax. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23(2):132–137. doi: 10.1097/01.inf.0000109287.97518.67. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro ED, Vazquez M, Esposito D, et al. Effectiveness of 2 doses of varicella vaccine in children. J Infect Dis. 2011;203(3):312–315. doi: 10.1093/infdis/jiq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA. 2004;292(6):704–708. doi: 10.1001/jama.292.6.704. [DOI] [PubMed] [Google Scholar]